Long-term albumin infusion in decompensated cirrhosis:A review of current literature
Yu Jun Wong,Rahul Kumar,Tiing Leong Ang,Department ofGastroenterology and Hepatology,Changi General Hospital,Singapore 529889,Singapore
Yu Jing Jonathan Chua,Department of Internal Medicine,Yong Loo Lin School of Medicine,Singapore 117597,Singapore
Abstract Decompensated cirrhosis is characterized by chronic inflammation and severe portal hypertension leading to systemic circulatory dysfunction. Albumin infusion has been widely used in decompensated cirrhosis in patients with spontaneous bacterial peritonitis,large-volume paracentesis and hepatorenal syndrome. Emerging data suggest long-term albumin infusion has both oncotic and non-oncotic properties which may improve the clinical outcomes in decompensated cirrhosis patients. We review the current literature on both the established and potential role of albumin,and specifically address the controversies of long-term albumin infusion in decompensated cirrhosis patients.
Key Words: Albumin; Cirrhosis; Hepatic encephalopathy; Hepatorenal syndrome; Acuteon-chronic liver failure; Spontaneous bacterial peritonitis; Large-volume paracentesis
INTRODUCTION
Long-term albumin infusion in decompensated cirrhosis:A critical review of current literature
Concept of compensated and decompensated cirrhosis:Cirrhosis represents the common pathway of all chronic liver disease resulting in over a million deaths every year[1]. The natural history of liver cirrhosis includes an asymptomatic compensated stage and a decompensated cirrhosis stage with clinically overt complications as ascites,jaundice,variceal bleeding and hepatic encephalopathy (HE)[2]. The median survival reduces significantly from 12 to 2 years as patients progress from the compensated to decompensated cirrhosis at an annual rate of 5%-7%[2,3].
Decompensated cirrhosis is characterized by chronic inflammation and severe portal hypertension leading to systemic circulatory dysfunction[4]. As a corrective response to portal hypertension,excessive nitric oxide secretion results in both splanchnic and arterial vasodilatation,which thus impairs organ perfusion[5,6]. To ensure adequate organ perfusion,the arterial pressure is maintained by increased activity of the renin-aldosterone-angiotensin system[7]. The understanding of circulatory dysfunction in patients with decompensated cirrhosis has led to the use of albumin and vasoconstrictors to improve circulatory dysfunction and prevent kidney injury[8]. Such an approach is paramount because the presence of acute kidney injury(AKI) is associated with significantly longer hospitalization stay and higher mortality in patients with decompensated cirrhosis[9].
Human albumin has widely been used in decompensated cirrhosis patients for varying indications. While the established indications of albumin infusion as endorsed by the current societal guidance include spontaneous bacterial peritonitis (SBP),largevolume paracentesis (LVP) and hepatorenal syndrome (HRS)[10],albumin infusion is often used beyond these indications in the daily clinical practice. Although some of the recently published studies have reported the beneficial effect of regular long-term albumin infusion in patients with decompensated cirrhosis[11-13],regular long term albumin infusion is not completely innocuous. Not only is albumin more expensive than crystalloids as volume expander,serious adverse events as pulmonary oedema and even death have also been reported[14].
With this background,we aim to critically review the current literature on both the established and potential role of albumin,and specifically addressed the controversies of long-term albumin infusion in decompensated cirrhosis patients.
WHAT IS ALBUMIN?
Albumin is the main circulating protein in healthy adults. Structurally it is a small(66500 Dalton),negatively-charged protein that consists of 2 sub-domains[15,16].Albumin is exclusively synthesized within the liver. It is up-regulated by hormones(insulin,cortisol and growth hormone)[17-19]and down-regulated by inflammatory mediators (tumor necrosis factor and interleukin-6)[20]. Once produced,up to 40% of albumin is released into the bloodstream. The half-life of albumin ranges from 12 to 19 d[21]. The degradation of albumin occurs mostly within the liver,kidney and muscle[22].
