Waterlogging stress in cotton:Damage,adaptability,alleviation strategies,and mechanisms
Ynjun Zhng,Gungy Liu,b, Hezhong Dong,d,, Cundong Li
aCotton Research Center,Shandong Academy of Agricultural Sciences,Jinan 250100,Shandong,China
bAgronomy College of Shandong Agricultural University, Tai'an 271018,Shandong,China
cHebei Agricultural University,Baoding 071001,Hebei,China
dCollege of Life Sciences,Shandong Normal University,Jinan 250014,Shandong,China
Keywords:
ABSTRACT Over the last few decades, waterlogging stress has increasingly threatened global cotton production.Waterlogging results in reduced soil oxygen, impairing the growth and development of this valuable crop and often resulting in severe yield loss or crop failure.However, as cotton has an indeterminate growth habit, it is able to adapt to waterlogging stress by activating three mechanisms: the escape, quiescence, and self-regulating compensation mechanisms.The escape mechanism includes accelerated growth, formation of adventitious roots, and production of aerenchyma.The quiescence mechanism involves reduced biomass accumulation and energy dissipation via physiological, biochemical, and molecular events.The self-regulation compensation mechanism allows plants to exploit their indeterminate growth habit and compensatory growth ability by accelerating growth and development following relief from waterlogging stress.We review how the growth and development of cotton is impaired by waterlogging, focusing on the three strategies associated with tolerance and adaptation to the stress.We discuss agronomic measures and prospects for mitigating the adverse effects of waterlogging stress.
1.Introduction
As a result of change in climate,accelerated land degradation,and inadequate drainage systems, flooding or waterlogging incidents have become increasingly frequent and unpredictable worldwide in recent years [1].Generally, flooding stress includes submergence stress,in which the plant is completely under water, and waterlogging stress, in which the plant leaf and stem are partially submerged[2].Under submergence,the plant experiences poor environmental conditions,such as the limitation of gas exchange, low light intensity, increased susceptibility to fungal diseases, and impairment of nutrient uptake from the soil [2,3].Waterlogging stress frequently occurs in response to external environmental factors such as heavy rainfall, excessive irrigation, storms, and overflow of rivers.It leads to the depletion of oxygen, a major source of injury because the rate of oxygen diffusion in waterlogged soil is 10,000 times lower than that in well-drained soil [4].Waterlogging-caused damage is due mainly to oxygen deficiency, hypoxia, or anoxia.Hypoxia occurs in soil when the oxygen content is reduced to below the optimal level,whereas anoxia usually refers to complete oxygen deprivation[4,5].Anoxia is always preceded by hypoxia in waterlogged environments [6,7].For major crops including cotton,waterlogging is more common than submergence.
Cotton (Gossypium hirsutum L.), a valuable economic crop,possesses an indeterminate growth habit and is often affected by waterlogging.Waterlogging stress impairs cotton plant growth and development as well as nutrient uptake [8-10],ultimately resulting in yield reduction [11-13].However, the plant is able to make certain physiological and molecular adjustments to adapt to water oversaturation.The influence or extent of the damage to plant growth and development,as well as on the yield and fiber quality of cotton,is determined by the interplay between waterlogging severity and the ability of the cotton to adapt to waterlogging.In this paper, the morphological, physiological, and molecular biological responses of cotton to waterlogging stress are systematically reviewed, with special focus on adaptation mechanisms.Measures for mitigating waterlogging stress in cotton are also discussed.
2.The effect of waterlogging on cotton
Hypoxic stress, caused by waterlogging, changes the energy metabolism of cotton plants from aerobic to anaerobic respiration, which is detrimental to root growth, especially gravitropic growth, and renders cotton plants more prone to lodging.It also leads to an imbalance between carbohydrate production and consumption, and even plant death when coupled with an accumulation of toxic metabolites under anaerobic respiration.The lack of energy supply hinders plant growth and development, in turn affecting lint yield and quality[11,13].
2.1.Photosynthesis
Decreased photosynthesis is a common physiological response of cotton to waterlogging stress and is also the cause of cotton yield reduction [14].Chlorophyll (Chl) content,activity of ribulose-1,5-bisphosphate carboxylase/oxygenase(Rubisco), and net photosynthetic rate (Pn) of waterlogged cotton plant leaves were significantly decreased, leading to premature senescence and severe yield loss [15-17].Waterlogging sensitivity has been strongly associated with photosynthetic inhibition in cotton [18].A significant drop in the photosynthetic rate was observed under sustained waterlogging for 72 h[19].It was showed that the Chl content in cotton leaves was reduced by 8.9% after waterlogging for 3 days and by 11.5% and 17.2% under submersion for 3 and 12 days at the squaring stage,respectively[16].Dong et al.[20]and Zhang et al.[15]also reported that the leaf Pnand Chl content of waterlogged cotton were greatly reduced after waterlogging for 10-20 days.Luo et al.[21]showed that flooding reduced the stability of the leaf thylakoids, possibly accounting for a significant decline in Pnunder waterlogging.Yordanova et al.[22]and Bradford [23]similarly attributed decreased photosynthesis under waterlogging to stomatal closure and decreased leaf Chl concentration.This conclusion was supported by Meyer et al.[24]and Luo et al.[21]who found that stomatal conductance,transpiration rate,and leaf water potential were also decreased by waterlogging stress.The activity of Rubisco in the leaves of cotton seedlings decreased significantly after 8 days of waterlogging [17].Although Meyer et al.[24]and Christianson et al.[25]suggested that waterlogging reduced stomatal conductance in cotton, others did not report any reductions [26-28].Thus,the photosynthesis decrease under waterlogging in cotton could not be attributed to changes in stomatal conductance or transpiration rate.The effects of transpiration and stomatal conductance may depend on different factors.
It can thus be concluded that waterlogging stress leads to decreases in chlorophyll content, leaf water potential, and Rubisco activity, ultimately reducing photosynthetic capacity and accelerating leaf senescence and abscission.
