Prospects for a better diagnosis and prognosis of NAFLD: a pathoIogist's view
CaroIin Lackner
Institute of Pathology, Medical University of Graz, Graz 8010, Austria.
Abstract Despite the development of surrogate non-invasive methods, histological evaluation remains an important tool for reliable classification, grading and staging, as well as prognosis in non-alcoholic fatty liver disease (NAFLD).However, histological evaluation has been criticised because it requires a liver biopsy, its propensity for sampling,and inter-observer variation. This article highlights the future developments in the morphological interpretation of liver biopsy in NAFLD, so as to aid in improving its diagnostic and prognostic utility.
Keywords: Non-alcoholic fatty liver disease, histology, prognosis, grading and staging
CURRENT PROPOSALS FOR A CHANGE IN TERMINOLOGY OF FATTY LIVER DISEASES
Traditionally, the liver manifestation of the metabolic syndrome has been known as non-alcoholic fatty liver disease (NAFLD). Recently, a group of experts proposed to change the name of this condition into metabolic-dysfunction-associated fatty liver disease (MAFLD) to highlight the underlying systemic metabolic dysfunction as the one of the primary causes of fatty liver disease[1,2]. In addition, some experts also suggested that for patients with both MAFLD and other risk factors for fatty liver disease such as alcohol abuse, the term “dual-etiology fatty liver disease” should be used. Currently the validity and utility of the proposed new terminology is debated[3,4]. In the light of ongoing discussion and since neither of the terms, MAFLD or “dual-etiology fatty liver disease”, has yet been endorsed by major liver societies the traditional term, NAFLD, is used in this review.
EPIDEMIOLOGY OF NON-ALCOHOLIC FATTY LIVER DISEASE
NAFLD is a major health concern. The ongoing epidemic of obesity provides the basis for the striking rise in incidence and prevalence of NAFLD around the world. Currently, the estimated global prevalence of NAFLD is 25%[5]and it is expected to increase substantially in the next decade: mortality and advanced liver disease due to NAFLD have been estimated to more than double during 2016-2030[6]. Cirrhosis, end-stage liver disease, and/or hepatocellular carcinoma due to NAFLD are also the leading indications for liver transplantation. NAFLD-related morbidity will continue to negatively impact public health and contribute to the escalating health-care cost[7].
TYPES OF NAFLD
NAFLD comprises a spectrum of diseases ranging from steatosis [non-alcoholic fatty liver (NAFL)] to nonalcoholic steatohepatitis (NASH) and NAFLD-associated fibrosis or cirrhosis. The types of NAFLD are defined by histology[8]. Morphological lesions of NAFLD are accentuated in centrilobular regions. NAFL refers to the accumulation of fat vesicles in hepatocytes (macrovesicular steatosis), affecting ≥ 5% of hepatocytes. In most cases, fatty change is associated with some degree of lobular inflammation.Inflammatory infiltrates are typically mild consisting mostly of mononuclear cells and eventually few admixed neutrophils. NASH is characterized by three key features: fatty change of hepatocytes,hepatocellular ballooning, and lobular inflammation.
Ballooning refers to a special form of hepatocellular degeneration that is characterized by enlargement and rounding of the cytoplasm contributed by the loss of the intermediate filament (IF) cytoskeleton,accumulation of small droplet fat, dilation of the ER as well as fluid retention. Ballooned hepatocytes often contain irregularly garland-shaped hyaline cytoplasmic inclusions, which consist of aggregated K8, K18, and a number of other stress-related proteins (reviewed in[9]).
Fatty change and inflammation- and ballooning-associated hepatocellular injury contribute to endoplasmatic reticulum (ER) stress and the activation of a tightly regulated cellular network consisting of macrophages, natural killer cells, B cells, and natural killer T cells, all of which control hepatic stellate cell activation. This results in the deposition of extracellular matrix containing collagen as well as other proteins around small groups and individual hepatocytes [reviewed in[10], giving rise to the so-called perisinusoidal or pericellular fibrosis (PSF and PCF, respectively); for simplicity the latter term is used in this manuscript],which is typical for fatty liver diseases. PCF can progress and extend from centrilobular areas to involve other portions of hepatic lobules connecting central veins and portal tracts (bridging the fibrosis).Eventually, in an ongoing liver injury, dense fibrous septa develop and destroy the lobular architecture.Parenchymal nodules without portal-central relations surrounded by fibrous septa are hallmarks of cirrhosis. In NAFLD-associated cryptogenic cirrhosis[11], the features of disease activity-steatosis, ballooning and inflammation-may subside and PCF too may no longer be a feature[12].
