Bile acid indices as biomarkers for liver diseases I:Diagnostic markers
Jawaher Abdullah Alamoudi,Wenkuan Li,Nagsen Gautam,Yazen Alnouti,Department of Pharmaceutical Sciences,College of Pharmacy,University of Nebraska Medical Center,Omaha,NE 68198,United States
Jawaher Abdullah Alamoudi,Department of Pharmaceutical Sciences,College of Pharmacy,Princess Nourah Bint Abdulrahman University,Riyadh 11564,Saudi Arabia
Marco Olivera,Department of Internal Medicine,College of Medicine,University of Nebraska Medical Center,Omaha,NE 68198,United States
Jane Meza, Department of Biostatistics,College of Public Health,University of Nebraska Medical Center,Omaha,NE 68198,United States
Sandeep Mukherjee,Department of Internal Medicine,College of Medicine,Creighton University Medical Center,Omaha,NE 68124,United States
Abstract BACKGROUND Hepatobiliary diseases result in the accumulation of toxic bile acids (BA) in the liver,blood,and other tissues which may contribute to an unfavorable prognosis.AIM To discover and validate diagnostic biomarkers of cholestatic liver diseases based on the urinary BA profile.METHODS We analyzed urine samples by liquid chromatography-tandem mass spectrometry and compared the urinary BA profile between 300 patients with hepatobiliary diseases vs 103 healthy controls by statistical analysis. The BA profile was characterized using BA indices,which quantifies the composition,metabolism,hydrophilicity,and toxicity of the BA profile. BA indices have much lower interand intra-individual variability compared to absolute concentrations of BA. In addition,BA indices demonstrate high area under the receiver operating characteristic curves,and changes of BA indices are associated with the risk of having a liver disease,which demonstrates their use as diagnostic biomarkers for cholestatic liver diseases.RESULTS Total and individual BA concentrations were higher in all patients. The percentage of secondary BA (lithocholic acid and deoxycholic acid) was significantly lower,while the percentage of primary BA (chenodeoxycholic acid,cholic acid,and hyocholic acid) was markedly higher in patients compared to controls. In addition,the percentage of taurine-amidation was higher in patients than controls. The increase in the non-12α-OH BA was more profound than 12α-OH BA (cholic acid and deoxycholic acid) causing a decrease in the 12α-OH/ non-12α-OH ratio in patients. This trend was stronger in patients with more advanced liver diseases as reflected by the model for end-stage liver disease score and the presence of hepatic decompensation. The percentage of sulfation was also higher in patients with more severe forms of liver diseases.CONCLUSION BA indices have much lower inter- and intra-individual variability compared to absolute BA concentrations and changes of BA indices are associated with the risk of developing liver diseases.
Key Words: Hepatobiliary diseases; Bile acids; Bile acid indices; Diagnosis; Biomarker;Liver diseases
INTRODUCTION
Bile acids (BA) have many physiological functions such as cholesterol absorption and elimination,fat absorption,and maintenance of healthy microbiome[1,2]. BA are also signaling molecules/hormones,which are involved in the regulation of their own homeostasis,thyroid hormone signaling,glucose and lipid metabolism,energy expenditure,and cellular immunity[2-5]. Conversely,certain BA are also cytotoxic at high concentrations and have deleterious effects on hepatocytes and cholangiocytes,which play a major role in liver injury during various liver diseases[5-8].
Cholestatic liver diseases are associated with a reduction in bile flow due to impairment of bile flow or defects in bile production[9]. This causes accumulation of BA in the liver,which spills out into the systemic circulation,extrahepatic tissues,and eventually into urine. Numerous clinical and preclinical studies have shown up to a 100-fold increase in BA concentrations in the blood and urine during various liver diseases[8,10-13]. Elevated BA concentrations were shown to correlate with the progression of damages to the liver and bile duct in cholestatic rats,rabbits,and in humans[14-18].
Biomarkers currently used in the clinic for the diagnosis and prognosis of liver diseases are primarily serum liver enzymes such as aspartate aminotransferase (AST)and alanine aminotransferase (ALT) as well as bilirubin[19,20]. However,they are not specific to the liver or bile duct injuries,may increase in non-hepatobiliary diseases,and require severe cell injury at advanced disease stages before their blood levels increase[19,20]. BA were extensively investigated for decades as biomarkers for numerous hepatobiliary diseases[13,21-23]. However,these efforts never translated into the clinic,with the few exception of limited use in the diagnosis of intrahepatic cholestasis of pregnancy and biliary atresia in infants. This could be attributed to the marked differences in the physiological and pathological properties of the different individual BA. For example,detailed profiling of the more toxic and relevant individual BA rather than total BA concentration may better correlate with the liver condition during hepatobiliary diseases[10,12,24]. Also,the extreme inter-and intraindividual variability of total and individual BA concentrations due to many factors such as food ingestion and diurnal variation,makes it challenging to determine the normal baseline ranges[25,26].
We have developed the concept of “BA indices”,which are ratios calculated from the absolute concentrations of individual BA and their metabolites (Table 1). These ratios provide comprehensive quantification of the composition,metabolism,hydrophilicity,formation of secondary BA,and toxicity of the BA profile[9,26]. BA indices have much lower variability than the absolute BA concentrations used to calculate them. Indeed,we have demonstrated that BA indices offered numerous advantages over absolute total and individual BA concentrations including low interand intra-individual variability and were resistant to covariate influences such as age,gender,body mass index (BMI),food consumption,and moderate alcohol consumption[9,26].
We have expanded on our previous pilot study,where we have recruited 300 patients with liver diseases and 103 control subjects over a period of 7 years. This study includes a series of two papers. In this article,we have shown the utility of BA indices as diagnosing markers for liver diseases by compared the urinary BA profile between healthy controls and patients and between patients with different severity levels of liver disease. In the 2ndarticle,we have built a survival model,the Bile Acid Score (BAS),to predict the prognosis of liver diseases using significant BA indices identified in this article.
MATERIALS AND METHODS
Study participants
For controls,103 healthy subjects (32 male and 71 female) without liver diseases between the ages of 19 and 65 years were recruited by the Clinical Research Center at the University of Nebraska Medical Center (UNMC) (Omaha,NE,United States). The registry URL was (https://www.clinicaltrials.gov/ct2/show/NCT01200082?term=aln outi&draw=2&rank=1). The clinical trial number was NCT01200082. Inclusion criteria for the healthy controls included normal liver functions,as verified by ALT < 50 U/L,AST < 56 U/L,gamma-glutamyl transferase < 78 U/L,absence of diabetes,and no- or moderate alcohol drinking[27]The study was approved by Institutional Review Board at UNMC and written informed consents were provided for all participating subjects.Thirty milliliters urine samples were collected from controls at fasting conditions in the first visit,and 1,2,and 4 wk thereafter.