2.2.Carbon and nitrogen metabolism in cotton
Waterlogging stress can disrupt the carbon (C) and nitrogen(N)metabolism balance of cotton plants,leading to changes in soluble sugar and protein content.Guo et al.[29]reported that the content of soluble protein and soluble sugar in cotton leaves decreased significantly after waterlogging for 8 days at the flowering and boll stages.The reduction was associated with a decreased rate of leaf photosynthesis and carbohydrate synthesis as well as a subsequent reduction in protein synthesis.Song et al.[30]found that 10 days of waterlogging decreased soluble protein and Bt protein content by 10.3%and 14.1%, respectively; however, it increased soluble sugar content by 8.5%.It is thus evident that waterlogging stress not only inhibits soluble protein synthesis,but also stimulates soluble sugar synthesis, and as a result destroys the C and N metabolism balance.It should be noted that the altered C and N metabolisms under waterlogging are not only a result of stress, but also adaptation mechanisms of cotton to stress,which will be discussed in a later section.
2.3.Cotton cell membrane system
When plants are subjected to abiotic stress such as chilling[11], mineral deficiencies [31], drought [32], or waterlogging[24], active oxygen generation is increased, usually leading to oxidative damage[16].Reactive oxygen species(ROS),such as superoxide anion radical (O2−), hydroxyl radical (OH-), and hydrogen peroxide (H2O2), are produced in plants under normal or non-stressed conditions, but at very low concentrations.However, ROS concentrations under abiotic stress are elevated to a quantity that is harmful to many cellular metabolic reactions, including photosynthesis and photosystem (PS) II efficiency [26].ROS-scavenging enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione(GTH), can also be produced after short-term waterlogging stress,and thus some ROS can be scavenged.However,under continuous or severe waterlogging stress, their structure is destroyed,resulting in increased lipid peroxidation of the cell membrane.Guo et al.[33]reported that malondialdehyde(MDA) content in cotton roots increased by 12.8%-93.1% after 8 days of waterlogging at the flowering and boll-setting stages.Since MDA is a major indicator of cell membrane damage,excessive MDA accumulation in cotton leaves indicates that the cell membrane has been seriously damaged.Such damage will further lead to a series of negative physiological and biochemical events, including reduced photosynthesis.All relevant studies [34,35]have reported that waterlogging results in the production of ROS and the accumulation of MDA.It can be concluded that cell membrane damage is an indicator of waterlogging stress, with which yield- and quality-based photosynthesis, transpiration, and nutrition are closely associated.
2.4.Plant nutrition
Waterlogging inhibits the uptake of most essential nutrients in the soil and thus leads to deficiencies in N,phosphorus(P),potassium (K), magnesium (Mg),and calcium(Ca) [36].Milroy et al.[9]reported that almost all mineral nutrients in fully expanded young leaves were decreased after 5 days of waterlogging at 65(flowering stage)and 112 days after sowing(DAS) (boll setting stage).The effects of waterlogging on N, P,and K nutrition were greater at the early development stage than at a later growth stage.N content in cotton leaves was decreased by 30% after waterlogging at 65 DAS, but less affected at 112 DAS[9].By 112 DAS(at the late stage of cotton growth), there were adequate reserves in the vegetative tissue, so that the plant was less dependent on nutrient absorption.Ashraf et al.[26]reported that waterlogging stress reduced the concentrations of N, K, and Ca2+in the roots,stems, and leaves of cotton, but increased those of Mn2+and Fe2+in those organs and of Mg2+in the roots.The reason for this nutrient imbalance may be the decrease of oxygen supply in the root zone,resulting in the reduction of root respiration and in turn the ability to absorb nutrients.In addition, under waterlogging conditions,the energy stored in roots is reduced,inhibiting the active transport of these nutrients to other organs [26].Under hypoxia, the decrease of root respiration may also lead to the death of root cells, decrease of cell permeability, and, under long-term hypoxia, even complete death of roots.Thus, it is evident that waterlogging inhibits the uptake of most major nutrients, leading to an imbalance in nutrition, although the increased accumulation of some essential nutrients has also been observed.Thus, nutrition imbalance is a contributor to yield loss under waterlogging.
2.5.Yield and yield components
Reduced plant growth is a common response to waterlogging,typically resulting in lower lint productivity [11,25,37].Numerous studies[11,18,25,35,37]have shown that waterlogging stress has a marked inhibitory effect on dry matter accumulation and yield formation (Table 1).Yield loss increases with waterlogging duration [20,38].Hodgson [38]proposed that waterlogging of 4 to 32 h reduced lint yield by 300 kg ha−1or 18.3%, while Song et al.[30]observed that yield decreased by respectively 23.5% and 26.5% after 8 and 10 days of waterlogging at flowering and boll stages.Zhang et al.[20]reported that lint yield was reduced by 52.7%, 58.9%, and 62.5% after 10, 15, and 20 days of waterlogging at squaring;27.2%,37.2%,and 54.5%after 10,15,and 20 days waterlogging at flowering; and 12.1%, 15.1%, and 24.0% after 10, 15, and 20 days waterlogging at the boll-setting stage.However,Meyer et al.[24]suggested that a single 7-day waterlogging event at 67 DAS did not reduce yield.Bange et al.[11]found that cotton yield did not vary with 72-h waterlogging treatments imposed at 47, 74, 87, 101, and 137 DAS compared with a nonwaterlogged control.They also reported a highly significant interaction between waterlogging treatment and ridge height,suggesting that cotton responses to waterlogging varied with the stress severity or management practices such as earthingup.
