Pathologic and molecular features of hepatocellular carcinoma:An update
Mukul Vij,Department ofPathology,Dr Rela Institute and Medical Center,Chennai 600044,Tamil Nadu,India
Julien Calderaro,Department of Pathology,Groupe Hospitalier Henri Mondor,Creteil F-94010,France
Abstract Morphological diversity and several new distinct pathologic subtypes of hepatocellular carcinoma (HCC) are now well-recognized. Recent advances in tumor genomics and transcriptomics have identified several recurrent somatic/genetic alterations that are closely related with histomorphological subtypes and have therefore,greatly improved our understanding of HCC pathogenesis. Pathologic subtyping allows for a diagnosis which is clinically helpful and can have important implication in patient prognostication as some of these subtypes are extremely aggressive with vascular invasion,early recurrence,and worst outcomes. Several targeted treatments are now being considered in HCC,and the reporting of subtypes may be quite useful for personalized therapeutic purpose. This manuscript reviews the recently identified histomorphological subtypes and molecular alterations in HCC.
Key Words: Pathology; Hepatocellular carcinoma subtypes; Macrotrabecular massive;Steatohepatitic; Fibrolamellar; Molecular alterations
INTRODUCTION
The incidence of hepatocellular carcinoma (HCC) has been increasing steadily over the past two decades and currently ranks as the fifth most common cancer in men and seventh in women[1,2]. HCC is now the fourth-most common cause of cancer-related deaths and the most frequent primary liver neoplasia,causing more than 80%-85% of liver cancer cases globally[3]. Major risk factors associated with HCC are chronic infection with hepatitis B virus and hepatitis C virus,chronic alcohol consumption,and non-alcoholic fatty liver disease associated with metabolic syndrome,diabetes and obesity. Prognosis of patients with HCC remains poor with 5-year survival rate of 18%,as the majority of these tumors are detected at a clinically advanced stage[3].Hepatocarcinogenesis is a multistep process of malignant transformation of hepatocytes through the sequential accumulation of multiple genomic and epigenomic alterations. HCC is a histologically and genetically diverse cancer[4]. Indeed,several new pathologic subtypes of HCC have been reported recently and new underlying genetic alterations have been described. HCC histological growth patterns are closely related to molecular alterations and oncogenic pathways.
PATHOLOGY OF PRECANCEROUS LESIONS AND CONVENTIONAL HCC
It is now well-established that HCC evolves from precancerous lesions (dysplastic foci/dysplastic nodules). By consensus,the sequence of hepatocarcinogenesis includes low-grade dysplastic nodule (LGDN),high-grade dysplastic nodule (HGDN),early HCC,and small progressed HCC[5,6](Figure 1). This classification is also supported by molecular studies on increasing accumulation of clonal molecular alterations[7].Dysplastic foci (< 1 mm in size) are identified incidentally in chronic liver disease(CLD) and are microscopic lesions composed of dysplastic hepatocytes (Figure 2A).The nature of dysplasia is similar to that observed in dysplastic nodules:Large cell change,small cell change,or focal iron free area. Large cell change is characterized by cellular enlargement with enlarged pleomorphic nuclei,abundant cytoplasm,and frequent multinucleation of hepatocytes. The nuclear-cytoplasmic ratio is preserved in large cell change. Small cell change is characterized by decreased cell volume,increased nuclear-cytoplasmic ratio,cytoplasmic basophilia,mild nuclear pleomorphism,and hyperchromasia. Iron-free foci in patients with marked hepatic iron overload show immunohistochemical evidence of proliferative activity and are associated with a high incidence of HCC. Dysplastic nodules are usually identified in livers with cirrhosis but are also occasionally found along with CLD without cirrhosis.These are around 5-15 mm in diameter and can be single or multiple lesions. A LGDN is a distinctly nodular lesion displaying a monotonous cell population with a mild increase in cellular density,a clear trabecular arrangement,and no architectural atypia in comparison to the neighbouring cirrhotic liver. HGDNs are characterized by hepatocyte proliferation with atypical cytological and/or architectural features that are not sufficient for a diagnosis of HCC. HGDN show higher cellular density and frequently demonstrates small cell change.