Patients diagnosed with one or multi-hepatobiliary conditions due to chronic hepatitis C (n= 71) ,hepatitis B (n= 15),alcoholic liver disease/alcoholic cirrhosis (n=117),primary biliary cholangitis (PBC) (n= 12),primary sclerosing cholangitis (n= 17),autoimmune hepatitis (n= 27),alpha-1-antitrypsin deficiency (n= 6),nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (n= 56),carcinoma (n= 26),cryptogenic cirrhosis (n= 11),polycystic liver disease (n= 5),elevated liver function test (LFT) (n= 22),and unknown etiology (n= 5),were enrolled in the hepatology clinic in UNMC. A total of 300 patients (157 male and 143 female) between the ages of 19 years and 83 years were recruited. Thirty milliliters of urine samples were collected on their first and follow-up visits to the hepatology clinic. All urine samples were stored in -80 °C until analyzed by liquid chromatography-tandem mass spectrometry(LC-MS/MS). Patients were divided into three disease-severity groups based on their model for end-stage liver disease (MELD) score:low-MELD (6-15 score),medium-MELD (16-25),and high-MELD (26-40). High MELD group was not included while performing the statistical analysis,because there were only four subjects in that group.In addition,patients were also categorized according to hepatic decompensation(presence or history of encephalopathy,bleeding varices,ascites,or jaundice)[28].
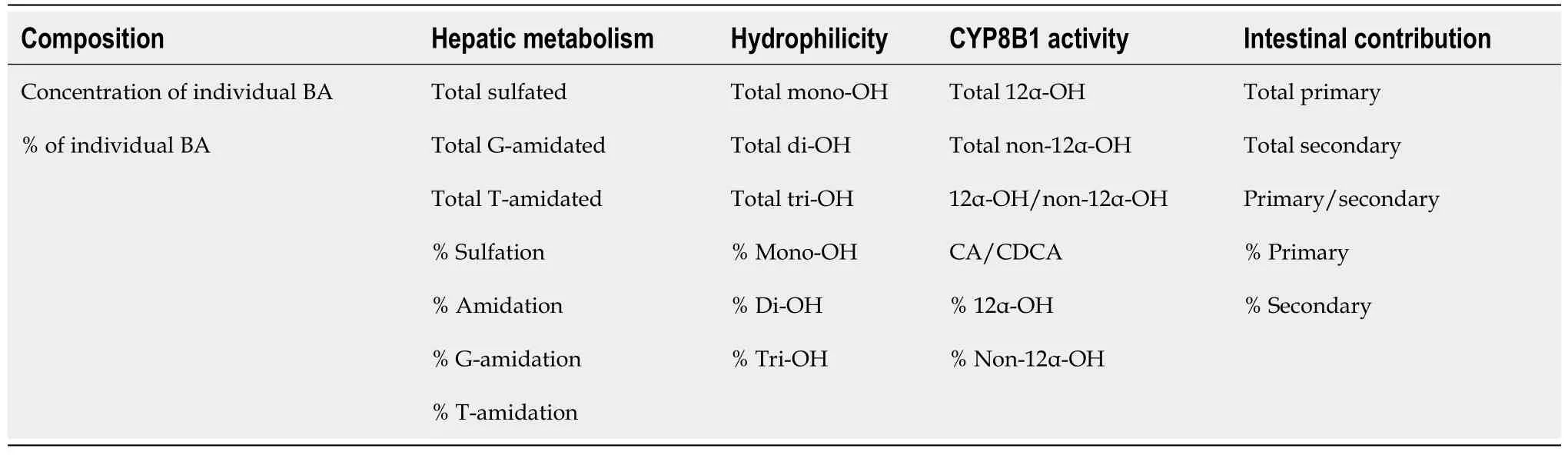
Table 1 List of bile acid indices
Non-BA parameters
AST,ALT,albumin,and serum creatinine were measured using the Beckman Coulter reagents (Beckman Coulter,Inc,Brea,California). Protime and international normalized ratio (INR) were measured using STANeoplastine “CI PLUS 10” reagent kit (Diagnostica Stago Inc,Parsippany,New Jersey). Total bilirubin in serum was analyzed using QuantiChromTM Bilirubin assay kit (BioAssay Systems,Hayward,CA,United States). AST/ALT ratio and AST/platelet ratio index (APRI) were calculated.
BA quantification by LC-MS/MS
Urine samples were extracted using solid phase extraction as described previously[9,26,29,30]. BA concentrations were quantified by LC-MS/MS,as we described previously[31].
Calculation of BA indices
In addition to the absolute concentration of individual and total BA,the BA profile in urine was characterized using “BA indices” (Table 1),and as we have described previously[9,26,30,31]. BA indices describe the composition,hydrophilicity,formation of 12α-OH BA by CYP8B1,metabolism,and formation of secondary BA by intestinal bacteria. The composition indices were calculated as the ratio of the concentration of individual BA in all of their forms (sulfated,unsulfated amidated,and unamidated) to the total concentration of BA. The percentages of mono-OH BA:[lithocholic acid(LCA)],di-OH BA:[ursodeoxycholic acid (UDCA),murideoxycholic acid (MDCA),chenodeoxycholic acid (CDCA),hyodeoxycholic acid (HDCA),and deoxycholic acid(DCA)],and tri-OH BA:[cholic acid (CA),muricholic acid (MCA),and hyocholic acid(HCA)] were calculated as the ratio of the concentration of the sum of the respective BA in all their forms to the total concentration of BA.
The 12α-OH BA are formed by CYP8B1 in the liver and include DCA,CA,nor-DCA,and 3-dehydroCA. Therefore,CYP8B1 activity can be measured by the ratio of 12α-OH BA to the remaining of all other BA (non-12α-OH BA). Another marker for CYP8B1 is the ratio of CA to CDCA because CA is formed by the 12α hydroxylation of CDCA. In the same way,the ratio of 12α-OH (DCA,CA,nor-DCA,and 3-dehydroCA in all of their forms) to non-12α-OH (CDCA,HDCA,LCA,UDCA,MDCA,HCA,MCA,12-oxo-CDCA,6-oxo-LCA,7-oxo-LCA,12-oxo-LCA,isoLCA,isoDCA in all of their forms)was calculated.