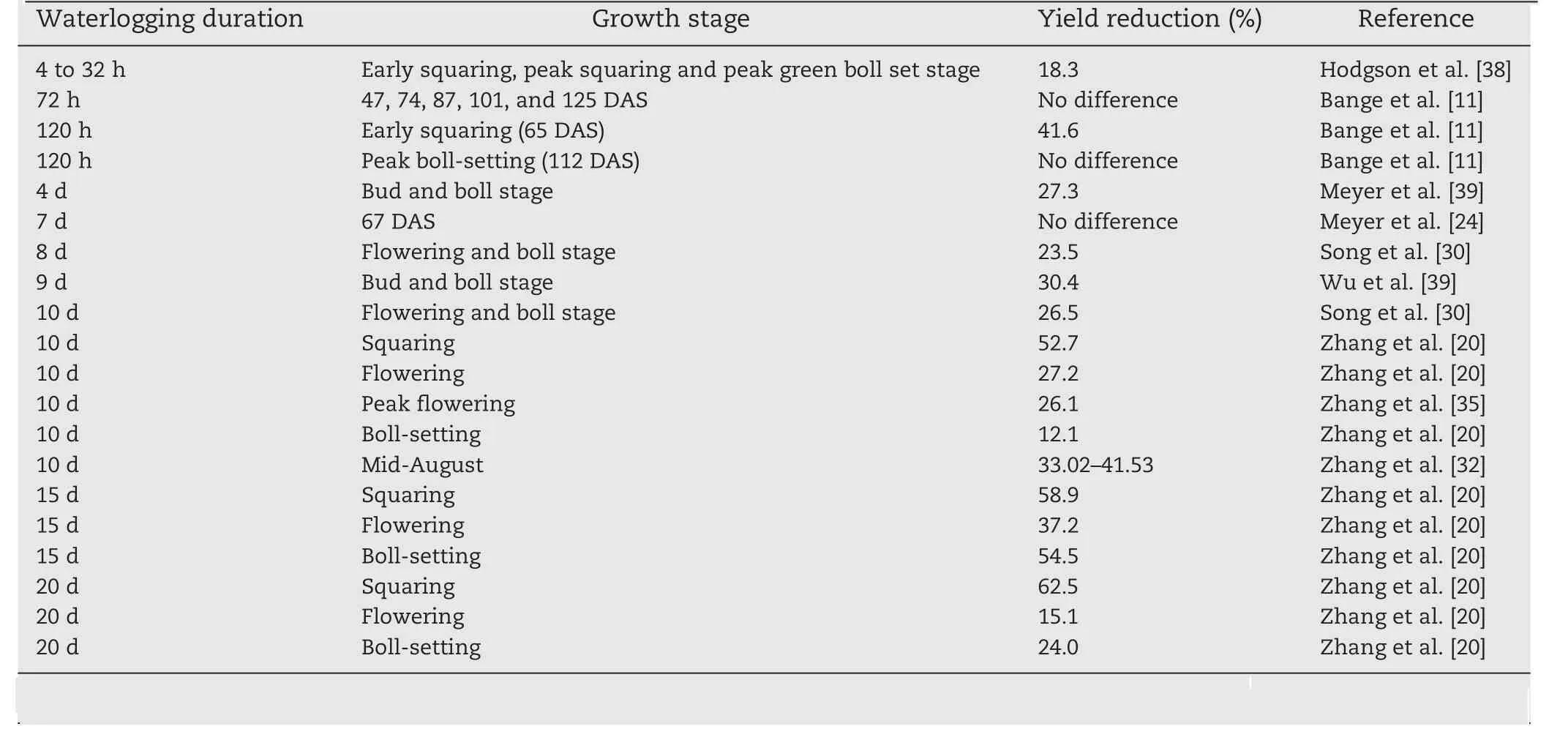
Table 1–Cotton yield reduction related to growth stage or duration of waterlogging stress.
The effect of waterlogging on yield is also determined by cotton plant age or the stage of growth and development.Zhang et al.[20]suggested that cotton plants at early stages were more sensitive to waterlogging than those at late stages, as waterlogging at squaring caused a greater yield loss than at the flowering or boll-setting stages.They also reported that lint yield decreased by respectively 52.7%, 27.2%, and 12.1% after 10-day waterlogging at squaring, flowering, and boll-setting.Such different sensitivities to waterlogging were also reported by Bange et al.[11].Wu et al.[39]found that seed cotton yield decreased significantly after waterlogging for 4 and 9 days during the squaring and boll stage.In an open-field experiment,Bange et al.[11]found that yield was significantly lower when waterlogging occurred at early squaring (65 DAS) than at peak boll-setting (112 DAS).These studies consistently showed that cotton yield reductions caused by early waterlogging were greater than those caused by late waterlogging stress, suggesting that younger plants are more sensitive to waterlogging than older plants.
Cotton lint yield is dependent on boll density (number of bolls per unit area), boll weight, and lint percentage [40].In some reports [11,31,32,38], cotton yield reduction under waterlogging is attributed to the reduced number of bolls rather than to reduced boll weight or lint percentage [11].However, Li et al.[41]found that yield reduction under low oxygen was due mainly to decreased boll weight.Zhang et al.[35]attributed the decline in lint yield caused by waterlogging to both decreased boll weight and boll density.An increased shedding rate under waterlogging contributed greatly to the reduction in boll number [11].No reports have cited lint percentage as a cause of yield reduction under hypoxia or anoxia.The differences of yield components and their contribution to yield decrease may be due to the changes of waterlogging duration and the growth stage at which the stress was imposed [11,19,20].The differential response of yield components to waterlogging may also be associated with the indeterminate growth habit of cotton, which allows the plant to adapt to waterlogging and to reduce yield loss.
3.Cotton adaptability to waterlogging
When subjected to waterlogging stress, cotton plants quickly perceive and gradually adapt to the stress using morphological,physiological,and molecular strategies to reduce damage or yield loss.Our assessment of the existing research on cotton and other crops has led us to propose that cotton adapts to waterlogging using three primary mechanisms:escape, quiescence adaptation, and self-regulation and compensation (Fig.1).The escape strategy includes accelerated elongation growth, formation of adventitious roots, and production of aerenchyma.The quiescence strategy involves reduced biomass accumulation and energy dissipation through a series of physiological, biochemical, and molecular strategies,and the self-regulation and compensation strategy involves accelerated growth and development after relief of the stress via compensatory and indeterminate growth.These strategies are discussed in detail below.
3.1.Escape strategy
Plants trigger the escape strategy when subjected to short-term waterlogging stress by increasing adventitious root growth,producing aerenchyma, and accelerating stem elongation.Root growth and function is determined by root morphological and anatomical traits in anoxic waterlogged soils [42].Many plant species produce adventitious roots[43],with some emerging into the soil and others growing along the soil surface.Aerenchyma provides an internal path for low resistance gas-phase diffusion of oxygen into and along roots.Stem elongation of crops under waterlogging stress can allow part of the plant to stay above the water surface, helping it to reach oxygen.Although there is limited research on the escape strategy of cotton under waterlogging conditions, this strategy is seen in field-grown or pot-grown cotton (unpublished data).It appears to be a mechanism by which cotton adapts to waterlogging stress.