Macroscopically,lesions with foci of malignant transformation may demonstrate variable features like vaguely nodular,expansile nodular,multinodular,multicentric,cirrhotomimetic,nodular with perinodular extension,and infiltrative types (Figure 2BD). Small HCCs,≤ 2 cm,are divided into two groups. Early HCC are vaguely nodular with indistinct margins and usually show higher cellular density than the surrounding cirrhotic tissue with increased nuclear to cytoplasmic ratio,irregular trabeculae,pseudoacini formation,and unpaired arterioles (Figure 3A). Stromal invasion is one of the most important characteristics to differentiate early HCC from HGDN,but is however difficult to identify. Progressed HCC are distinctly nodular with distinguishable margins,frequently capsulated,and show infiltrative or expansile growth pattern. Morphologically,conventional HCC show 4 major architectural growth patterns:Trabecular,solid,pseudoglandular/acinar,and macrotrabecular (Figure 3B-D) and several cytological features (clear cell,steatosis,pleomorphism,multinucleation,foamy cells,oncocytic cells,spindle cells),with frequent co-existence of several features (Figure 4)[8,9]. Various intra-hepatocytic inclusions may be seen like hyaline globules (Figure 5A),Mallory-Denk bodies,bile,and pale bodies. Two histological grading systems for HCC are available. The WHO three-tiered grading system is based on a combination of cytological features and differentiation,and further grades the tumor into well,moderately,and poorly differentiated types[10].Primary hepatic undifferentiated carcinoma is not included in the WHO grading system as it shows no evidence of either hepatic or biliary differentiation. It is the system most commonly used by pathologists[10,11]. Edmondson and Steiner grading system divides HCC into four grades based on histological differentiation with grade 1 being very well differentiated[12]. A correlation between the histological grade and patient prognosis has been reported[13]. Poorly differentiated HCC are associated with higher recurrence after surgery[14].

Figure 1 International consensus group for hepatocellular neoplasia classification of small hepatocellular lesions. HCC:Hepatocellular carcinoma.
ANCILLARY STUDIES FOR THE PATHOLOGIC DIAGNOSIS OF HCC
Differentiation of HCC from other malignancies can be difficult; immunostaining can be helpful to differentiate between these lesions. Arginase-1 is a binuclear manganese metalloenzyme and is the most sensitive and specific marker of hepatocytic differentiation[15]. It shows diffused nuclear and cytoplasmic staining. Carcinoma with hepatoid differentiation and rare cases of adenocarcinoma (including colorectal,pancreatic,breast,and prostatic primaries),cholangiocarcinoma,and may however,show focal or weak Arginase-1 positivity[16]. Hepatocyte paraffin 1(Hep-Par 1) is a monoclonal antibody that reacts with the urea cycle enzyme carbamoyl phosphate synthetase 1 of liver mitochondria. It shows diffuse granular cytoplasmic staining in normal and neoplastic hepatocytes[17]. Hep-Par 1 is unfortunately frequently negative for poorly differentiated HCCs. Few cholangiocarcinoma and metastatic adenocarcinoma may show Hep-Par 1 immunopositivity. Glypican 3 is excellent marker for neoplastic hepatocytes with cytoplasmic,membranous,or golgi-zone pattern of immunopositivity[18]. Other immunostains like polyclonal carcinoembryonic antigen(pCEA),CD10 and villin shows a distinct canalicular immunostaining pattern in HCC[16]. Alpha-fetoprotein (AFP) immunohistochemistry is not very useful in diagnosis of HCC as it has low sensitivity and is often only focally positive. Albumin RNAin situhybridization has been shown to be a highly sensitive maker for hepatocellular differentiation[19]. Its specificity is however suboptimal and it can be positive in tumors demonstrating hepatocytic differentiation,such as hepatoid carcinomas of various sites,intrahepatic cholangiocarcinoma (iCCA),gall bladder adenocarcinoma,and yolk sac tumour[20]. Well-differentiated HCCs may also be difficult to distinguish from dysplastic nodules. Loss of reticulin,stromal invasion,and neoarteriolization are particularly useful in these cases. The combination of 3 immunomarkers-glypican 3,glutamine synthetase (GS),and heat shock protein 70-can be used to differentiate early HCC from HGDN[12].
DISTINCT PATHOLOGICAL SUBTYPES WITH MOLECULAR FEATURES
Table 1 summarizes distinct pathological subtypes and their molecular features.
MACROTRABECULAR MASSIVE HCC
The Macrotrabecular-Massive HCC (MTM-HCC) subtype represents a novel histomorphological subtype of HCC. It represents 10%-20% of all cases of HCC.Histologically,it is defined by a macrotrabecular (> 6 cells thick) architectural pattern involving > 50% of the entire tumour,regardless of the associated cytological features(Figure 5B)[4]. Most trabeculae in MTM-HCC are ≥ 10 cells thick[10]. On trucut biopsy analysis,MTM-HCC case is classified if at least 1 focus of macrotrabecular pattern is observed,and the percentage of the macrotrabecular pattern is not taken into account.Pathologists robustly identify MTM-HCC with good inter-observer agreements. MTMHCC is also characterized by an association with tumor protein 53 (TP53) mutations and fibroblast growth factor 19 (FGF19) amplifications[21]. Being an aggressive form of HCC,it is associated with poor prognostic factors,such as higher Barcelona Clinic Liver Cancer (BCLC) stage B or C,higher AFP levels (> 100 ng/dL),larger tumor size,

Table 1 Hepatocellular carcinoma distinct subtypes with pathological and molecular features

Figure 4 Hepatocellular carcinoma cytological features. A:Hepatocellular carcinoma (HCC) with fatty change [hematoxylin and eosin (H&E)]; B:Marked pleomorphism in an HCC (H&E); C:Foamy cell cytoplasm in an HCC (H&E); D:HCC with oncocytic cells (H&E).