BA are metabolized primarily by sulfation,glycine (G),and taurine (T) amidation in the liver. The percentage of individual BA sulfation was calculated as a ratio of the concentration of sulfated BA,in both the amidated and unamidated forms,to the total individual BA concentration in all their forms (amidated,unamidated,sulfated,and unsulfated). In both the sulfated and unsulfated forms,the percentage of individual BA amidation have been calculated as the ratio of the concentration of amidated BA,to the total concentration of individual BA in all of their forms (amidated,unamidated,sulfated,and unsulfated). Additionally,percentages of amidation were divided into the percentages of BA existing as G or as T amidates.
The ratio of primary (CA,CDCA,MCA and HCA in all of their forms) to secondary BA (DCA,LCA,UDCA,HDCA,MDCA,Nor-DCA,12-oxo-CDCA,3-dehydroCA,6-oxo-LCA,7-oxo-LCA,12-oxo-LCA,isoLCA,and isoDCA in all of their forms) was calculated.
Statistical analysis
Independent sample-t-test and Mann-Whitney test were used to study the demographic differences between controls and patients because the sample size was >30[32]. Independent sample-t-test was used for continuous variables and Mann-Whitney test was used for categorical variables. The demographic variables were (age,BMI,gender,and race). Subjects were divided into four age groups (19-29,30-41,42-53,54-83 years),and the variable age was studied as both a continuous and a categorical variable. Subjects were also divided into three BMI groups (normal:BMI < 25,overweight:BMI 25-29.9,and obese:BMI ≥ 30) and the effect of BMI was studied as both a continuous and a categorical variable. Also,subjects were divided into five race groups (White,Black,Asian,Hispanic,others),and the variable race was studied as a categorical variable.
Urine samples were collected from controls and patients on their first visit and follow-up visits. Mixed effects models were used to compare patientsvscontrols and the demographic variables were included as covariates. Statistically significant covariates were returned to the mixed effects models as interaction terms with the primary group,i.e.,patientsvscontrol.
BA indices were compared between controls,low-MELD (patients),and medium-MELD (patients) groups using mixed effects models followed by pairwise comparisons using Bonferroni’s adjustment if thePvalue was < 0.05. BA indices were compared between compensated and decompensated patients using mixed effects models. Mixed effects models were also used to determine the association between non-BA parameters including (AST,ALT,bilirubin,MELD score,AST/ALT,creatinine,INR,APRI,protime,and albumin) and BA indices. Receiver operating characteristic curve (ROC) analyses were used to determine cut-off values of BA as markers for the diagnosis of liver diseases with optimum sensitivity and specificity.The areas under the ROC curve (AUC) values were compared between urinary BA profiles and non-BA parameters. The mixed effects models were used to compare BA indices with AUC > 0.7 between controls and the patients with specific disease subtypes described in the “Study Participants” section (same patients can belong to different disease groups). Polycystic liver disease and unknown etiology subtypes were not included in the comparison between the disease subtypes because they had <six subjects.
Univariate logistic regression analysis was used to determine the association between BA concentrations and indices and the likelihood of developing a liver disease. From logistic regression analysis,the odds ratios (ORs) were calculated for a 10% and 20% change from the mean value of BA indices in the healthy controls.
Pvalue of 0.05 was considered significant for all the statistical tests described above.All statistical analysis was performed using the Statistical Product and Service Solutions (SPSS) software,version 25 (IBM corporation,Armonk,NY,United States).
RESULTS
Demographics
Table 2 shows a summary of the demographics of both patients and controls participants. We enrolled 103 controls (32 males and 71 females) and 300 patients (157 males and 143 females),who were treated for cholestatic liver diseases in UNMC,over the period from November of 2011 to December of 2018. To compare the demographics between the two groups,age and BMI covariates were compared as both continuous and categorical variables usingt-test,and Mann-Whitney test,respectively. While gender and race were compared as categorical variables using Mann-Whitney test. Age,gender,and BMI were significantly different between control and patients (Pvalue < 0.05),while race was not different. Therefore,the statistically significant demographic variables (age,BMI,and gender) were included as covariates in the mixed effects models to compare BA indices between patients and controls.

Table 2 Demographics
Differences in BA between patients vs controls are not due to differences in demographics
Because some of the covariates (age,BMI,and gender) were significantly different between the two groups (Table 2),we reran the univariate mixed effect analysis with these covariates (multivariate analysis). First,association between these covariates and BA indices was identified,and then the covariates with significant association with BA indices were incorporated in the multivariate mixed effect analyses as interaction terms with the group (patients and controls). We did not find any difference in the association between covariates and BA indices between the two groups except for the% primary and % secondary BA with gender (Supplementary Table 1).
BA profiles in controls vs patients
Table 3 shows the absolute concentrations of major urinary BA in controls and patients. Table 4 compares representative absolute BA concentrations and indices between controls and patients. Supplementary Table 2 shows the full list of BA concentrations and indices. Total BA was 5.9-fold higher in patients compared with controls. All individual BA concentrations were also higher in patients,except MDCA,but to different extents. The highest increase was in UDCA (11.9-fold),while the lowest increase was for DCA and HDCA (1.6-fold). The percentage of UDCA,CDCA,MCA,CA,and HCA were higher (1.2-1.6-fold),while the percentage of LCA,DCA,HDCA,and MDCA were lower (0.5-0.8-fold) in patientsvscontrols.
Unamidated,G-amidated,and T-amidated BA which were 3.3-,5.9-,and 9.4-fold higher in patients than controls. Therefore,the overall % amidation and % Gamidation did not change or slightly decreased in patients,whereas % T-amidation increased from 8.0% in controls to 10.8% in patients. Similarly,the concentrations of both sulfated and unsulfated were approximately 6-fold higher in patient; so that the% sulfation of BA was unchanged.

Table 3 Absolute concentrations of major bile acids in controls and patients

1Other bile acids:Nor-deoxycholic acid,12-oxo-chenodeoxycholic acid,3-dehydrocholic acid,6-oxo-lithocholic acid,7-oxo-lithocholic acid,12-oxolithocholic acid,isolithocholic acid,and isodeoxycholic acid.ND:Not detected; -:Not quantified; BA:Bile acids; G:Glycine; T:Taurine; CDCA:Chenodeoxycholic acid; CA:Cholic acid; LCA:Lithocholic acid; UDCA:Ursodeoxycholic acid; DCA:Deoxycholic acid; HDCA:Hyodeoxycholic acid; MDCA:Murideoxycholic acid; MCA:Muricholic acid; HCA:Hyocholic acid.
The absolute concentrations of mono-,di-,and tri-OH BA were also higher in patients compared with controls,but the % mono-OH decreased (0.8-fold),di-OH remained unchanged,and % tri-OH increased (1.4-fold) due to increasing % CA (1.2-fold),% MCA (1.6-fold),and % HCA (1.5-fold).