Function of albumin
Albumin has both oncotic and non-oncotic properties[15,23]. The potent oncotic property of albumin is primarily derived from the direct oncotic effect from high plasma concentration,which accounts for about two-thirds of its osmotic effect. The Gibbs-Donnan effect,where the negatively-charged albumin molecule also attracts positively charged molecules such as sodium within the bloodstream,is responsible for the remaining one-third of the osmotic effect of albumin[23].
Albumin transports hydrophobic molecules (such as bilirubin,bile acid,long-chain fatty acids) to hepatocytes for detoxification and elimination[24]. Recent evidence suggests that the effect of albumin goes beyond the oncotic functions and transport,but also include immunomodulatory and antioxidant functions as well. Albumin is shown to attenuate prostaglandin E2 mediated immune-dysfunction in patients with decompensated cirrhosis[25]. It also exerts immunomodulatory effect by downregulating the expression of tumor necrosis factor-alpha and pro-inflammatory nuclear factor-kappa B[26]. Another property attributed to albumin is that it also functions as an antioxidant to scavenge reactive oxygen and nitrogen species in our body[27,28].
Albumin in decompensated cirrhosis
Hypoalbuminemia is a known predictor of poor survival in decompensated cirrhosis and serves well as a constituent of Child-Turcotte-Pugh score. What is less well appreciated is the fact that abnormalities with serum albumin in decompensated cirrhosis patients are both quantitative and qualitative[29]. The quantitative reduction of serum albumin concentration is a result of dilution from sodium and water retention,reduced synthesis from hepatocytes as well as increased trans-capillary leak,particularly amongst patients with refractory ascites[30,31]. The quality of albumin is further compromised in decompensated cirrhosis due to a higher proportion of oxidized albumin[32]. The oxidized albumin differs from native albumin because it has a lower binding capacity,impaired antioxidant properties and a shortened half-life[31].Oxidized albumin not only correlates with the severity and complication of cirrhosis but also with short and long-term mortality[29,32]. This understanding on both the quantitative and qualitative alterations of albumin has resulted in the concept of"effective albumin concentration" in decompensated cirrhosis,which takes into account both the amount of albumin and its structural integrity[33].
ESTABLISHED INDICATION OF ALBUMIN IN DECOMPENSATED CIRRHOSIS
SBP
SBP is defined based on the presence of > 250 polymorphonuclear cells/mm3or positive ascitic fluid cultures,in the absence of an intraabdominal source of infection or malignancy[34]. Renal impairment is reported in up to 33% patients following SBP and is associated with inpatient mortality,despite resolution of infection[34,35]. In the first randomized trial which investigated the role of intravenous albumin infusion in SBP,Sortet al[36]demonstrated that albumin infusion and cefotaxime significantly reduced the risk of renal impairment (33%vs10%),inpatient mortality (29%vs10%)and 3-month mortality (41%vs22%)[36]. The benefits of albumin especially in patients at high risk of developing renal impairment (baseline serum bilirubin ≥ 4 mg/dL or creatinine ≥ 1 mg/dL) were subsequently confirmed in a meta-analysis of randomized trials[37].
Is albumin mandatory in SBP patients with low risk of renal impairment,particularly those who did not fulfil the above criteria? A meta-analysis reported a low pooled incidence of renal impairment and death (2.8% and 3.8%,respectively) among the patients with low risk of renal impairment[37]. The number needed-to-treat to prevent one case of renal impairment and death is 45 and 27,respectively. Given the limited data in low-risk SBP patients,further prospective randomized trials are required to confirm the benefit of albumin infusion in SBP patients with low risk of renal impairment.
Post-paracentesis circulatory dysfunction
Paracentesis-induced circulatory dysfunction (PICD) is a known complication of LVP in patients with decompensated cirrhosis. The reported incidence varies widely between 17.1% to as high as 72.7%,depending on whether albumin infusion was given during LVP[38]. PICD classically has been defined as at-least 50% or more rise in serum renin levels up to 6 d following a large volume paracentesis[39]. PICD can lead to arterial hypotension and the resultant renal impairment has been associated with readmissions and mortality[39].