frequent satellite nodules,substantial necrosis,and macro or microvascular invasion;hence,there is a higher risk of early tumor recurrence and poor disease-free and overall survival rate (Figures 5C and D)[22]. These findings have been further validated by several groups. The other characteristics are its association with viral hepatitis B infection and profound activation of angiogenesis[23]. Presence of the satellite nodule on the multiphase liver magnetic resonance imaging (MRI) has been described as independent factor associated with both early and overall tumor recurrence[24]. Rheeet al[25]reported imaging findings of MTM-HCC by gadoxetic acid-enhanced MRI. With gadoxetic acid-enhanced MRI findings,including arterial phase hypovascular component,they were able to stratify the probability of MTM-HCC and obtain prognostic information[25]. The gene expression profile associated with the MTM-HCC subtype is characterized by the activation of neoangiogenesis,with overexpression of angiopoietin 2 and Vascular Endothelial Growth Factor A (VEGFA). Angiopoietin 2 is responsible for the destabilization of established vasculature and subsequent neoangiogenesis,and also disturbs interactions between endothelial and periendothelial cells,which results in an increased receptiveness to VEGFA[4]. These tumors have high expression of neoangiogenesis-related genes,which led to the discovery of Endothelial-Specific Molecule 1 as a reliable immunostaining marker[26].Immune assessment of MTM-HCC using expression of the programmed death ligand 1 (PD-L1) and Chemokine-like factor MARVEL transmembrane domain containing 6(CMTM6) protein coded immune-checkpoint inhibitors showed higher tumoral PD-L1 expression,higher density of inflammatory cells,and higher CMTM6 expression.Therefore,combined expression of PD-L1 and CMTM6 were associated with shorter overall and disease-free survival[27].
STEATOHEPATITIC HCC

Figure 5 Conventional and macrotrabecular massive hepatocellular carcinoma. A:Hyaline globules in a conventional hepatocellular carcinoma (HCC)[arrow,hematoxylin and eosin (H&E)]; B:Macrotrabecular massive HCC (H&E); C:Large macrotrabecular massive HCC with satellite nodule; D:Macrotrabecular massive HCC with vascular invasion (arrow,H&E).
Steatohepatitic HCC (SH-HCC) first described in 2010 by Salomaoet al[28],and is a distinct histological subtype strongly associated with underlying steatosis and/or steatohepatitis and metabolic syndrome[28]. SH-HCC demonstrates morphological features similar to steatohepatitis with macrovesicular steatosis,hepatocellular ballooning with cytoplasmic clarification,Mallory-Denk bodies,pericellular fibrosis,and patchy inflammation (Figure 6A)[29]. The steatohepatitis should be a dominant part of the tumor morphology,and at least 50% of the tumor should show this pattern.Fibrosis can be best demonstrated on histochemical stain like Masson trichrome. The immunophenotyping of SH-HCC is similar to conventional HCC; however,it shows increased immunostaining with markers of inflammation like C-reactive protein due to interleukin (IL)-6/Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway activation[21]. SH-HCC are well-differentiated to moderately differentiated tumors and are associated G4 transcriptomics subclass. In a recent transcriptomic analysis by Van Treecket al[30]SH-HCC demonstrated a distinctive differential gene expression profile with upregulation of the sonic hedgehog signal transduction pathway based on GLI1 family zinc finger 1 (GLI1)overexpression.GLI1gene encodes a protein that functions as a transcription factor protein and plays a role in the regulation of stem cell proliferation. There was reduced expression of carnitine palmitoyltransferase 2 (CPT2) transcripts. CPT2 is a mitochondrial enzyme with an essential role in fatty acid β-oxidation and carnitine metabolism. In a mouse model of obesity-driven and non-alcoholic steatohepatitisdriven HCC,metabolic reprogramming mediated by the downregulation of CPT2 enables protection of neoplastic hepatocytes from lipotoxicity[31]. Therefore; reduced level of CPT2 is believed to facilitate survival of malignancy in obesity-associated HCC. Leeet al[32]recently suggested that alteration of the tumor stroma might play an important role in SH-HCC development,and as compared to classical HCC,cancerassociated fibroblasts in SH-HCC and non-tumoral stellate cells were characterized by increased expression of IL-6,a key governor of the JAK/STAT pathway[32]. SH-HCC appears to have similar overall and disease-free survival,development of metastasis,or local recurrence compared with conventional HCC[29].