3.1.1.Adventitious root formation under waterlogging
Plants develop adventitious roots typically under waterlogging conditions.Since adventitious roots are close to the water surface, connected to the stem, and close to the aerated tissue,they are more likely to acquire oxygen than the primary roots.Adventitious roots can temporarily replace the submerged primary roots to respire aerobically and generate energy for the stressed plants [44].Their formation is closely associated with ethylene signal transduction or production [45].The stability of ethylene-responsive transcription factors(ERFs)is dependent on oxygen availability,which is closely associated with waterlogging stress[25].They promote the formation of adventitious roots by inducing the expression of related genes.Zhang et al.[35]and Christianson et al.[25]reported that the expression level of ERFs in roots and leaves of cotton are differentially regulated under waterlogging,suggesting that ERF plays an important role in the waterlogging tolerance of cotton[35].This activity has also been reported in many other plant species,including soybean(Glycine max), maize (Zea mays L.) [46], rice (Oryza sativa L.) [47], barley(Hordeum vulgare L.) [22], and tomato (Solanum lycopersicum) [48].The formation of adventitious roots in tomato under waterlogging stress was regulated by the expression of a key ETH synthesis gene,acetyl CoA synthetase(ACS),as well as the interaction between ETH and auxin [48].It was observed in a microarray study that a key ETH synthesis gene, ACC oxidase(ACO)was up-regulated in waterlogged cotton roots[25],suggesting that ethylene may act as a key signal mediating response to waterlogging in cotton.Our unpublished data from a greenhouse experiment showed that potted cotton plants subjected to partial submergence for 7 days at seedling stage produced adventitious roots at the base of the main stem.Thus, the formation of adventitious roots is a mechanism by which cotton can adapt to waterlogging stress.
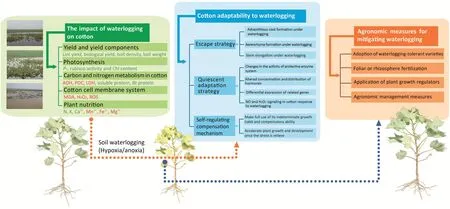
Fig.1– Changes in the response of cotton to waterlogging stress and agronomic measures for mitigating waterlogging.The green box describes the impact of waterlogging on cotton.The metabolites printed in red are typically up-regulated and increased in abundance under waterlogging stress.The words in green indicate metabolites with reduced abundance.The blue box describes cotton adaptability to waterlogging.The orange box describes agronomic measures for mitigating waterlogging.
3.1.2.Aerenchyma formation under waterlogging
Aerenchyma is composed of continuous gas-filled channels,which provide a low-resistance internal pathway for the movement of O2from aerobic shoots to anaerobic roots to permit aerobic respiration under waterlogging stress.Many crop plants can form aerenchyma in their roots to adapt to hypoxic stress caused by waterlogging [49].The formation may be associated with increased ETH concentration after waterlogging, increased cellulose activity, and subsequent cell division or programmed cell death.Expansins [50,51]and xyloglucan endotransglucosylase/hydrolases (XTHs) [52-54]are two protein families implicated in cell elongation.They can alter the shape and size of cells and affect cell regeneration and differentiation.As cell-wall loosening proteins, XTHs play important roles in waterlogging-induced cell elongation in maize(Zea mays L.)and Arabidopsis [46,53].It has been reported by Thirunavukkarasu et al.[46]that XTH13,XTH32,XTH8,XTH9,and XTH23 are involved in the formation of aerenchyma in maize under waterlogging stress.A large amount of ETH was produced in maize roots under waterlogging stress,promoting the formation of larger cavities in the cells and greater tissue gaps and finally resulting in the formation of aerenchyma.Unlike in maize or Arabidopsis, no visible formation of aeration tissue from submerged cotton has been observed, however, several related genes have been reported to be up-regulated.Zhang et al.[35]found that the expression of GhXTH1 and GhXTH3 was significantly reduced in the leaves of waterlogged cotton plants relative to that in nonwaterlogged plants, whereas their expression was up-regulated in the roots of waterlogged plants.Similar expression patterns of GhEXPAs in cotton have also been observed under waterlogging stress [35].Najeeb et al.[13]reported that cotton can improve survival under hypoxia by developing hypertrophic lenticels at the base of the stems under long-term waterlogging,allowing gas exchange between internal tissues and the atmosphere.We accordingly suggest that although there is no conspicuous aerenchyma forming,waterlogging regulates the expression of a set of GhXTH and GhEXPA genes involved in the formation of aerenchyma in cotton that are necessary for cell wall loosening and subsequent cell expansion in roots.
3.1.3.Stem elongation under waterlogging
Upon complete submergence, some plant species exhibit accelerated elongation rates of the petioles, stems, or leaves[47,55].Stem elongation is accelerated by waterlogging stress and allows part of the plant to remain above the water surface at all times.Oxygen in the air is transported to the roots to maintain the growth of the root system as well as the growth and development of the entire plant.Numerous studies have reported elongated growth in rice after flooding.Hattori et al.[47]reported that under waterlogging stress, two genes SNORKEL1 (SK1) and SNORKEL2 (SK2) interact with ERFs in the ETH signal transduction pathway, thus triggering stem elongation in which gibberellin (GA) participates.However,how the SK gene directly or indirectly participates in GA accumulation or GA signal transduction remains unclear.The two hypoxia-responsive genes in Arabidopsis are HYPOXIA RESPONSIVE ERF1 and 2 (HRE1 and HRE2), both of which are regulated by ETH and promote plant elongation and growth under hypoxic conditions.Several plants including rice and Arabidopsis have been reported to rely on this strategy.Although no reports of cotton stem elongation under waterlogging or submergence exist, differential expression of the above-mentioned stem elongation-associated genes has been found in cotton [56].This finding suggests that stem elongation may constitute an escape mechanism for waterlogging, but it is unlikely to be a major strategy in cotton,because the accelerated elongation may also result in plant death if energy reserves are depleted before relief of the stress.
3.2.Quiescent adaptation strategy
The quiescent adaptation strategy differs from escape adaptation.It minimizes energy consumption by retarding plant growth and development via a series of metabolic changes under waterlogging(Fig.2).
3.2.1.Changes in the activity of protective enzyme systems
Generally, waterlogging increases the activity of the antioxidant enzyme system in crops to reduce stress damage[15,57].The SOD activity of cotton leaves first increased and then decreased under waterlogging stress from 10 to 20 days [20].By contrast, POD activity in cotton leaves increased significantly after waterlogging for 12 days [15].The increased SOD and POD activities could remove a large amount of ROS and reduce membrane damage of cotton under waterlogging.