Total 12α-OH and non-12α-OH BA were 2.3-fold and 8.2-fold higher in patients,so that the ratio of 12α-OH/ non-12α-OH and the % 12α-OH decreased (approximately 0.5-fold),while % non-12α-OH BA increased (1.2-fold).
Total primary and secondary BA were 8.1-fold and 4.6-fold higher in patients,so that the ratio of primary/secondary BA was 3.6-fold higher. Therefore,% primary BA was 1.4-fold higher,while % secondary BA was 0.80-fold lower in patientsvscontrols.
BA profile in low vs medium-MELD patients
Table 5 compares representative urinary BA concentrations and indices between lowand medium-MELD patients. Total BA concentrations was twice and individual BA concentrations were (1.15-fold to 3.9-fold) higher in mediumvslow-MELD patients(Table 5).
Unamidated BA concentration was lower,while G-amidated and T-amidated BA were higher in the medium-MELD patients. Therefore,% T-amidation was 1.5-fold higher,while there was minimal difference in the % amidation and % G-amidation between medium and low-MELD patients. Similarly,the concentrations of both sulfated and unsulfated were 1.3- and 2-fold higher in mediumvslow-MELD. On the other hand,the % sulfation of BA was only 1.07-fold higher,but it was statistically significant.
The absolute concentrations of mono-,di-,and tri-OH BA were also (1.8-2-fold)higher in medium-MELD patients,but the % mono-OH decreased (0.86-fold); while %di- and % tri-OH remained unchanged.
Total 12α-OH and non-12α-OH BA were both higher in mediumvslow-MELD patients,but to different extents so that % non-12α-OH BA remained unchanged,while % 12α-OH decreased and the ratio of 12α-OH/ non-12α-OH was approximately 0.7-fold lower.
Total primary BA were 3.4-fold higher,while total secondary BA were slightly (0.9-fold) lower in medium-MELD patients,so that the ratio of primary/secondary BA was 2.3-fold higher. Similarly,% primary BA was 1.4-fold higher,while % secondary BA was 0.6-fold lower in medium- MELD patients.
BA profile in compensated vs decompensated patients
Table 6 compares representative urinary BA concentrations and indices between decompensated and compensated patients. In general,the same trend in the highervslower MELD patients comparison was observed in the decompensatedvscompensated patients. Total BA was 1.3-fold higher,all individual BA were higher,but to variable extents. The percentage of CDCA,HDCA,CA,and HCA were higher(1.3-2.1-fold),while the percentage of LCA,UDCA,DCA,and MDCA were lower (0.3-0.7-fold) in decompensatedvscompensated patients.
The % T-amidation was 1.3-fold higher in decompensated vs. compensated patients,while there was no difference in the % amidation,% G-amidation,or % sulfation. The% mono-OH decreased (0.73-fold),% di-OH remained unchanged,and % tri-OH slightly increased (1.13-fold) due to increasing % CA and % HCA. The ratio of 12α-OH/ non-12α-OH lower,the % 12α-OH,and CA/CDCA ratio decreased (0.7-0.8-fold),while % non-12α-OH BA remained unchanged.
Total primary BA were two-fold higher,while total secondary BA were 0.8-fold lower,so that the ratio of primary/secondary BA was 2.6-fold higher in decompensated patients. Therefore,% primary BA was 1.5-fold higher,while %secondary BA was 0.56-fold lower in decompensated patients.

Table 4 Representative bile acids concentrations and indices in controls vs patients

1Other bile acids:Nor-deoxycholic acid,12-oxo-chenodeoxycholic acid,3-dehydrocholic acid,6-oxo-lithocholic acid,7-oxo-lithocholic acid,12-oxolithocholic acid,isolithocholic acid,and isodeoxycholic acid.NA:Not available; BA:Bile acids; G:Glycine; T:Taurine; CDCA:Chenodeoxycholic acid; CA:Cholic acid; LCA:Lithocholic acid; UDCA:Ursodeoxycholic acid; DCA:Deoxycholic acid; HDCA:Hyodeoxycholic acid; MDCA:Murideoxycholic acid; MCA:Muricholic acid; HCA:Hyocholic acid.
ROC curve analysis
Supplementary Table 3 lists the AUC for BA concentrations and indices. Supplementary Table 4 shows the full list of BA concentrations and indices. Total BA,CDCA,CA,% DCA,% HDCA,% MDCA,total G-Amidated,total unsulfated,total sulfated,total di-OH,total tri-OH,total non-12α-OH,12α-OH/non12α-OH,% 12α-OH,% non-12α-OH,total primary,primary/secondary,% primary,and % secondary produced AUC > 0.7.Figure 1 shows ROC curves of BA indices with AUC > 0.7. Potential cut-off values selected based on the optimum specificity and sensitivity for BA indices with AUC > 0.7 are listed in Supplementary Table 5.
Risk analysis:Logistic regression analysis
Table 7 shows the results of logistic regression analyzes for BA indices with ROC(AUC) > 0.7. Logistic regression analysis detects whether there is a risk of liver disease associated with changes in BA indices. The risk of liver disease increased with changing levels of all BA indices (P< 0.05) except (% HDCA and % MDCA).Additionally,the OR from the logistic regression analysis quantifies the magnitude of the risk of developing liver diseases per unit (10% and 20% of the normal value)changes in BA indices. For example,for every 20% increase in the % non-12α-OH BA,the likelihood of having a liver disease increases 2.72-folds (OR:2.72;P< 0.05). In contrast for every 20% increase in the % 12α-OH BA,the likelihood of having a liver disease decreases 0.56-folds (OR:0.56;P< 0.05).
BA profile in different liver disease subtypes
Table 8 compare BA indices with ROC-AUC > 0.7 between controlsvspatients with specific liver disease subtype. Mixed effects models were used to compare disease subtypes individuallyvscontrols. The goal was to identify BA indices that can serve as diagnostic biomarkers for specific liver disease subtypes.
We have found that most BA indices were significantly different between controlsvsall individual liver disease subtypes. Total BA,total CDCA,total CA,total Gamidated,total unsulfated,total sulfated,total di-OH,total tri-OH,Total non-12α-OH,% non-12α-OH and total primary were higher (1.1- to 39.5-fold) in every liver disease group compared with controls. % Primary and primary/secondary were higher (1.1-fold to 9.27-fold) in all liver disease group compared with controls except in PBC. %DCA,% HDCA,% 12α-OH,and 12α-OH/non-12α-OH were lower (0.07-fold to 0.85-fold) in every liver disease group compared with controls. % MDCA and % secondary was lower in all liver disease group compared with controls except in elevated LFT and PBC,respectively.