SCIRRHOUS HCC
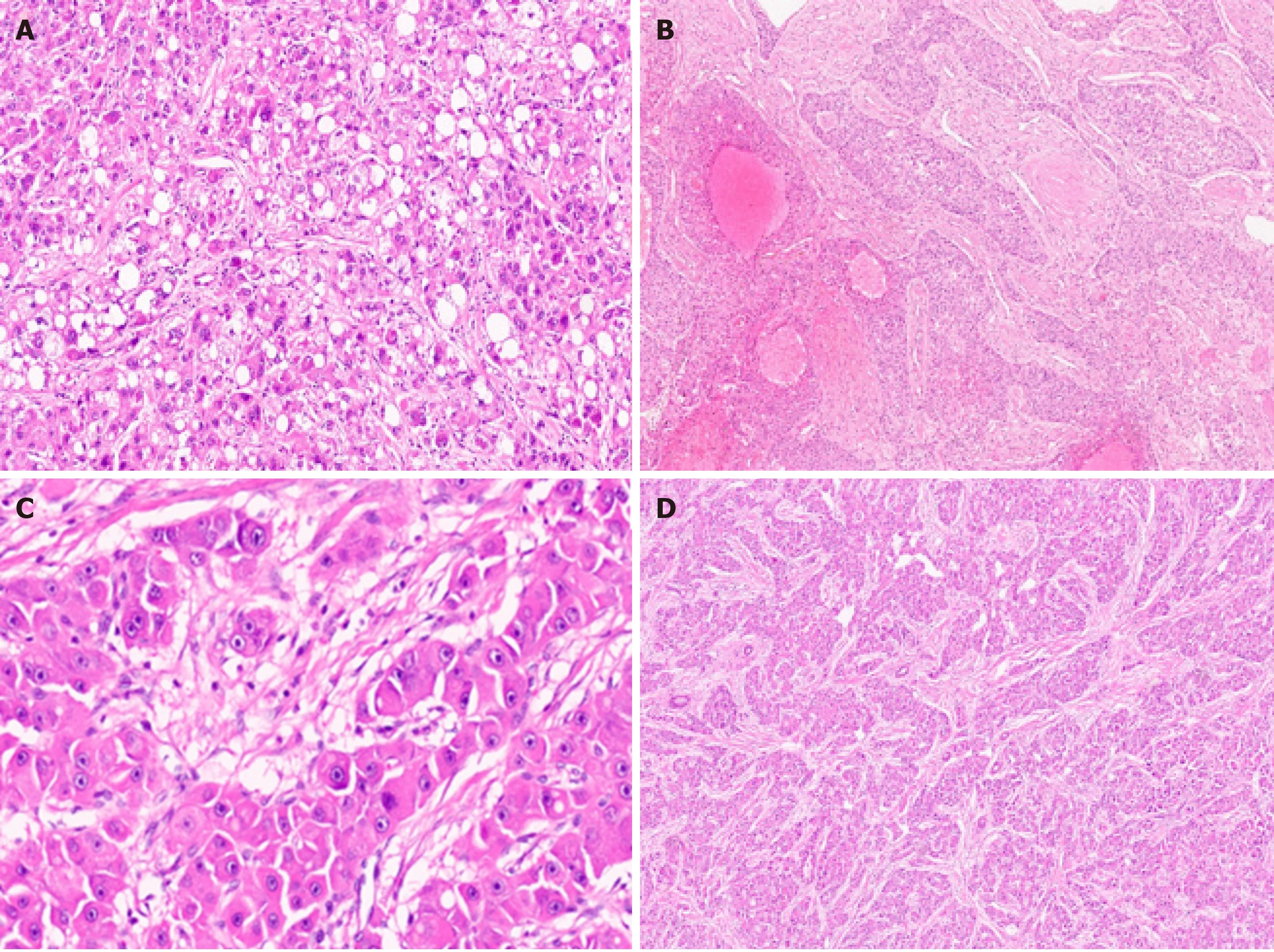
Figure 6 Hepatocellular carcinoma subtypes. A:Hepatocellular carcinoma (HCC) with steatohepatitic pattern [hematoxylin and eosin (H&E)]; B:Sclerotic HCC (H&E); C:Fibrolamellar HCC with large cells and prominent nucleoli (H&E); D:Fibrolamellar HCC with lamellar fibrosis (H&E).