3.2.2.Altered concentration and distribution of hormones
Waterlogging altered the synthesis,metabolism,and transport of endogenous hormones, which resulted in the accumulation of ETH, auxin [indole-3-acetic acid (IAA)], and ABA, as well as inhibited synthesis of cytokinin(CTK)and GA in the root cells of mangroves[58].He et al.[59]further confirmed that waterlogging inhibited the synthesis of IAA, GA, and CTK, and increased the synthesis of ABA in ginkgo(Ginkgo biloba L.)leaves.Accumulation of ABA led to decreased stomatal conductance, which maintained the balance of leaf water potential.In a waterlogging pot experiment, the ABA content in cotton leaves increased, while contents of IAA,ZR,and GA as well as their ratios(GA/ABA,IAA/ABA,and zeatin riboside/ABA),decreased substantially[34].
ETH is considered one of the most important signaling molecules in plants under hypoxia stress.Waterlogging stress increases ETH concentration, which further stimulates shoot elongation in wetland plants and possibly plays a key role in the interactions among hormones such as ABA and GA[60-63].ACC oxidase 1 (ACO1), a gene involved in ETH synthesis, was up-regulated in the leaves of waterlogged cotton [56].Nine of the 13 differentially expressed ERF TFs were also up-regulated, suggesting that ETH plays an essential role in the response of cotton to waterlogging.
GA increased waterlogging tolerance in rice and Rumex palustris under waterlogging stress [64,65].It promotes internode elongation and thus causes rice leaves to emerge from the water surface and continue aerobic respiration[62,66-68].GA is sensed by its nuclear receptors GA INSENSITIVE DWARF1s (GID1s), which then trigger the degradation of its downstream repressors DELLAs.DELLAs regulate GA signal transduction by integrating multiple hormone signaling pathways via interactions with transcription factors or regulatory proteins of different families [69].The expression of GA biosynthesis genes and three GID1s was up-regulated in waterlogged cotton plants.Thus, GA plays an active role in maintaining cotton growth under waterlogging stress.
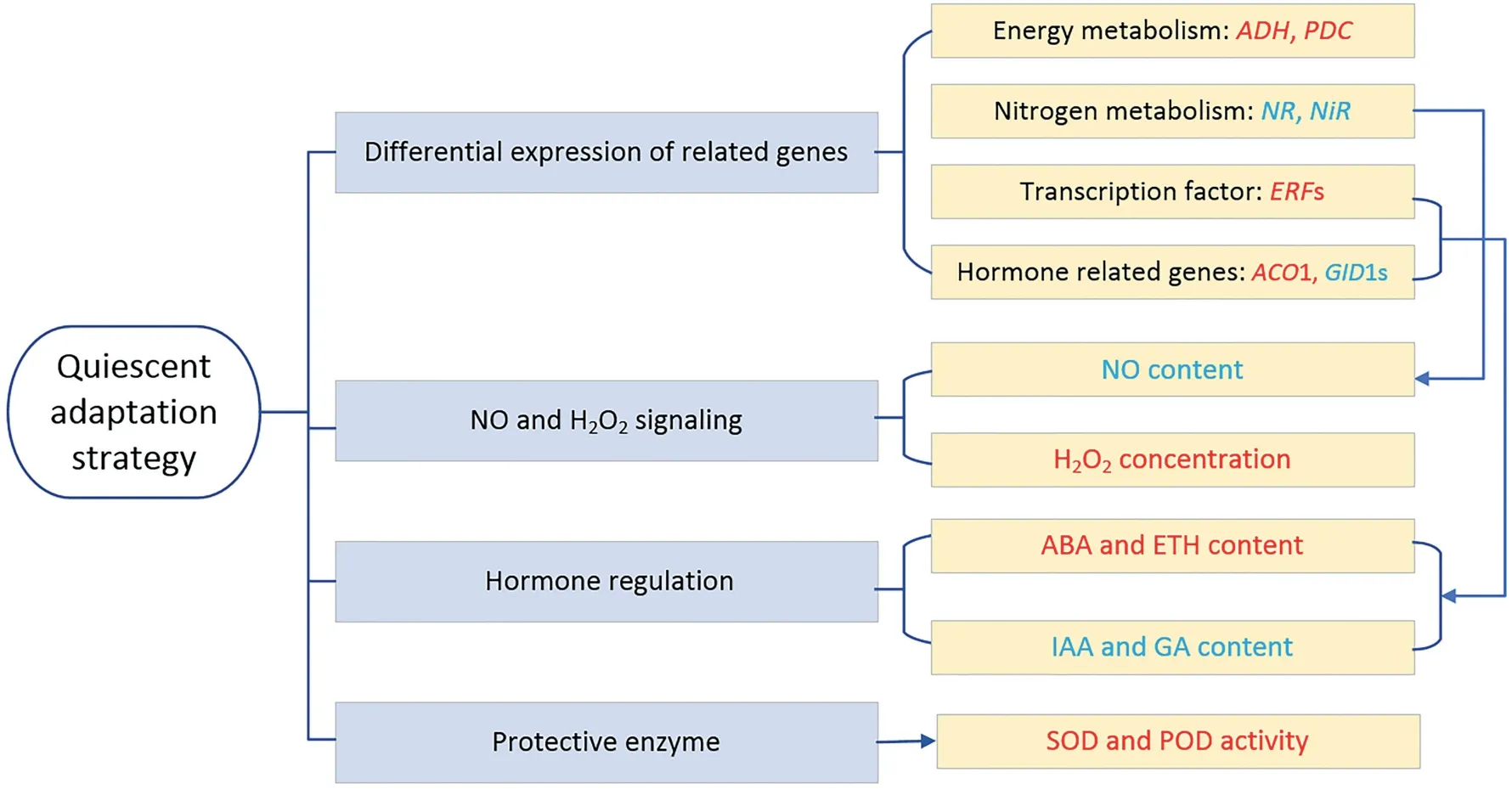
Fig.2–Quiescent adaptation strategy of cotton in response to waterlogging stress.The metabolites printed in red are typically up-regulated or increased in abundance.The words in blue indicate metabolites with reduced abundance.