Several studies have evaluated the role of albumin infusion in large volume paracentesis. Albumin infusion (given at 6-8 g/L of ascitic fluid drained) has shown to prevent PICD in paracentesis beyond 5 L[39,40]. In a meta-analysis of randomized trials,albumin infusion is associated with a lower risk of PICD (OR = 0.39,95%CI:0.27-0.55)and mortality (OR = 0.64,95%CI:0.41-0.98) following paracentesis[38]. Specifically,all the included trials removed beyond 5 L of ascitic fluid; the majority of the studies administered 6-8 g of albumin 20%perL of ascitic fluid removed. With this understanding,the current guidelines recommending albumin replacement in paracentesis beyond 5 L to prevent PICD[38].
HRS
HRS is the functional renal failure secondary to intrarenal vasoconstriction in patients with decompensated cirrhosis or acute liver failure[10]. Emerging data suggest HRS to be driven by both renal hypoperfusion from systemic circulatory dysfunction as well as increased circulating pro-inflammatory cytokines[41].
Currently,most of the evidence for albumin infusion in HRS is derived from HRS type 1 (also known as HRS-AKI). In a prospective,non-randomized study to investigate the role of albumin infusion,with and without terlipressin,in patients with HRS-AKI,Ortegaet al[42]demonstrated that albumin infusion significantly improves HRS-AKI in addition to terlipressin alone (albumin:77%vs25%)[42]. Ever since then,albumin has become an integral part of HRS treatment with vasoactive drugs such as terlipressin,noradrenaline or octreotide[42-53]. Most studies administer 20-40 g of albuminperday and titrate according to fluid status to avoid fluid overload.Combination of albumin and terlipressin reverse HRS-AKI in up to 56% of patients in randomized clinical trials[43-45]. However,treatment-related adverse events leading to treatment discontinuation still occur in up to 43% of patients during the clinical trials.These complications (namely acute coronary syndromes and peripheral vascular ischemia) are mostly caused by intense systemic vasoconstriction attributable to terlipressin and can be partially mitigated by continuous terlipressin infusion(complication rates of 35%vs62%),without compromising the treatment efficacy[46].
Even though albumin and terlipressin infusion achieves reversal of HRS-AKI in up to 60% of patients,it may not eventually result in reduced mortality. Several notable studies have evaluated the mortality benefit of albumin and vasoconstrictor in HRSAKI with conflicting results[43-45,47-53]. Based on two of the recent meta-analyses,there is no conclusive survival benefit of albumin and vasoconstrictor infusion in HRS-AKI when compared to placebo[54,55].
Type-2 HRS is different from type-1 as it has a more subtle course and lower shortterm mortality. Albumin and terlipressin infusion has also been shown to improve renal function in HRS type 2. However,the recurrence rate of HRS type 2 after treatment discontinuation is high and there is no clear benefit on mortality of these patients[56-58].
THE ROLE OF ALBUMIN IN DECOMPENSTAED CIRRHOSIS:BEYOND GUIDANCE
Non-SBP infection
As the circulating human albumin is less than optimal both quantitatively and qualitatively in decompensated cirrhosis. Theoretically,the benefit of albumin infusion may be expanded to non-SBP infection,especially those with renal impairment. It is also widely accepted that while renal impairment is often reversible in patients with decompensated cirrhosis with non-SBP infection,the 3-mo mortality is significantly higher compared to patients without renal impairment (55%vs13%)[59]. Some notable literatures have tried to answer this quandary with help of randomised clinical trials(RCT). In a single-center RCT,Guevaraet al[60]randomized 110 patients with non-SBP infections to receive standard antibiotics with or without albumin[60]. The dose of albumin administered was similar to SBP (1.5 g/kg on day 1 and 1 g/kg on day 3)regimen. Despite a reduction in serum creatinine,renin and aldosterone (which indicates an improvement in renal and circulatory function),the 3-mo survival rates were similar between the two groups[60]. In another RCT,Thévenotet al[61]randomized 191 patients with decompensated cirrhosis (Child-Pugh score > 8) with sepsis to receive albumin in addition to antibiotic. The rate of renal failure and mortality at three months were similar in both groups (albumin:14.3%vs13.5%,and,albumin:70.2%vs78.3%,respectively)[61]. However,8.3% of patients developed pulmonary oedema following albumin infusion,and two patients died as a result of pulmonary oedema. These findings were confirmed in a recent meta-analysis of randomized trials,which showed that albumin infusion did not reduce the risk of renal impairment or death in non-SBP infection[14]. As albumin infusion did not improve renal function or survival,yet may result in adverse events such as pulmonary oedema or even death,the current guideline does not recommend albumin infusion for patients with non-SBP infection[10].