Scirrhous HCC represent approximately 5% of all cases[33]. Radiologic findings are atypical and often show arterial phase peripheral enhancement and venous phase persistent enhancement[34]. Scirrhous HCC is characterized by tumor cell clusters surrounded by abundant fibrous stroma which should constitute at least 50% of the tumor (Figure 6B)[11]. The presence of marked intratumoural fibrosis may lead to a faulty impression of intrahepatic CCA on radiology and macroscopic examination.Scirrhous HCC are mostly well to moderately differentiated HCC. Steatosis,clear cell change,pale bodies,and hyaline bodies have also been reported. Immunohistochemically,there is lack of positive staining for primary hepatocellular stains like HepPar-1 and pCEA in more than 60% of scirrhous HCC,with arginase and glypican 3 positivity in around 80% of cases[35]. Immunostains used for adenocarcinoma,like cytokeratin (CK) 7,CK19,and epithelial cell adhesion molecule,are positive in more than 60% of cases and can lead to erroneous diagnosis of adenocarcinoma[36]. Scirrhous HCC may resemble fibrolamellar HCC histologically,and molecular testing for DNAJ heat shock protein family member B1 (DNAJB1) and protein kinase 3'-5'-cyclic adenosine monophosphate (cAMP)-activated catalytic subunit alpha (PRKACA)fusion can be performed in histologically difficult cases[37]. There is no significant difference in prognosis in Scirrhous HCC compared with conventional HCC[38].Expression of various cholangiocarcinoma-like and stem-cell-like genomic traits,including CK7 (KRT7),CK19 (KRT19),THY1,and CD133/Prominin-1,have been reported in scirrhous-HCC,and it has therefore been suggested that scirrhous HCC harbour intermediate molecular features,between HCC and cholangiocarcinoma[4,39].Scirrhous HCC genomic profile also shows activation of transforming growth factor beta pathway/epithelial-to-mesenchymal transition related genes,with overexpression of Vimentin,SNAIL family transcriptional repressor 1,SMAD family member 4,and Twist-related protein[21].
FIBROLAMELLAR HCC
Fibrolamellar HCC (FL-HCC) is a rare and unique histologic subtype of liver cancer with a predilection for adolescent and young adults (male:female,1:1) without underlying liver disease,a characteristic morphological pattern with large neoplastic cells,distinct immunostaining,and recurrent genomic abnormalities typically involving PRKACA[40]. FL-HCC comprises approximately only 1% of primary liver cancer[41]. FL-HCC commonly presents as an abdominal mass with enlargement of liver,pain in abdomen,and features of biliary obstruction secondary to external compression by the mass lesion[42]. Rarely FL-HCC can present with paraneoplastic manifestations. These tumors are mostly solitary,large,and well circumscribed grossly with a yellow tan colored cut surface,and areas of central scarring are identified in almost 70% of cases[43,44]. Importantly,FL-HCC are much more likely to invade regional lymph nodes. Histologically,the tumor cells are large,polygonal with abundant eosinophilic granular cytoplasm (because of numerous mitochondria),centrally located nuclei with vesicular chromatin,and prominent nucleoli (Figure 6C).Focal bi-or multi-nucleation are also reported. Dense bands of intratumoural fibrosis arranged in lamellar (parallel arrangement) pattern separates the trabeculae and clusters of tumor cells (Figure 6D). FL-HCC also show presence of pale or hyaline bodies; however,these are not specific and may be observed in conventional HCC.Immunophenotyping shows neoplastic cells are positive of CD68 and CK-7 (biliary lineage) apart from markers of hepatic differentiation (Arginase 1,Hep-Par 1 and albumin mRNA as detected byin situhybridization). Honeymanet al[37]first reported a specific 400-kilobase deletion on chromosome 19 in FL-HCC leading to recurrent chimericDNAJB1-PRKACAgene fusion,genetic footprint of FL-HCC. DNAJB1 encodes a member of heat shock protein 40 which is involved in protein folding within cells,while PRKACA codes for the cAMP-dependent protein kinase catalytic subunit alpha; the molecular alteration results in upregulation of PRKACA activity by a promoter switch mechanism[45,46]. Both fluorescencein situhybridization (FISH) or reverse transcription polymerase chain reaction are available now to detect DNAJB1-PRKACA fusion for confirming the diagnosis of FL-HCC. Recently,the genetic alteration (DNAJB1-PRKACAgene fusion) has also been identified in a set of oncocytic pancreaticobiliary neoplasm; however,DNAJB1-PRKACA fusion is still the most accurate test when the diagnosis of FL-HCC is doubtful[47,48]. FL-HCC has a unique gene expression profile,with Erb-b2 receptor tyrosine kinase (ERBB) 2 overexpression and glycolysis upregulation leading to compensatory mitochondrial hyperplasia,and various neuroendocrine genes,including Proprotein Convertase Subtilisin/Kexin Type 1,Neurotensin,Delta/Notch Like EGF Repeat Containing and Calcitonin Related Polypeptide Alpha[49].