IAA is also involved in regulation of plant growth and development under waterlogging[70].Zhang et al.found that several auxin-related genes were down-regulated in cotton leaves under waterlogging, and changes in the transcript levels of auxin-associated genes were consistent with changes in the IAA content in main-stem leaves [20].The plant hormones jasmonic acid (JA), salicylic acid (SA) and brassinosteroid(BR)are also regulators of plant growth.Zhang et al.[56]further suggested that JA,SA,and BR were involved in waterlogging response, given that many differentially expressed genes associated with JA, SA, and BR were upregulated in stressed cotton plants.Nguyen et al.[71]speculated that these three hormones were participating in a signal cascade network to help plants adapt to abiotic stress.
Waterlogging stress thus causes substantial changes in endogenous hormone metabolism in cotton tissues.There may also be complex interactions among endogenous hormones in regulating plant growth and development in response to waterlogging stress.We accordingly suggest that changes in concentration of endogenous hormones and their interactions play important roles in the quiescent strategy of cotton adaptation to waterlogging stress.
3.2.3.Differential expression of related genes
Although the quiescent adaptation strategy based on differential gene expression was established for the first time in rice[67,72,73], it also plays an important role in the waterlogging adaptation of many other crops, including cotton.It is believed that waterlogging stress alters the expression of certain genes in cotton.The expression of genes associated with glycolysis,fermentation, and mitochondrial electron transport chains was up-regulated,while that of genes involved in cell wall synthesis,carbohydrate metabolism,nitrogen metabolism,photosynthesis,production of secondary metabolites, and amino acid synthesis in the aspartic acid and serine-glycine-cysteine pathways was decreased [25,56].Genes with potential roles in ETH synthesis and perception and transcriptional regulation were also markedly up-regulated in cotton under waterlogging stress[63].
3.2.3.1.Expression of genes involved in energy metabolism.
Waterlogging-stressed plants usually transform their metabolic mode from oxidative phosphorylation to anaerobic fermentation to maintain ATP levels by suppressing storage metabolism and mitochondrial respiration [66,74,75].Anaerobic fermentation is activated under low-oxygen stress:the pyruvate dehydrogenase complex(PDC)and alcohol dehydrogenase(ADH)are two major enzymes involved in anaerobic fermentation.First,PDC converts pyruvate to acetaldehyde,and then ADH converts acetaldehyde to ethanol.The induction of ADH during anaerobic processes has been observed in many plant species such as mung bean(Vigna radiata)[76],pigeon pea(Cajanus cajan L.)[77],rice[78],and cotton[20,25].ADH activity significantly increased under anoxia in roots[7], shoots [79,80], coleoptiles [81], and seedlings [82].In a floodtolerant sorghum (Sorghum bicolor L.) cultivar, ADH activity was much higher than that in a sensitive variety[83].A maize mutant deficient in one of its ADH genes was more sensitive to waterlogging injury and died earlier than wild-type plants [84].Transgenic lines overexpressing ADH produced significantly more ethanol than wild-type plants, indicating an increase in ethanol fermentation [85].In leaves of waterlogged cotton, the expression of ADH and PDC was greatly increased [20], in agreement with the increased activity of ADH in waterlogged cotton.Under waterlogging conditions, the expression of many genes involved in the synthesis of sucrose and starch is decreased[56].This finding suggests that ADH and other carbon metabolism-related genes play an important role in maintaining cotton growth under waterlogging stress.
Nitrogen metabolism is a basic physiological process in plant growth and development.Its associated enzymes play an important role in plant resistance to abiotic stress [86].Nitrogen metabolism has been shown to improve adaptation of plant cells to hypoxia stress [66].Low redox potential in waterlogged soils may result in nitrogen deficiency, which is beneficial to denitrification of nitrate (NO3−) [87].Nitrate reductase (NR), glutamine (GS), glutamate synthase (GOGAT),and glutamate dehydrogenase (GDH) are key enzymes in N metabolism whose activities have been used as representative biochemical markers to assess N status.Waterlogging results in decreased N content and decreased rates of N accumulation [88].Li et al.[89]reported that, in waterlogged cotton, soluble protein content and NR, GOT, GPT, GS, and GOGAT activities decreased and that free amino acid content,peptidase, and protease activities increased.Zhang et al.[56]further showed that the expression of N metabolism-related genes was increased and the expression of amino acid biosynthesis-related genes was decreased in waterlogged cotton.This result suggests that waterlogging reduces the activity of key N metabolism enzymes in cotton plants,resulting in the inhibition of normal N metabolism.
3.2.3.2.Transcription factor response to waterlogging.Many transcription factors (TFs) are differentially regulated under waterlogging stress and involved in plant response to this stress.HRE-and RAP-type ERF genes played important roles in regulating plant responses to hypoxia and anoxia [90-94].The N-end rule pathway of protein degradation has been shown[90,91,93]to act as a homeostatic sensor of hypoxia in Arabidopsis through the regulation of critical hypoxia-responsive TFs.Licausi et al.[95]identified two Arabidopsis hypoxia-inducible ERF genes(HRE1 and HRE2)which could induce the expression of anaerobic genes and ethanol fermentation to improve plant resistance to anaerobic stress.RAP2.2 in Arabidopsis and SUB1A in rice play important roles in plant survival under low oxygen conditions and can be induced by ERF [67,91].Waterlogging stress up-regulated the expression of nine plant ERFs [56].The increased expression of ERF may improve the tolerance of plants to waterlogging stress by inducing the expression of waterlogging-responsive genes, as mentioned above [56].Further investigation of TFs induced by waterlogging could reveal more functional candidate genes involved in plant adaptation to waterlogging stress.
3.2.4.NO and H2O2signaling in the cotton response to waterlogging
Besides the plant hormones believed to be involved in signaling under waterlogging, nitric oxide (NO) and hydrogen peroxide(H2O2)are also thought to play key signaling roles in the response of cotton to waterlogging.NO is an essential endogenous signaling molecule that is involved in multiple physiological processes in plants [96], including acting as a secondary messenger in environmental stress signal transduction [97,98].NO increased the photosynthetic pigments and net photosynthesis rate of Chinese cabbage (Brassica chinensis L.)leaves[99]and facilitated the anaerobic survival of plants by coupling to anaerobic ATP production and NAD(P)+regeneration,thus constituting an alternative to fermentation[100,101].Chen and Song [45,102]showed that the foliar supply of NO derived from sodium nitroprusside (SNP)alleviated the damage to the euhalophyte Suaeda salsa and cotton under waterlogging stress.Plant survival after long periods of low oxygen was achieved when NO−3was continuously supplied, owing to the synthesis of NO from NO−3via NR and NiR [100,103,104].In cotton, the expression of NR and NiR was decreased under waterlogging stress [63], indicating that NO has an important signaling role in the response of cotton to waterlogging.