Non-BA parameters
In addition to BA indices,we have also examined other biomarkers currently used in the clinic to evaluate liver functions. These non-BA parameters include AST,ALT,AST/ALT,bilirubin,albumin,INR,protime,creatinine,APRI,and MELD. Table 9 compares the non-BA parameters in controls and patients using mixed effects models.All the non-BA parameters were higher in patients compared to controls except albumin and protime,which were lower in patients. Within the patient population,all non-BA parameters were higher in medium compared to low- MELD patients except albumin,and ALT. The same results also applied to decompensatedvscompensated patients.

Table 5 Representative bile acids concentrations and indices in medium- vs low- model for end-stage liver disease patients

BA:Bile acids; MELD:Model for end-stage liver disease; G:Glycine; T:Taurine; CDCA:Chenodeoxycholic acid; CA:Cholic acid; LCA:Lithocholic acid;UDCA:Ursodeoxycholic acid; DCA:Deoxycholic acid; HDCA:Hyodeoxycholic acid; MDCA:Murideoxycholic acid; MCA:Muricholic acid; HCA:Hyocholic acid.
The AUC for non-BA parameters was > 0.7 for all of them except creatinine,protime,and AST/ALT ratio. Also,per logistic regression analysis,the risk of being diagnosed with a liver disease increased to various extents with changing levels of all non-BA parameters (P< 0.05) except creatinine and AST/ALT. For example,for every 20% increase in the albumin and protime,the likelihood of having a liver disease decreases 0.28-fold and 0.85-fold,respectively. In contrast for every 20% increase in the other non-BA parameters,the likelihood of having a liver disease increases 1.13-fold to 3-fold (Supplementary Table 6).
In addition,we have found that most non-BA parameters were significantly different between controlsvs. all individual liver disease subtypes (Supplementary Table 7). Creatinine,INR,AST,ALT,bilirubin,AST/ALT,and MELD were higher in most liver disease group compared with controls. In contrast,albumin and protime were lower in most liver disease group compared with controls.
Association between non-BA parameters and BA indices
Supplementary Table 8 shows the association between non-BA parameters and BA indices using mixed effects models. We have found that all non-BA parameters were significantly associated with most BA concentrations/indices,except creatinine (P>0.05).
DISCUSSION
To ensure that the difference in the BA profiles between patients and controls are not due to the differences in the demographics we showed that:(1) Most of BA were not associated with demographic covariates,and (2) The ones that were associated had the same extent of association in the patient and control groups (Supplementary Table 1).
Patients were categorized based on the severity of the liver disease using MELD[33-37]and the compensation status[28]. Accordingly,we have compared the BA profiles between entire patientvscontrol populations as well as among the patients with different levels of disease severity. Most BA (except MDCA) were higher,but to different extents,in patientsvscontrols (Table 4) and in the more-severe patient groups,i.e.,mediumvslow-MELD (Table 5) as well as decompensatedvscompensated(Table 6). In particular,the percentages of the primary BA (CDCA,CA,and HCA)were higher,while the percentage of the secondary BA (DCA) was lower. The %primary BA was 1.4-fold higher,while % secondary BA was 0.8-fold lower and the ratio of primary/secondary BA was 3.6-fold higher in patientsvscontrols (Table 4).The same trend was also observed in the patients with more severe form of the disease,where the % of primary BA also increased with the severity of the liver disease (medium-MELD > low-MELD > controls) and (decompensated > compensated> controls),whereas % secondary BA decreased with the severity of the disease.(Tables 5 and 6).
Cholestatic diseases are associated with impaired bile flow to the intestine,which translates into reduced transformation of primary into secondary BA by intestinal bacteria[9,25,38-40]. Therefore,while all BA concentrations were higher in patients due to the impairment of bile flow,the proportion of secondary BA (formed in the intestine)decreased with the severity of the cholestatic disease,which may reflect the extent of bile flow impairment.
The conjugation of BA with G and T decreases their pKa,increases their ionization and solubility,enhances their urinary elimination,and decreases their toxicity[30,41-44].However,T-amidated BA are generally less cytotoxic and more ionized than G-amidated BA[43,45,46]. Even though unamidated as well as T-and G-amidated BAs were higher in patients,the increase in T-amidated BA was the most profound. Therefore,%T-amidation increased,while % G-amidation decreased in patientsvscontrols(Table 4) as well as in medium-MELDvslow-MELD (Table 5) and decompensatedvscompensated patients (Table 6). The preferential accumulation of T-amidated BA can be interpreted as an adaptive compensating response to protect the liver from BA toxicity by increasing elimination of the more toxic G-amidated and unamidated compared to the less toxic T-amidated BA[9,26,47]. In addition,T-amidated BA has the highest affinity as substrates for the canalicular transporter,Bile Salt Export Pump(BSEP) (T-amidated > G-amidated > unamidated BA)[48-50]. Therefore,an impairment of the BA transport by BSEP,as documented in some cholestatic diseases[51-53],is expected to preferential accumulation T-amidated BA.