HE
HE is a neuropsychiatric manifestation associated with poor prognosis in decompensated cirrhosis resulting from the complex interplay between effective circulatory volume,ammonia,systemic inflammation and portosystemic shunting. As albumin is known to improve systemic circulatory dysfunction and oxidative stressmediated tissue injury,there has been growing interest in using albumin to treat or prevent HE.
The preventive role of albumin infusion was investigated in a single center cohort study by Riggioet al[62]The author enrolled 23 patients following Transjugular intrahepatic portal-systemic shunt (TIPSS) to receive albumin infusion for three weeks.The risk of developing new HE was similar to a historical cohort which did not receive albumin infusion[62],suggesting that infusion of albumin may not have any role in preventing TIPSS or systemic shunting-related HE.
The role of albumin for the treatment of HE was first studied in 15 alcoholic cirrhosis patients with diuretic-induced HE. Patients were randomized to receive albumin or colloid infusion titrated accordingly to the central venous pressure[63].Despite having a similar reduction in serum ammonia in both groups,the albumin group has a greater improvement in HE grade. Similar beneficial effects were observed in a prospective,open-labelled randomized study,Sharmaet al[64]enrolled 120 patients with overt HE (graded based on the West Haven criteria) to receive either lactulose,with and without albumin[64]. Albumin was administered at 1.5 g/kg/d until the resolution of HE or day 10 of admission. Albumin group was more likely to achieve complete resolution of HE (albumin:75%vs53%),shorted hospitalization stays(albumin:6.4 dvs8.6 d) and lower mortality (albumin:18%vs32%). Furthermore,the albumin group had a greater decline in the serum tumor necrosis factor alfa,interleukin-6 and endotoxin level when compared to lactulose alone. However,this beneficial effect of albumin is not consistently demonstrated across studies. In a multicenter,double-blind,randomized controlled study,56 patients with HE were randomized to receive albumin infusion (1.5 g/kg on day 1 and 1 g/kg on day 3)vs0.9% saline[65]. This study remarkably did not find any significant difference in HE resolution by day 4,even though albumin infusion was associated with better transplant-free survival in patients with HE [hazard ratio (HR) 0.27,95%CI:0.11-0.74].The current societal guidelines do not endorse the use of long-term albumin infusion for either the treatment or prevention of HE in patients with decompensated cirrhosis[10].
Acute-on-chronic liver failure
Acute-on-chronic liver failure (ACLF) is a distinct clinical entity characterized by systemic inflammation associated with multiorgan failure and high short-term mortality among decompensated cirrhosis patients[66]. As systemic inflammation is the hallmark of ACLF,the pleiotropic properties of albumin to rapidly expand the intravascular volume and ameliorate systemic inflammation makes albumin a promising treatment option in ACLF. Although clinical studies in past investigating the role of extracorporeal devices[67,68]provide the proof of concept that albumin infusion could play an effective role in the management of patients with ACLF,only a few studies have been carried out to specifically investigate the effect of albumin infusion in patients with ACLF.
In a recent multicenter randomized study (INFECIR-2 trial),Fernándezet al[69]randomized 108 patients with decompensated cirrhosis and non-SBP infection resulting in ACLF to receive albumin or placebo in addition to antibiotic[69]. More patients in the albumin group experienced resolution of ACLF (82.3%vs33.3%),even though the overall mortality were similar to patients receiving antibiotics alone[69].Though promising,more robust data is required to support the use of albumin in ACLF.