The increase of cellular ROS caused by abiotic stress impairs metabolic function [105,106].At high concentrations,H2O2is a strong oxidizing agent that injures cells and reduces photosynthesis when produced internally or applied externally [105,107,108], but at low levels, it acts as a stress signal[107,109-111].H2O2can act as an intermediate signal upstream of both ETH and salicylic acid during plant stress response or can serve as a secondary messenger in signal transduction pathways, leading to stress acclimation [107].Andrade et al.[112]found that pretreatment of seeds with H2O2promoted the tolerance of soybean seedlings to waterlogging.The available information suggests that H2O2directly regulates the expression of numerous genes in plants,including cotton,some of which are involved in plant defense and hypersensitive response [113], as well as in antioxidants and signaling proteins such as kinase, phosphatase, and TFs[63,114].Thus, H2O2signaling is of potential significance to any mechanism aimed at improving cotton tolerance to waterlogging stress.
3.3.Self-regulating compensation mechanism
Cotton has an indeterminate growth habit and a strong compensation ability.When an organ or tissue is damaged, a new one is formed.For example, when the apical bud of the cotton plant is injured, the axillary buds are activated.This recovery mechanism following the loss of the apical bud involves the release of apical dominance and the activation of axillary buds [115].Similarly, columnar sheath cells start to differentiate and form new roots when the root is injured,demonstrating strong regeneration ability [116].Cotton also shows high spatiotemporal compensation capacity in growth and fruiting.If the growth of the cotton plant is blocked at an early growth stage, accelerated growth will occur in the later stages.There may also be an increase in the photosynthetic rate and assimilates of the undamaged leaves in a damaged cotton plant,such as following hailstorm damage at squaring.It has been confirmed that the response of young cotton plants to waterlogging stress is different from that of old cotton plants because of different storage resources,morphological changes and recovery time.Different stress intensity requires different coping strategies, which also depends on the development stage.This leads to different yield potentials of cotton plants at different developmental stages.Thus,yield potential, as affected by reproductive damage, recovery time,and growing conditions, explains the extensive yield responses of crops suffering developmental damage [115,116].The indeterminate growth habit and compensatory ability of cotton also play an important role in adaptation to waterlogging.The growth and development of cotton are inhibited under waterlogging stress, leading to increased square and boll abscission.However, plant growth and development will be substantially accelerated once the stress is eliminated [56,117].Zhang et al.[56]reported that the recovery rate of the dry matter weight of cotton plants stressed at squaring, flowering, and boll-setting stages was increased by 28.6%, 70.9%, and 29.0%, respectively, at 10 days after waterlogging relief,compared with the non-waterlogged control.The recovery rate of leaf area was also increased by 43.3%, 46.8%, and 65.5%, respectively.It has also been noted that the extent of compensation depends largely on the stress severity and cotton growth conditions.The recovery growth rate becomes lower after longer periods of waterlogging.Under waterlogging for less than 6 days, the morphological and yield parameters recovered faster and more completely than at a waterlogging duration of more than 6 days[118].Ten days after waterlogging stress at the flowering stage, the leaf area had returned to normal growth levels and the growth rate was higher than that of the control plants,indicating that cotton can resume growth after short-term waterlogging stress.Physiological activity as measured by photosynthetic gas exchange parameters and antioxidant enzyme activities recovered earlier and more rapidly than the morphological responses like formation of aerenchyma and adventitious roots [42].Waterlogged cotton will thus utilize its indeterminate growth habit and compensatory ability to accelerate plant growth and development once the stress is relieved in order to compensate for losses due to waterlogging stress.However,when the duration of waterlogging stress is too long(e.g.>20 days,as reported by Zhang et al.[20])at an early age(at seedling or squaring),stressed cotton will not recover from waterlogging damage.
4.Agronomic measures for mitigating waterlogging
Understanding the mechanisms of cotton adaptation to waterlogging is necessary for the planning of strategies and technical measures to mitigate waterlogging damage.Measures should be taken to improve waterlogging tolerance of cotton as well as exploit its compensation ability to reduce yield losses(Table 2).
4.1.Adoption of waterlogging-tolerant cultivars
Although there are few reports on the selection of waterloggingtolerant crop cultivars, genotypic differences in tolerance to waterlogging have been observed in several crops, including cotton[119].Key variables such as Soil Plant Analytical Development (SPAD) readings, leaf nitrogen and potassium concentrations, and leaf Pnof poorly waterlogging-tolerant cultivars decreased under stress conditions [119].Thus, it is feasible to identify or develop cotton cultivars with better waterlogging tolerance.However,given the limited efficiency of conventional screening and breeding methods, modern molecular tools may prove more useful.Some genes have been reported to be involved in plant tolerance to waterlogging stress[85].The overexpression of ACC deaminase activity in transgenic tomato plants reduced ETH production by 70% and improved waterlogging tolerance[120].Liu et al.noted that the overexpression of RAP2.6 L promoted stomatal closure by participating in the ABA signal transduction pathway,thus delaying premature senescence in of Arabidopsis under waterlogging stress [121].Some differentially expressed genes were also identified in cotton under waterlogging stress [25,56].Ellis et al.[85]transformed cotton with constructs for the overexpression of ADH and PDC,but found no increase in waterlogging tolerance in cotton.This result suggested that the expression of a single gene was insufficient for the plants to resist hypoxia stress.Further research should investigate the relationship between anaerobic fermentation and anaerobic viability in plant roots.
4.2.Foliar and soil fertilization
Foliar or rhizosphere fertilization following waterlogging has long been considered an efficient measure for reducing yield loss in field crops,including cotton[88,122].Zhou et al.[88]reported that foliar spraying with N fertilizer alleviated waterlogging damage in winter rape (Brassica napus L.) by retarding chlorophyll degradation and improving photosynthesis.In maize,nitrogen fertilizer application after waterlogging can increase photosynthetic efficiency by increasing photosynthetic pigment content and leaf area index, alleviating photo-damage to PSII, and reducing the degree of photosynthetic damage under waterlogging stress at the seedling stage[123].