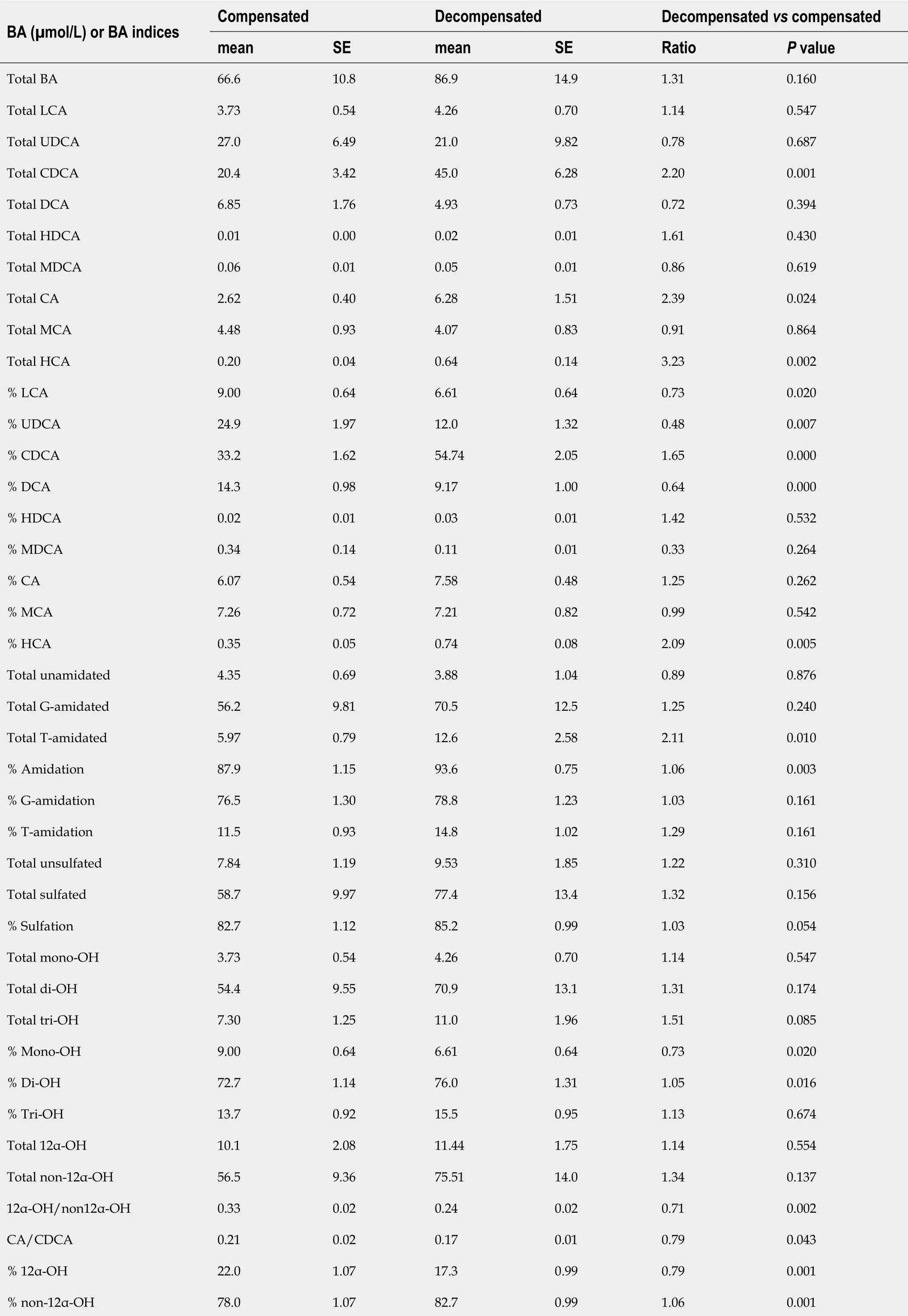
Table 6 Representative bile acids concentrations and indices in compensated vs decompensated patients

BA:Bile acids; G:Glycine; T:Taurine; CDCA:Chenodeoxycholic acid; CA:Cholic acid; LCA:Lithocholic acid; UDCA:Ursodeoxycholic acid; DCA:Deoxycholic acid; HDCA:Hyodeoxycholic acid; MDCA:Murideoxycholic acid; MCA:Muricholic acid; HCA:Hyocholic acid.
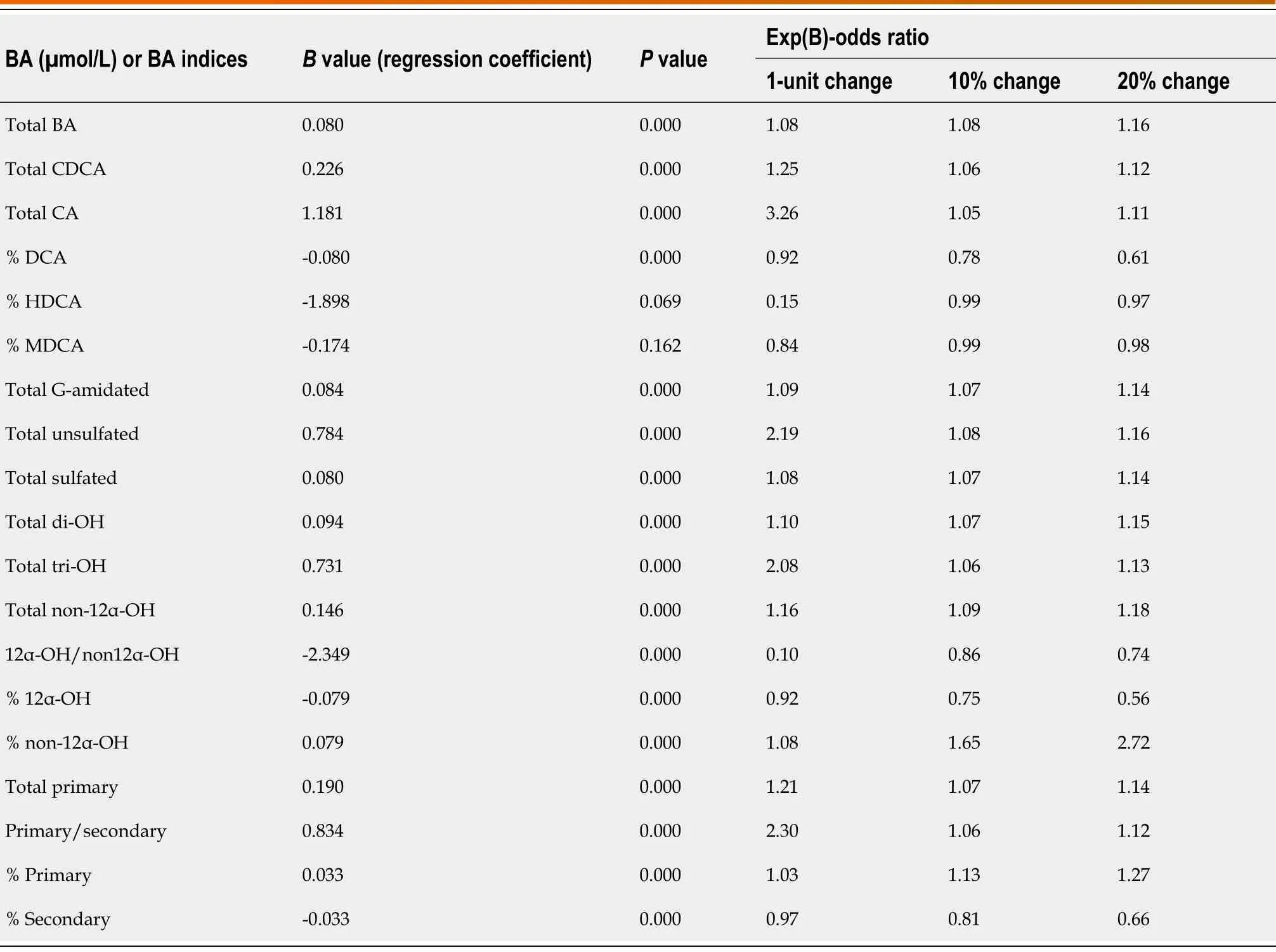
Table 7 Univariate logistic regression analysis of bile acids concentrations and indices1

Table 8 Bile acids concentrations and indices in controls and patients with specific liver disease subtype1
Both sulfated and unsulfated BA were higher in patients (Table 4),but % sulfation was slightly higher in medium- compared with low-MELD and in decompensated compared with compensated patients (Tables 5 and 6). The upregulation of sulfation of BA by SULT2A1 in patients with liver diseases is thought as a compensatory response to eliminate and detoxify the accumulated toxic BA[8-13,54,55]. However,it is also possible that sulfation activity in these patients may eventually decrease due to exhaustion or defects of these recovery mechanisms. Therefore,while liver insults can be remediated by upregulating BA sulfation under normal conditions and in milder forms of liver diseases,but subjects who fail to upregulate this defensive mechanism or exhaust it under more severe forms of the diseases are at higher risk of developing the disease and/or may have worse prognosis[26]. Another explanation for the preferential accumulation of BA-sulfates could be related to the inhibition of their canalicular transport into bile by efflux transporters,mainly the multidrug resistance-associated proteins 2-4 (MRP 2-4). These transporters preferentially transport divalent amidated and conjugated (sulfated and glucuronidated) BA[56-59]. MRPs including MRP2 activity is known to be compromised in various cholestatic liver diseases due downregulation of their expression and/or membrane localization[60-62],which may lead to the preferential retention of their substrates including BA-sulfates in the liver and systemic circulation.