Prognostic reIevance and chaIIenges in the histoIogicaI diagnosis of NASH
The diagnosis of NASH is of clinical relevance[13]since it helps to identify patients who are at risk for the development of cirrhosis, liver failure, hepatocellular carcinoma, cardiovascular disease, and extrahepatic malignancies, which eventually result in elevated liver-related as well as overall mortality[14,15]. The diagnosis of NASH requires a liver biopsy, an invasive procedure that is subject to a small, but not insignificant risk of morbidity and mortality. A plethora of non-invasive methods to diagnose NASH have been developed.However, presently, the diagnostic accuracy of these approaches is not optimal. Therefore, current clinical practice guidelines issued by international scientific societies recommend liver biopsy and histological evaluation for patients in whom a diagnosis of NAFLD is uncertain or NAFLD-related advanced liver disease is suspected[16].
Hepatocellular ballooning is pivotal for the distinction between NAFL with relatively benign prognosis and NASH with considerably worse prognosis. It is also an integral feature of all current morphological grading and staging systems of NAFLD. Furthermore, clinical studies in NAFLD evaluating the utility of novel therapeutic strategies frequently include only those patients who have a histologically confirmed NASH.
Although the morphological features of NASH are well described, its histological diagnosis can be challenging. A great proportion of the diagnostic difficulties is related to the reliable recognition of ballooned hepatocytes in H&E-stained sections. Ballooning refers to a spectrum of morphological changes in the hepatocellular cytoplasm and its contents (see above) rather than a single feature that is subject to a moderate inter-observer agreement in the morphological interpretation[17,18]. However, several immunohistochemical markers for ballooned hepatocytes have been described. Oxidative stress-related rearrangement of the IF cytoskeleton leads to a loss of K8- and K18-associated staining of the cytoplasm. In contrast to normal-sized hepatocytes, the cytoplasm of ballooned cells is negative with antibodies against K8 and K18; whereas Mallory-Denk bodies containing the aggregated forms of K8 and K18, are positive[19].Ballooned hepatocytes have also been shown to express sonic hedgehog promoting pathways responsible for fibrogenesis in hedgehog-responsive stroma cells in the vicinity of ballooned hepatocytes and the development of PCF[20][Figure 1]. In addition, expression of aldose reductase, AKR1B10, presumably involved in the detoxification of oxidative stress-associated reactive aldehydes in NASH can also be detected by respective antibodies in ballooned hepatocytes[21]. These markers could be used to confirm ballooning by immunohistochemistry in doubtful cases and increase inter-observer agreement.
PROGNOSTIC IMPLICATIONS OF FIBROSIS STAGES IN NAFLD
Although NASH is associated with an increased risk for the development of fibrosis, several studies have identified fibrosis stages as the most important prognostic factor for NAFLD[22,23]. A large meta-analysis including 1495 NAFLD patients with approximately 17-year of follow-up showed that stage 1 NAFLD patients have an increased all-cause mortality risk, and this risk increases with progression to higher stages,whereas liver-related mortality increases exponentially after progress to stage 2. These data indicate that all cause- and liver-related mortality is predicted by individual fibrosis stages[14]. Furthermore, individual fibrosis stages are associated with cause-specific mortality in NAFLD. In a multi-national study including 458 NAFLD patients, liver-related events were predominantly associated with the cirrhosis stage, whereas patients with the bridging fibrosis stage were more prone to develop non-hepatic cancers and vascular events[15]. However, non-invasive fibrosis tests are not suited to accurately discriminate between individual histological fibrosis stages, thereby impacting accurate prognosis as well as efficient patient management.
Histology also offers information about the quality of fibrosis and has potential important implications in predicting the prognosis of NAFLD. Fibrogenesis is a complicated process involving hepatocytes,mesenchymal, and hematological cells, which leads to an excess accumulation and insufficient degradation of extracellular matrix (ECM) in the presence of a persistent liver injury (reviewed with emphasis on NAFLD in[24]). In addition, fibrogenesis can be propelled by platelets, endothelial, and stellate cells via liver injury-associated activation of the coagulation cascade and thrombin production (reviewed in[25]). However,if liver injury subsides, e.g., after antiviral therapy in patients with chronic viral hepatitis, degradation of ECM and hepatocellular regeneration leads to resorption of ECM and restitution of the lobular architecture.Fibrosis regression can occur in pre-cirrhotic as well as, to a certain extent, in the cirrhotic stage[26-28].Histological features of fibrosis vary between progressive and regressive disease processes. Recently, the Beijing classification of liver fibrosis was developed (reviewed in[29]) [Table 1]. This classification system provides a tool to not only stage fibrosis, but also to differentiate between progressive and regressive fibrosis types that are associated with outcomes. In contrast to patients with a fibrosis pattern that indicated regression, individuals with a progressive or indeterminate (balance of pro- and regressive features) fibrosis pattern continued to progress despite successful suppression of a viral infection[30]. Although the Beijing classification has been developed for the interpretation of biopsies of patients with viral hepatitis, it could also be applied in other chronic liver diseases like NAFLD[29]. However, studies specifically addressing this issue are currently lacking.