LYMPHOEPITHELIOMA-LIKE HCC
Lymphoepithelioma-like HCC (LEL-HCC) also known as lymphocyte-rich-HCC is an uncommon variant of HCC and comprises < 1% of primary liver cancer[11]. LEL-HCC are associated with lower rates of recurrence after surgery and has an overall favorable survival rate when compared with conventional HCC[50]. LEL-HCC morphologically resembles lymphoepithelioma-like carcinomas,a poorly differentiated epithelial tumor first described in nasopharynx,characterized by a prominent immune stroma/microenvironment[4]. Subsequently it has been diagnosed in various organs such as stomach,colon,salivary glands,lungs,thymus,uterus,and ovaries[51]. These liver tumors are composed of poorly or undifferentiated neoplastic epithelial cells with a prominent lymphoid infiltrate[52]. A study of 11 cases of LEL-HCC by Wadaet al[53]proposed quantitative criteria > 100 tumor infiltrating lymphocytes in 10 high power microscopic filed to define significant lymphocytic infiltration[53]. WHO defines LELHCC subtype as the condition in which lymphocytes outnumber pleomorphic neoplastic cells in most microscopic fields,but no clear cutoffs for lymphocyte number has been provided[10]. In contrast to LEL cholangiocarcinoma,which are frequently associated with EBV infection and are well described in literature,LEL-HCC are not associated with EBV infection and are not well characterized in literature[52,54,55].Grossly,these are well circumscribed tumors with variable encapsulation.Histologically,the tumors are composed of atypical cells with syncytial cytoplasm and nuclei with prominent nucleoli and infiltrated by abundant lymphocytes (Figure 7A).Tumor cells show positivity for markers like Hep-Par 1 and Glypican 3 indicating hepatocellular origin. Immunohistochemical profile of the infiltrating immune cells shows a predominance of cytotoxic CD8+ lymphocytes[52]. Rare molecular studies are available on LEL-HCC. A recent study by Chanet al[56]showed marked focal amplification of chromosome 11q13.3 in LEL-HCC. Calderaroet al[57]showed high level of PD-L1 and programmed cell death 1 expression in intratumoural inflammatory cells in LEL-HCC. These findings indicate LEL-HCC might be sensitive to drugs targeting immune checkpoint inhibitors. No association of LEL-HCC with a transcriptomic subclass has been identified. Immune class of HCC reported by Siaet al[58]characterized by markers of an adaptive T-cell response or exhausted immune response was also not associated with increased number of somatic mutations[58].

Figure 7 Hepatocellular carcinoma subtypes. A:Lymphoepithelioma like hepatocellular carcinoma (HCC) [hematoxylin and eosin H&E)]; B:Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) with hepatocytic and cholangiocytic component (H&E); C:cHCC-CCA with stem/progenitor cell features (H&E); D:Cirrhotomimetic HCC with numerous tumor nodules.
PROGENITOR HCC
The progenitor subtype of HCC is defined by the immunohistochemical expression of biliary marker CK19,in more than 5% of neoplastic cells[59,60]. Dedifferentiation of malignant hepatocytes or malignant transformation of hepatic progenitor/stem cells may give rise to this histological subtype[4]. There is growing evidence that progenitor cells,activated during acute and CLD,can directly give rise to HCC. This phenotype is associated with mutation in TP53 and particular genomic subclasses (GI-G3,S2) of HCC[21]. CK19 expression is also reported in HCC after transarterial chemoembolization[61].
COMBINED HEPATOCELLULAR-CHOLANGIOCARCINOMA
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare primary liver cancer. Diagnosis of cHCC-CCA is challenging because of its pathological heterogeneity,unique molecular alterations,poorly defined radiological features,and non-specific clinical features. The WHO 2010 Classification defined a classical type of cHCC-CCA (tumor containing unequivocal,intimately mixed elements of both HCC and intrahepatic CCA),and 3 subtypes of cHCC-CCA with stem/progenitor cell features:Typical,intermediate cell,and cholangiocellular[62]. The WHO consensus classification published in 2019 removed the 3 different stem/progenitor cell subtypes and defined cHCC-CCA as a primary liver carcinoma with unequivocal presence of both hepatocytic and cholangiocytic differentiation (Figure 7B) within the same tumor[63]. This change was implemented because “stem/progenitor cells” identified as small cells with scant cytoplasm,a high nuclear/cytoplasmic ratio,and hyperchromatic nuclei may potentially be seen in all forms of cHCC-CCA; cholangiocellular carcinoma is not always associated with hepatocellular component and subtyping has no prognostic or clinical relevance.