CYP8B1 catalyzes 12α-hydroxylation of the di-OH CDCA to the tri-OH CA. The CA/CDCA or the 12α-OH/non-12α ratios are used as probes to measure CYP8B1 activity[63-65]. The 12α-OH/non-12α-OH ratio was 50% lower in patients compared with controls (Table 4). Also,both ratios were lower in medium-MELDvslow-MELD as well as decompensatedvscompensated patients (Tables 5 and 6). This indicates that CYP8B1 activity,which exclusively takes place in the liver[66,67],may be compromised during liver diseases in general and is further compromised with disease severity.Also,CDCA has a much higher affinity to BSEP than CA and other 12α-OH BA[49,68].Therefore,when BSEP activity is compromised in the more severe liver diseases,it is expected to lead to the preferential accumulation of its high-affinity substrates including CDCA,which will also decrease the CA/CDCA and 12α-OH/non-12α ratios.
Many BA concentrations and indices demonstrated AUC > 0.7 supporting their potential as biomarkers for the diagnosis of liver diseases (Supplementary Table 3). We identified three potential cut-off values,which achieve a good balance between specificity and sensitivity (Supplementary Table 5). BA indices have higher AUC values than the absolute BA concentrations,which indicates that BA indices are more accurate in distinguishing between controls and patients.
We found correlation between the risk of developing a liver disease and many BA indices using logistic regression analysis (P< 0.05). The univariate logistic regression associated with a 20% change from the mean value for the absolute BA concentrations ranged from 1.11 to 1.18,whereas it was as high as 2.72 for BA indices (Table 7). This suggests that BA indices are more sensitive than absolute BA concentrations in terms of predicting larger magnitudes of the risk of developing a liver disease.
All the above analyses demonstrate that BA indices can serve as a global marker to differentiate the pooled cholestatic liver disease population from controls in this study.In addition,we have divided the patients into different individual disease groups and performed similar analyses in these groups vs. controls,for the individual diseases.Most BA indices with ROC-AUC > 0.7 were significantly different between controlsvsmost of the individual liver disease subtypes (Table 8). In particular,hepatitis C and cirrhosis were the largest subpopulations in our study,and all global diagnostic BA indices from the pooled patients vs. control analyses (P< 0.05 and ROC-AUC > 0.7)were also specific diagnostic markers for these two particular liver diseases vs.controls (P< 0.05).
We have found a significant correlation between BA indices and non-BA parameters,except creatinine (P> 0.05) (Supplementary Table 8). However,BA indices,in general,outperformed non-BA parameters as biomarkers for liver diseases on many levels. Non-BA parameters were 0.76-fold to 2.5-fold higher (Table 9),whereas BA indices were as high as approximately 12-fold higher (total UDCA) in patients compared to controls (Table 4). Similarly,the magnitude of change within the MELD groups,compensation status,and among individual diseases were all much higher in BAvsnon-BA.
This study has the following limitations:(1) Severity of the liver diseases were assessed using MELD score,compensation status,and a panel of liver enzymes.However,liver histological evaluation was not included because it is not a routine practice to perform liver histology on all patients,but rather for specific patients as required by the hepatologists. And (2) we have enough subjects in this study toperform solid statistics,but smaller number of subjects in many individual disease subgroups. Also,distribution of subjects between disease groups was unbalanced.
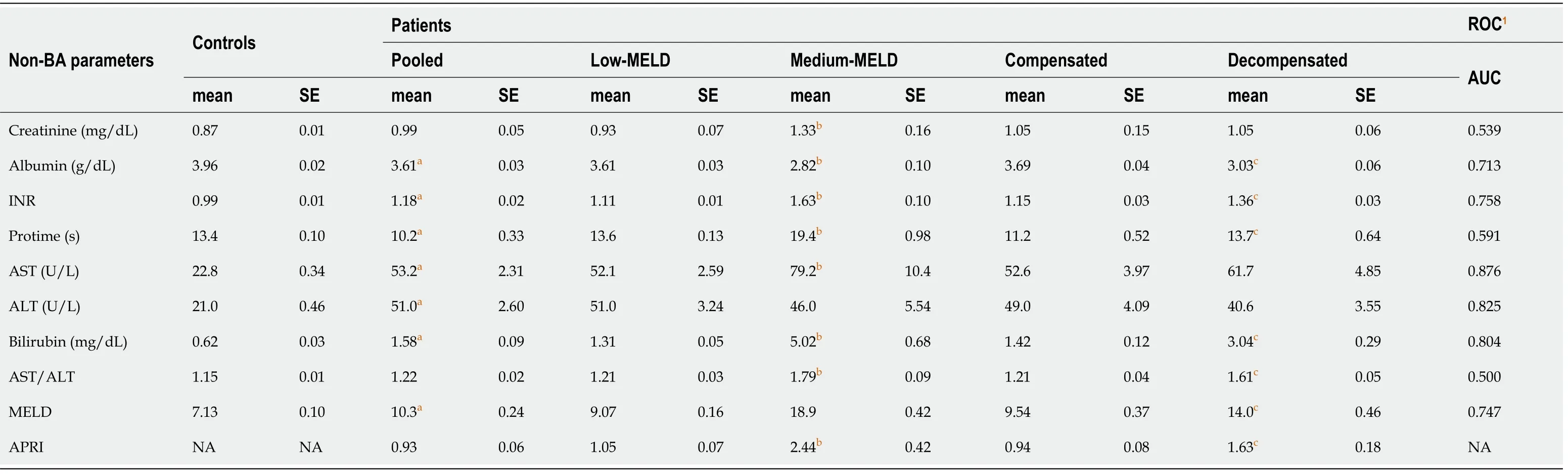
Table 9 Summary of non-bile acids parameters
CONCLUSION
In summary,the results of this study demonstrated that total and all individual BA increased in patients with 11 different cholestatic diseases. However,the high interindividual variability of BA absolute concentrations makes most of them statistically insignificant and prevent their utilization as diagnostic markers. In contrast,BA indices had much lower inter- and intra-individual variability,which allowed their use as diagnostic and prognostic markers for liver diseases. Furthermore,we have shown that several BA indices outperformed non-BA markers,currently used in the clinic,as diagnostic markers to differentiate our patient pool as well as individual cholestatic diseases against healthy controls.