Figure 1. Immunohistochemical markers for the detection of hepatocellular ballooning (A-D). (A) Ballooned hepatocytes (indicated by arrow heads) with enlarged lightly stained cytoplasm containing lipid droplets and Mallory-Denk bodies (MDBs) are present among hepatocytes with fatty change. (B) Consecutive section of the HE stained section in (A). Immunohistochemical analysis reveals loss of cytoplasmic and positive MDB-staining signals with K8/18 antibodies in the ballooned hepatocytes (marked by arrow heads). In addition, in this deeper section another small group of ballooned hepatocytes with MDBs not clearly detectable in the respective H&E stain is indicated by the characteristic K8/18 staining pattern (ballooned hepatocytes are indicated by white arrow heads). (C) Ballooned hepatocytes adjacent to a central vein with loss of cytoplasmic K8/18 staining and K8/18-positive MDBs are strongly marked in a consecutive section (D) with antibodies against sonic hedgehog whereas the cytoplasm of normal sized hepatocytes shows only weak reactivity.
Another factor defining the quality of fibrosis is the composition of ECM. Data from animal as well as human studies suggest that paucicellular scar tissue present in dense fibrous septa that bridges fibrosis and cirrhosis consist of, among other proteins, cross-linked collagen and elastic fibres, which are more resistant to degradation than the ECM of early fibrosis stages and/or PCF. It is conceivable that the composition of ECM may thus differ with respect to a progression- or regression-related biological setting[31-34].Unfortunately, data on the prognostic utility of ECM composition in patients with NAFLD are sparse.
Dual-photon microscopy-based quantitation of fibrosis-related parameters (q-FP) is an interesting novel method for the refinement of hepatic fibrosis assessment. The q-FP analysis measures the quantity of collagen and also provides information on the architectural features of collagen fibers. Quantitative collagen and the collagen fiber pattern are strongly correlated to the NASH Clinical Research Network (CRN)fibrosis stages and represent an accurate and reproducible way to characterize fibrosis along a continuous scale in NAFLD[35].
IMPACT OF STANDARDS FOR GRADING AND STAGING NAFLD
Grading and staging refer to the semiquantitative assessment of inflammation and hepatocellular injury(activity) and fibrosis (stage) by the application of numerical scores. Grading and staging systems are used in many chronic liver diseases to predict a prognosis, guide patient management, assess treatment effects in clinical trials, and as research tools[36-38]. Several grading and staging systems have been described for NAFLD, among which the NAFLD activity score (NAS), the Clinical Research Network (CRN) staging system[17]and the steatosis, activity and fibrosis (SAF) scores[39]are the most widely used. These are important tools assisting the diagnosis of NASH and have been validated for clinical practice[40,41][Table 2].The natural course of NAFLD may be reflected in an improvement or worsening of morphological disease activity (indicated by the NAS and possibly also the SAF activity score), which is associated with regression or progression of fibrosis, respectively[42]. In clinical trials of NAFLD, the currently accepted endpoints for a conditional approval of pharmaceutical agents include resolution of NASH without worsening of the fibrosis and/or improvement in fibrosis without worsening of NASH, as assessed by a standardized histological evaluation of paired liver biopsies using the NAS and CRN staging systems[43]. Therefore,reliable recognition of key features of activity as well as stage are pivotal for the interpretation of results of clinical trials.
However, morphological interpretation is prone to inter-observer bias[44]. In the NAS and SAF systems,descriptive terms define the scores for morphological changes of grade and stage. To some extent, these definitions allow for a range of interpretations. For example, macrovesicular steatosis, a component of the NAS, is assessed according to the degree of “parenchymal involvement”, which can be interpreted as the percentage of hepatocytes containing macrovesicular fat or the proportion of parenchymal area contributed by macrovesicular lipid droplets [Figure 2]. By the same token, an uncertainty with respect to the exact definitions of other morphological features of grade and stage exists. Therefore, scores will vary among individual observers depending on the definitions used, which impacts the diagnostic categories and therefore has obvious consequences in the clinical management as well as in the selection of patients for clinical trials. However, inter-observer variation can be markedly reduced by a settlement on morphological standards prior to the histological evaluation using tutorial guidelines, diagnostic algorithms[39], and guideline images[45]. In a study on the utility of the SAF score and the fatty liver inhibition of progression diagnostic algorithm[39], the rate of agreement with a reference diagnosis increased after the application of the algorithm from 77% to 97% among expert liver pathologists, and from 42% to 75% among pathologists with a more general training. Similarly, the inter-observer agreement for the detection of hepatocellular ballooning and lobular inflammation, NASH features traditionally plagued by only fair to moderate kappa values[17,18], reached almost perfect and substantial levels (κ = 0.8 and 0.72, respectively) with tutorial guidance. The same was true for the interpretation of stage, for which almost perfect agreement (κ = 0.84)was found.