The hepatocellular and cholangiocarcinoma components in cHCC-CCA may be intimately mixed or lie in separate regions of a tumor. Collision of HCC and iCCA arising separately in the same liver should not be included under cHCC-CCA. The diagnosis of cHCC-CCA should be based on hematoxylin and eosin staining only and immunophenotyping can be performed to confirm histologic components. However,IHC alone should not define the diagnosis of cHCC-CCA[64]. Stem/progenitor cell features (Figure 7C) can be mentioned in the comment section of the histology report.Intermediate cell carcinoma is a unique form of cHCC-CCA comprising of monomorphic tumor cells,smaller than hepatocytes but larger than stem/progenitor cells,and has features intermediate between hepatocytes and cholangiocytes. These malignant cells are arranged in strands or trabeculae in an abundant fibrous stroma.Molecular studies of cHCC-CCA are limited and the earlier reported literature suggested that these tumors have a distinct mutational profile with isocitrate dehydrogenase (IDH) mutations usually observed in intrahepatic CCA[4,65]. However this remains debated as a recent study performed by Josephet al[66]demonstrated that the genetics of cHCC-CCA classical type,are distinct from intrahepatic CCA but similar to conventional HCC with alteration in telomerase reverse transcriptase(TERT),p53,and cell cycle genes[66]. Few studies have also reported enrichment in stem/progenitor-like signatures,supporting the concept of a stem/progenitor cell origin of cHCC-CCA[67]. cHCC-CCA has a dismal prognosis,worse than that of either HCC or iCCA,and currently,there are no accepted international management guidelines for cHCC-CCA.
RARE AND PROVISIONAL PATHOLOGICAL SUBTYPES OF HCC
These pathological subtypes are rare and provisional because limited published literature is available.
FIBRONODULAR HCC
Fibronodular HCC (FN-HCC) is a recently described candidate variant[68]. FN-HCC histology is characterized by extensive fibrosis dividing a single tumor into multiple well circumscribed distinct nodules with no significant intranodular fibrosis between single or clusters of neoplastic cells[54]. These tumors show well to moderate differentiation with trabecular or solid growth pattern. Scattered pseudoacini are also described. FN-HCC are reported to be more likely to arise in liver with lower fibrosis stage and lower advanced BCLC stage. They have lower rates of tumor progression.Imaging analysis of FN-HCCs revealed higher rates of non-peripheral washout and a new distinct pattern of enhancement which is characterized by the presence of multiple rounded nodules within a lesion embedded in fibrotic-appearing parenchyma,called as ‘popcorn’ appearance of the lesion[68].
CHROMOPHOBE HCC WITH ABRUPT ANAPLASIA
This histological subtype is characterized by a unique set of morphological features:smooth chromophobic cytoplasm which can be either slightly eosinophilc or basophilic,abrupt focal nuclear anaplasia (small tumor cell clusters with marked nuclear anaplasia in a background of tumor cells with bland round nuclei and inconspicuous nucleoli),and scattered microscopic pseudocysts[9,69]. This subtype is associated with distinct molecular features with respect to telomere maintenance resulting in alternative lengthening of telomeres (ALT),which can be detected by telomere FISH. ALT is a telomerase-independent mechanism of telomere maintenance and is found in > 90% of chromophobe HCC with abrupt anaplasia and < 10% of unselected HCCs. Woodet al[69]also investigated somatic mutations of alphathalassemia/mental retardation,X-linked,Histone H3,and Death Domain Associated Protein identified in various ALT positive tumors reported at other sites in two cases of chromophobe HCC with abrupt anaplasia; however,no mutations were identified[69-71].
GRANULOCYTE COLONY-STIMULATING FACTOR PRODUCING HCC/NEUTROPHIL-RICH HCC
This rare subtype is characterized by production of granulocyte colony-stimulating factor (G-CSF),leading to diffuse infiltrates by neutrophils[72-74]. There is no clear histological definition for this variant. Morphologically,these tumors are poorly differentiated HCC,usually with areas of sarcomatous differentiation and numerous neutrophils. These generally occur in older individuals,grow rapidly,have a high probability of distant metastases,and the overall prognosis seems to be poor as compared with conventional HCC. The mechanism of the production of G-CSF in HCC remains unclear; a close relationship between G-CSF production in malignant cells and their dedifferentiation has been reported[74].