Figure 1 Receiver operating characteristics curves of bile acids concentrations and indices with area under the receiver operating characteristics curve > 0.7. The area under the receiver operating characteristics curve (AUC) for differentiating patients from healthy controls. The scale of both the Y-axis (sensitivity) and the X-axis (1-specificity) is 0-1. Bile acids (BA) indices are higher in patients vs. controls,and the positive actual state was patients except the ones annotated with “*”,where BA indices were lower in patients compared to controls. For these BA indices,“1-AUC” instead of “AUC” was calculated. AUC:Area under the receiver operating characteristics curve; BA:Bile acids; CDCA:Chenodeoxycholic acid; CA:Cholic acid; DCA:Deoxycholic acid; HDCA:Hyodeoxycholic acid; MDCA:Murideoxycholic acid; G:Glycine.
The increase in the total BA concentration in patients can be attributed to specific changes in the BA pool composition. This increase primarily resulted from primary BA(CDCA,CA,and HCA),while the % of the secondary BA (LCA and DCA) were lower.This lead to about 4-fold increase in the primary/secondary BA ratio. Consequently,the BA pool has drastically shifted in patients from being 37% primary to approximately 50% primary BA. The increase in T-amidated BA was more profound than that of G-amidated BA,which lead to a marked increase in the % T-amidation.Furthermore,this trend of elevated primary and amidated BA was exacerbated with disease severity. This pattern can be a sign of less transformation of primary into secondary and less deconjugation of amidated BA by intestinal bacteria associated with more impairment of bile flow associated with more severe cholestatic diseases. %Sulfation was higher in patients with more severe forms of liver diseases indicating the upregulation of sulfation in these patients as a compensatory response to detoxify BA accumulation. Finally,the increase in non-12α-OH was more profound than that of 12α-OH BA,which indicates that hepatic CYP8B1 activity is compromised in liver diseases in general and is further compromised with disease severity.
In the 2ndpaper of this series,we have utilized BA indexes to build a survival model called “The Bile Acid Score”,which we showed was able to predict the prognosis into adverse events including death and liver transplant in liver patients.
ARTICLE HIGHLIGHTS
Research background
Bile acids (BA) have been extensively investigated for decades as biomarkers for numerous hepatobiliary diseases. However,these efforts never translated into a widespread in the clinic,due to the extreme inter-and intra-individual variability of total and individual BA concentrations and the marked differences in the physiological and pathological properties of the different individual BA. To this end,we have developed the concept of “BA indices”,which demonstrated their use as diagnostic biomarkers for cholestatic liver diseases.
Research motivation
Biomarkers currently used in the clinic are not specific to the liver or bile duct injurie.BA were extensively investigated for decades as biomarkers for numerous hepatobiliary diseases. This could be attributed to the marked differences in the physiological and pathological properties of the different individual BA. BA indices have much lower variability than the absolute BA concentrations used to calculate them. Indeed,we have demonstrated that BA indices offered numerous advantages over absolute total and individual BA concentrations including low inter- and intraindividual variability and were resistant to covariate influences such as age,gender,body mass index,food consumption,and moderate alcohol consumption.
Research objectives
The objective of this project was to discover and validate diagnostic biomarkers of cholestatic liver diseases based on the urinary BA profile. We have developed the concept of “BA indices”,which are ratios calculated from the absolute concentrations of individual BA and their metabolites. BA indices have much lower variability than the absolute BA concentrations used to calculate them,which enabled their use as diagnostic biomarkers for cholestatic liver diseases.
Research methods
We analyzed urine samples by liquid chromatography-tandem mass spectrometry and compared the urinary BA profile between patients with hepatobiliary diseases vs healthy controls by statistical analysis (independent sample-t-test,Mann-Whitney test,Mixed effects models,by pairwise comparisons using Bonferroni’s adjustment,receiver operating characteristic curve analyses,Univariate and multivariate logistic regression analysis).
Research results
The results of this study demonstrated that total and all individual BA increased in patients with 11 different cholestatic diseases. However,the high inter-individual variability of BA absolute concentrations makes most of them statistically insignificant and prevent their utilization as diagnostic markers. In contrast,BA indices had much lower inter- and intra-individual variability,which allowed their use as diagnostic and prognostic markers for liver diseases. Furthermore,we have shown that several BA indices outperformed non-BA markers,currently used in the clinic,as diagnostic markers to differentiate our patient pool as well as individual cholestatic diseases against healthy controls.
Research conclusions
BA indices demonstrated high area under the receiver operating characteristic curves,and changes of BA indices were associated with the risk of having a liver disease as determined by the logistic regression analysis,which demonstrated their use as diagnostic biomarkers for cholestatic liver diseases.
Research perspectives
We have developed survival models based on BA indices to predict the prognosis of hepatobiliary diseases which is illustrated in the second paper of this series.
ACKNOWLEDGEMENTS
The authors wish to thank the nurses of the CRC (Mary Ann Martin,Cindy Cowarden,Caroline Peterson,Claire Haier,and Mary Phillips) and the staff for their valuable contributions to managing the health control arm of the study,recruiting subjects,and collecting samples.
 World Journal of Hepatology2021年4期
World Journal of Hepatology2021年4期
- World Journal of Hepatology的其它文章
- Pathologic and molecular features of hepatocellular carcinoma:An update
- Infantile giant cell hepatitis with autoimmune hemolytic anemia
- Long-term albumin infusion in decompensated cirrhosis:A review of current literature
- Elderly patients (≥80years) with acute calculous cholangitis have similar outcomes as non-elderly patients (<80years):Propensity score-matched analysis
- Retrospective analysis of complications related to endoscopic retrograde cholangio-pancreatography in patients with cirrhosis vs patients without cirrhosis
- Fatal arterial hemorrhage after pancreaticoduodenectomy:How do we simultaneously accomplish complete hemostasis and hepatic arterial flow?