LONG-TERM ALBUMIN IN DECOMPENSATED CIRRHOSIS
There have been growing interests in long-term albumin use among decompensated cirrhosis patients. We summarize all the relevant studies describing the use of longterm albumin in decompensated cirrhosis in Table 1. Wilkinson and Sherlock[70]first studied the role of long-term albumin infusion in the 1960s. They randomized 16 patients with diuretic refractory ascites to receive albumin infusionvsstandard medical therapy (SMT)[70]. Albumin infusion was titrated based on serum oncotic pressure and maintained up to 19 mo. Apart from improving general "well-being",long-term albumin infusion did not improve overall survival or reduce the need for diuretics.
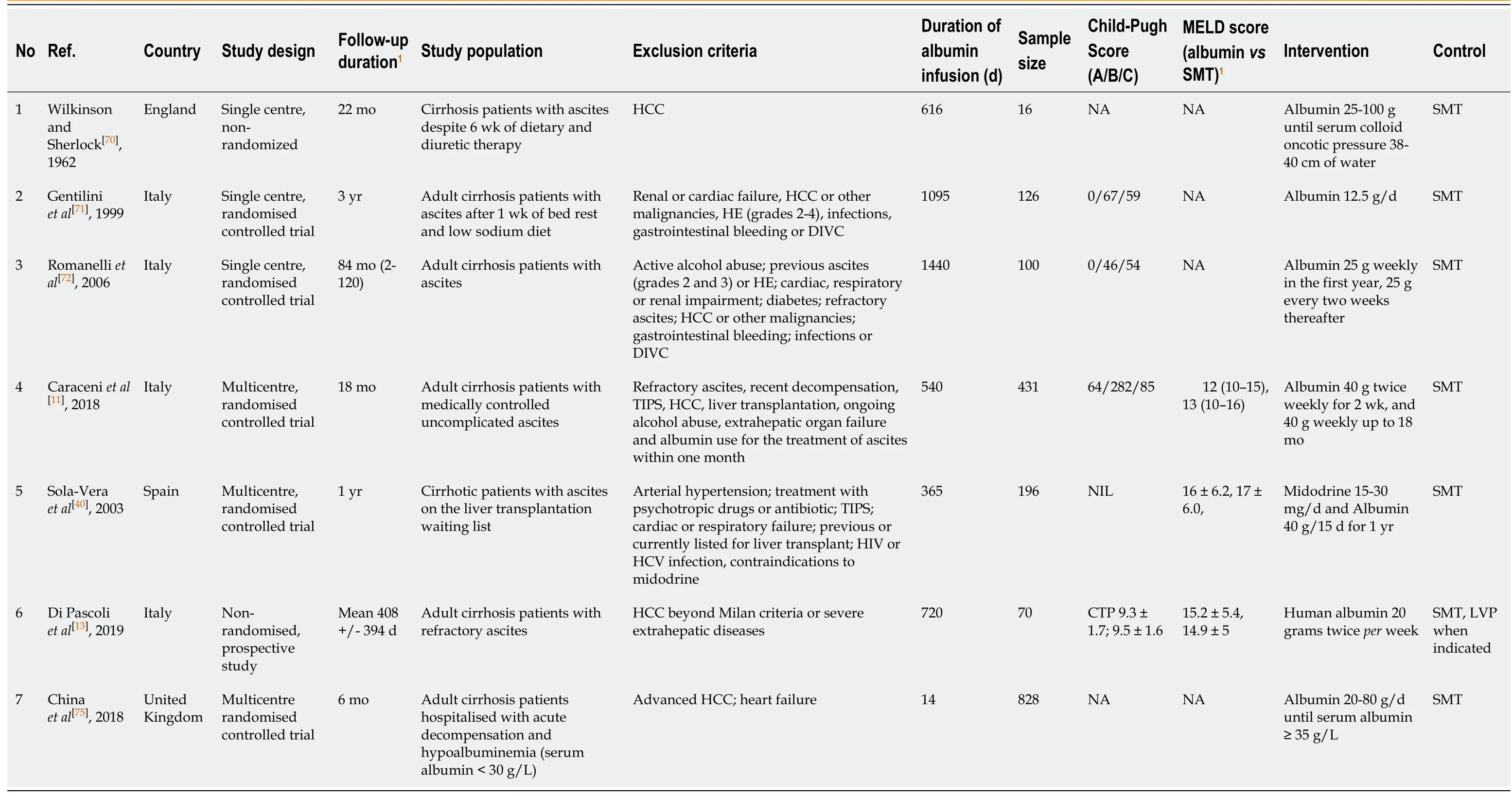
Table 1 Characteristics of studies on long-term albumin infusion in decompensated cirrhosis patients
In another single center randomized study,Gentiliniet al[71]enrolled 126 patients with refractory ascites to receive either albumin infusion or SMT[71]. Patients received weekly albumin infusion of 25 g in the first year,followed by 25 g every two weeks up to 3 years. Long-term albumin infusion reduced ascites recurrence and ascites-related readmission without improving the overall survival. Subsequently,the same group performed a follow-up study 7 years later in 2006,evaluating the long-term outcomes of long-term albumin infusion with an extension of the follow-up period to a median of 84 mo[72]. They recruited 100 patients with new-onset,clinically significant ascites and randomized them to receive either albumin or SMT. The effect of long-term albumin in ascites management was again demonstrated,with less ascites recurrence(39%vs85%) in the albumin group. More importantly,long-term albumin infusion improved 5-year transplant-free survival (albumin:62%vs26%) for the first time,even though the sample size was relatively small.
The ANSWER study (the human Albumin for the TreatmeNt of aScites in patients With hEpatic cirRhosis) enrolled 431 patients of decompensated cirrhosis with medically controlled ascites and compared the clinical outcomes in patients receiving long term albumin infusionvsSMT[11]. In this study,long term albumin infusion (40 g twice weekly for two weeks,followed by 40 g weekly) in addition to SMT was associated with significantly lower mortality (HR 0.62,95%CI:0.40-0.95). The ascites control were better in albumin group with a lower risk for paracentesis (HR 0.48,95%CI:0.35-0.54) and refractory ascites (HR 0.43,95%CI:0.29-0.62). Also,long-term albumin infusion was associated with a lower risk of both SBP and non-SBP related bacterial infection,grade III and IV HE,HRS,renal dysfunction and hyponatremia.Long-term albumin infusion was well-tolerated. Finally,long-term albumin infusion was also shown to be cost-effective,primarily by a reduction in hospital admission,risk of paracentesis and HRS.