LIPID-RICH HCC
Lipid-rich HCCs have a foamy cytoplasm resulting from lipid accumulation,with numerous very tiny droplets of fat[75-77]. These can be associated with few larger fat droplets. The differential includes lipid-rich variants of metastatic carcinoma.Immunostaining with Hep-Par 1 and Arginase is helpful in doubtful cases.
CIRRHOTOMIMETIC OR DIFFUSE CIRRHOSIS LIKE-HCC
Cirrhotomimetic (CM) or diffuse cirrhosis like-HCC is a rare variant of liver cancer characterized by small cirrhosis-like tumor nodules that are intimately admixed within the cirrhotic liver parenchyma[78-81]. This tumor pattern is often diagnosed incidentally on the native liver explanted at the time of transplantation or autopsy liver specimen,as most of the times,it is clinically and radiologically undetectable (Figure 7D). These tumors are well to moderately differentiated and majority of patients show no significant elevation in serum AFP values[79]. Pseudoacinar architectural growth pattern with bile production and numerous Mallory-Denk bodies have been demonstrated in these tumors. Few studies have investigated tumor nodules in CMHCC and suggested that these are synchronous multiclonal HCCs[82,83]. One recent study evaluated the liver explants post transcatheter arterial chemoembolization in CM-HCC and non-CM-HCC and reported lower rates of complete pathologic necrosis and poorer overall survival in CM-HCC after liver transplantation as compared with non-CM-HCCs[84].
CLEAR CELL HCC
Clear cell HCC is an uncommon histological variant of HCC. WHO defines this tumor as the condition when > 80% of the neoplastic cells show clear cell morphology[10].Glycogen accumulation leads to clearing of the cytoplasm; admixed minor steatosis is also acceptable. These are well to moderately differentiated tumors with similar or better prognosis than conventional HCC[85-87]. There is,however,no distinct definition of this subtype and clear cells may be observed in other subtypes.
HEPATIC CARCINOSARCOMA
Hepatic carcinosarcomas are composed of both malignant epithelial component and mesenchymal components[9]. These neoplasms are extremely rare. The carcinomatous component is moderately to poorly differentiated HCC. The sarcomatous component shows morphologic or immunohistochemical evidence of mesenchymal differentiation,such as leiomyosarcoma,rhabdomyosarcoma,chondrosarcoma,fibrosarcoma,or rarely osteosarcoma. There is scant data on molecular alterations[88,89]. One earlier study revealed mutation in TP53,Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha and FGFR3 genes[88]. One recent study using targeted nextgeneration sequencing with a panel of 329 cancer-related genes identified TP53,Neurofibrin 1/2 mutations,and VEGFA amplification in both carcinomatous and sarcomatous components[89]. Amplifications involving MET and platelet-derived growth factor receptor A were identified only in the sarcomatous components,whereas mutation affecting ERBB4 and amplifications of Cyclin D1 and FGF 3/4/19 were present only in the carcinomatous components.
MYXOID HCC
This rare morphological subtype of HCC shows well to moderately differentiated neoplastic cells with a trabecular growth pattern,separated by abundant extracellular myxoid/mucin material[9,90]. The neoplastic cells stain strongly with HepPar1 and Arginase-1,and are negative for biliary marker CK19. These tumors typically show loss of liver fatty acid binding protein and also immunostaining with strong and diffuse positivity for GS.
CONCLUSION
Pathology of HCC has evolved significantly in the last two decades. We are now well versed with various dysplastic liver lesions and multiple distinct pathologic subtypes of HCC. There is also remarkable improvement in our understanding of HCC pathogenesis as tumor genome sequencing has identified recurrent molecular alterations and oncogenic pathways and how this correlates with various morphological findings. Identification of genetic alterations also gives us an opportunity to develop targeted therapies that can prevent recurrence and improve patient survival.
 World Journal of Hepatology2021年4期
World Journal of Hepatology2021年4期
- World Journal of Hepatology的其它文章
- Infantile giant cell hepatitis with autoimmune hemolytic anemia
- Long-term albumin infusion in decompensated cirrhosis:A review of current literature
- Bile acid indices as biomarkers for liver diseases I:Diagnostic markers
- Elderly patients (≥80years) with acute calculous cholangitis have similar outcomes as non-elderly patients (<80years):Propensity score-matched analysis
- Retrospective analysis of complications related to endoscopic retrograde cholangio-pancreatography in patients with cirrhosis vs patients without cirrhosis
- Fatal arterial hemorrhage after pancreaticoduodenectomy:How do we simultaneously accomplish complete hemostasis and hepatic arterial flow?