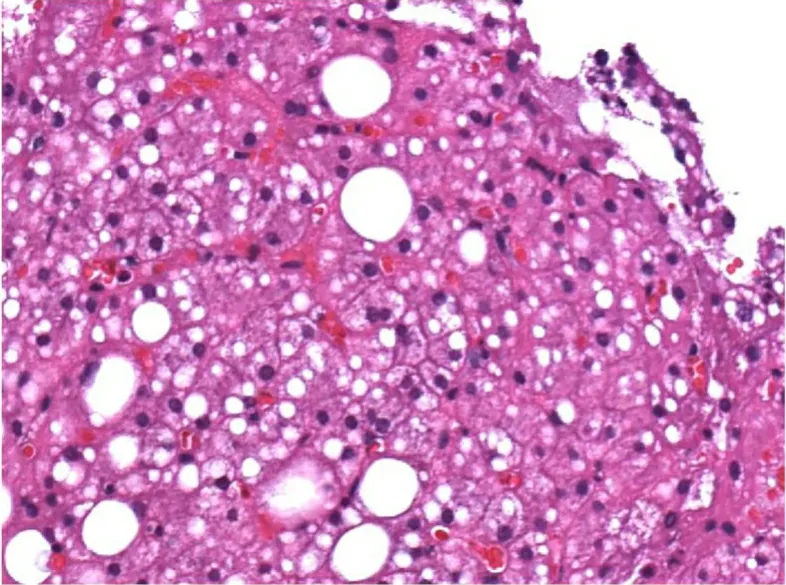
Figure 2. Impact of the morphological definition of steatosis on the results of semiquantitative grading. Approximately two thirds of the hepatocytes contain lipid droplets of variable sizes. However, if steatosis is defined by parenchymal areas contributed by lipid droplets the estimated degree of fatty change is considerably lower.
Recently, computer models have been developed to standardize the assessment of morphological features of grade and stage in NAFLD. Furthermore, semi-quantitative assessments can be replaced by quantitative measurements by using softwares that automatically evaluates steatosis, inflammation, ballooning, and fibrosis in liver tissue sections. It is expected that machine learning algorithms and quantification tools will improve reproducibility in the interpretation of grade and stage, which is required for clinical practice as well as for clinical trials of NASH[46,47].
SUMMARY
The classical histological interpretation of liver biopsies is an important tool for the diagnosis, classification as well as prognosis of NAFLD. Currently, histology is required for the exact diagnosis of NASH, the identification of individual fibrosis stages associated with diverging prognostic and therapeutic scenarios, as well as for the semi-quantitative assessment of grading and staging NAFLD. Developments in novel morphological methods such as computer-assisted digital image analysis, dual-photon microscopy, and the use of immunochemistry hold great promise in overcoming limitations related to the diagnosis and the inter-observer variability of grading and staging NAFLD. Future studies evaluating the applicability and utility of these novel methods for clinical practice and clinical trials are warranted.
DECLARATIONS
Authors’ contributions
The author contributed solely to the article.
AvaiIabiIity of data and materiaIs
Not applicable.
FinanciaI support and sponsorship
None.
ConfIicts of interest
The author declared that there are no conflicts of interest.
EthicaI approvaI and consent to participate
Not applicable.
Consent for pubIication
Not applicable.
Copyright
© The Author(s) 2021.
- Hepatoma Research的其它文章
- The impact of rituximab prophylaxis on hepatocellular carcinoma recurrence after living donor liver transplantation
- Laparoscopic ICG-guided RALPPS procedure for HCC on cirrhosis with 3D reconstruction implementation: a case report
- The narrow ridge from liver damage to hepatocarcinogenesis
- The evolution of minimally invasive surgery in liver transplantation for hepatocellular carcinoma
- Systematic review of existing guidelines for NAFLD assessment
- Mechanisms of protective effects of astaxanthin in nonalcoholic fatty liver disease