In another prospective but non-randomized study,Di Pascoliet al[13]enrolled 70 patients with cirrhosis and refractory ascites to receive either long-term albumin infusionvsSMT[13],with the primary endpoint of 24-mo survival. Subjects in the albumin group received 20 g of albumin twice weekly. The study demonstrated a significant improvement in 24-mo survival in the albumin group when compared to the SMT (58%vs35% in SMT) over a mean follow up of 408 d. Furthermore,the albumin group had a lower risk of emergency hospitalizations from SBP,non-SBP infection and HE. While the liver transplantation rate was similar in both groups (11%vs8% in SMT),it should be highlighted that none of the patients with refractory ascites received Transjugular intrahepatic portosystemic shunt (TIPS). More data is required to evaluate the comparative efficacy of long-term albumin and TIPS for refractory ascites.
The MACHT trial (midodrine and albumin for cirrhotic patients in the waiting list for liver transplantation) however offered a contrasting view on the survival benefit of long-term albumin in decompensated cirrhosis patients[12]. In this multicenter,randomized,double-blind,placebo-controlled trial,196 patients on the transplant waiting list were enrolled to receive either SMT or albumin infusion (40 g every 15 d for one year) plus midodrine with cirrhosis-related complications being the primary end-point. In contrast to the ANSWER trial,the cirrhosis-related complications,ascites control and overall survival were similar between albumin and SMT group. However,3 important features of the MACHT trial must be considered and the results interpreted in accordingly. First,a relatively higher proportion of patients in both groups received transplantation,(68% in albuminvs55% in SMT group). Second,the duration of albumin therapy was relatively short (median duration of 80 d). Thirdly,the dose of albumin therapy used was also lower than that used in all the other studies. A dosage of 40 g every 15 d was used,as compared to higher dosages in all the other trials. The failure of albumin therapy group to show a better outcome may potentially be attributed to these three factors.
IS LONG-TERM ALBUMIN READY FOR PRIME TIME?
The ANSWER study has provided valuable insights on using long-term albumin infusion as a pathophysiological approach to prevent cirrhosis related complications and death in stable cirrhosis patients with medically-controlled ascites. Nevertheless,it is worth noting that the ANSWER study excluded more advanced-cirrhosis patient with refractory ascites and recent decompensation (variceal bleeding,bacterial infection). In patients with refractory ascites,the comparative efficacy between longterm albumin infusionsvsTIPS,which is a one-off procedure with good efficacy,remains unanswered. Besides,only 3.2% (14/431) of patients with hepatitis C related cirrhosis received direct-acting antiviral therapy in the ANSWER study. As the treatment with direct-acting viral therapy is expected to improve the clinical outcomes in these patients[73,74],whether this specific subset of decompensated patients would benefit from long-term albumin infusion following sustained virological response remains unanswered.
The most recent published data,although in abstract form,evaluating the benefits of albumin infusion comes from the ATTIRE (Albumin to prevent infection in chronic liver failure) study which included patients with cirrhosis hospitalized for acute decompensation and hypoalbuminemia (serum albumin < 30 g/L)[75]. In this multicenter randomized trial which enrolled 778 patients to receive albumin infusionvsSMT,the primary endpoint was having a new infection,renal dysfunction or mortality from day 3 to 15 of treatment. The results of this study show that the risk of renal dysfunction and death were similar between albumin and SMT group and thus albumin infusion may not be beneficial in these patients. The PRECOISA (Effect of long-term administration of albumin in subjects with decompensated cirrhosis and ascites) study which aims to investigate the impact of long-term albumin on the 1-year mortality and ACLF,is currently ongoing (NCT03451282). The results of PRECOISA will hopefully provide robust evidence for the use of long-term albumin infusion in decompensated cirrhosis patients.
CONCLUSION
Decompensated cirrhosis is characterized by systemic circulatory dysfunction from portal hypertension and systemic inflammation. In decompensated cirrhosis,albumin dysfunction both in terms of quantity and quality. The established therapeutic role of albumin infusion in decompensated cirrhosis includes SBP,HRS and in patients undergoing LVP. Although long-term albumin seemed promising to prevent ascitesrelated complications in decompensated cirrhosis,the existing studies were heterogeneous in terms of their study population,follow-up duration,and the dose of albumin infusion,thus making the interpretation on the survival benefit particularly challenging. The positive results of long-term albumin infusion will likely increase the global demand for intravenous albumin,particularly among decompensated cirrhosis patients. Meanwhile,the cell-free concentrated ascites reinfusion therapy (CART) may be a novel alternative to intravenous albumin infusion in patients with ascites[76],however more data is required to evaluate the efficacy and safety of CART,particularly among cirrhosis patients with refectory ascites.
Future upcoming studies evaluating the role of long-term albumin infusion to ameliorate systemic inflammation and cirrhosis-related complications are expected in the next few years. Till then,the use of albumin beyond the established indication should be individualized. Future studies should focus on refining the dosages,schedule of long-term albumin infusion and on the specific population groups which would benefit the most.
 World Journal of Hepatology2021年4期
World Journal of Hepatology2021年4期
- World Journal of Hepatology的其它文章
- Pathologic and molecular features of hepatocellular carcinoma:An update
- Infantile giant cell hepatitis with autoimmune hemolytic anemia
- Bile acid indices as biomarkers for liver diseases I:Diagnostic markers
- Elderly patients (≥80years) with acute calculous cholangitis have similar outcomes as non-elderly patients (<80years):Propensity score-matched analysis
- Retrospective analysis of complications related to endoscopic retrograde cholangio-pancreatography in patients with cirrhosis vs patients without cirrhosis
- Fatal arterial hemorrhage after pancreaticoduodenectomy:How do we simultaneously accomplish complete hemostasis and hepatic arterial flow?