Guo et al.[33]further showed that different application rates of nitrogen had different effects on the waterlogging resistance of cotton.Appropriate N application (240 kg N ha−1) increased the activity of antioxidant enzymes and reduced lipid peroxidation,thus improving the waterlogging resistance of cotton;by contrast,excessive N supply(480 kg N ha−1)aggravated lipid peroxidation by accelerating the accumulation of O2−in cotton roots, thus reducing waterlogging resistance.Thus, the N fertilizer application rate should be carefully considered in order to reduce stress damage and promote the recovery of cotton growth after the relief of waterlogging stress.Ashraf et al.[26]found that K supplementation increased plant growth and increased photosynthetic pigments and photosynthetic capacity in waterlogged cotton.It also improved nutrient uptake and the accumulation of K+, Ca2+, N, Mn2+, and Fe2+in stressed cotton.Similar effects of NPK were found in rapeseed (Brassica napus L.) [124], with the length of the raceme, number of seeds per pod, and 1000-seed weight increasing in waterlogging-stressed plants.Wu et al.[39]reported that timely fertilization after waterlogging increased cotton yield relative to a fertilizer-free control,but the yield was still lower than that of the non-stressed plant.Timely fertilization soon after waterlogging might partially alleviate damage from waterlogging.
4.3.Application of plant growth regulators
The timely application of plant growth regulators is also a promising practice for mitigating waterlogging stress through regulating plant growth and development.Song reported increased cotton growth after waterlogging with an appropriate dose of SNP (an NO donor) [102].Jiang et al.[125]found that cotton plants treated with the plant growth regulators brassin(0.02 mg L−1)and diethyl aminoethyl hexanoate(DA-6)(10 mg L−1)in addition to foliar fertilization(1%urea and 0.5%potassium chloride) recovered better from waterlogging than the non-treated control by improving physiological performance through increasing SOD activity, which reduced lipid peroxidation of the cell membrane in cotton plants.It thus appears that a combination of fertilizer and growth regulators could be a promising approach for alleviating waterlogging stress.

Table 2–Main measures for alleviating waterlogging damage in cotton.
ETH production in the roots or shoots is a common response to waterlogging, and reducing ETH production could constitute a mechanism for reducing waterlogging stress.Anti-ETH chemicals,such as aminoethoxyvinylglycine(AVG), aminoethoxy acetic acid (AOA),1-methylcyclopropene(1-MCP), and cobalt (Co2+), have been found to be effective in limiting ETH biosynthesis[126]or ETH activity[127]in plants.Najeeb et al.[18]reported that AVG application increased total fruit number and retention by blocking ETH biosynthesis in cotton tissues under waterlogging.Similar positive effects of AVG[128]on cotton growth and yield have also been reported under waterlogging or non-waterlogging conditions.
The growth regulator mepiquat chloride (MC) is used worldwide to restrict vegetative growth and promote reproductive growth in cotton.MC application increases leaf thickness,reduces leaf area [129], shortens internodes, improves root system growth, and reduces plant height [130], resulting in a more compact plant architecture[131]with a well-developed root system[132].Well-developed roots improve resistance to lodging,thereby reducing waterlogging damage.Thus, foliar spraying with MC is a promising measure for controlling cotton lodging and overgrowth.
4.4.Agronomic management measures
Timely and appropriate field management is also desirable for reducing yield loss under waterlogging, since the extent of damage to cotton is largely dependent on the duration of the stress.Dredging channels and ditches ensures that main ditches,branch ditches,and chamber ditches are unimpeded,thus facilitating drainage.Rapid water drainage is the most effective way to alleviate waterlogging damage.
Lodging affects ventilation, light transmission,and photosynthesis,ultimately leading to yield reduction.Lodged plants should thus be straightened to restore the roots to their original position as soon as the water recedes but the soil is wet and loose [47].In the recovery period following waterlogging, cotton plants usually show excessive growth and reduced fruiting.Pruning by removing vegetative branches and growth terminals of the stem is desirable.However, plant straightening and pruning after waterlogging cannot yet be done mechanically.It is thus not practical for countries with a limited labor force, though can still be adopted in some developing countries with large labor forces.
5.Summary
Waterlogging is one of the most severe stresses limiting cotton production worldwide.Reduced soil oxygen impairs plant growth and development, resulting in severe yield loss or even crop failure.
Cotton has evolved adaptation and protection mechanisms that allow it to respond to waterlogging stress,including escape,quiescence, and self-regulating compensation mechanisms.Waterlogging stress can be well mitigated if the adaptive mechanisms are well understood.Given these three mechanisms,several measures that can improve the response of cotton to waterlogging stress, including the adoption of waterloggingtolerant cotton cultivars, foliar or rhizosphere fertilization, and the application of plant growth regulators and other agronomic management measures.However,the effects of these measures remain limited.We propose that future research should focus on the areas detailed below.
The mitigation of waterlogging tolerance in plants may still be hindered by an inadequate understanding of the molecular basis.However,the use of transgenic plants may reveal the physiological role of stress-related pathways and their contribution to waterlogging tolerance.cDNA libraries of stressed root and shoot may contain novel genes associated with low-oxygen response.Waterlogging stress can alter the expression of numerous genes in cotton.Research on the molecular basis will advance conventional and transgenic breeding approaches to increase cotton waterlogging tolerance.
Plant growth regulators,such as SNP,AVG,and 1-MCP,can improve plant tolerance to waterlogging stress, but the effect is very limited and the dosage is difficult to control, possibly resulting in toxicity.Optimizing or developing formulas or mixtures of various plant growth regulators might offer a solution.Combinations of plant growth regulators with fertilizers and nutrients also offer promise.
In combination,selecting resistant cultivars,improving agronomic measures, and applying suitable fertilization and chemicals can increase the adaptation of cotton plants and reduce damage caused by waterlogging stress.Given that improvements in waterlogging tolerance could be made by exploiting genotype×management×environment interactions,we suggest that bioengineering approaches in conjunction with appropriate crop management practices could be highly effective for improving waterlogging tolerance in cotton.
CRediT authorship contribution statement
Yanjun Zhang wrote the manuscript; Hezhong Dong and Cundong Li designed and structures the paper; Guangya Liu provided relative data.All authors read and approved the manuscript.
Declaration of competing interest
Authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (2018YFD1000907), National Natural Science Foundation of China (31771718, 31801307),Natural Science Foundation of Shandong Province(ZR2018BC033), and Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences(CXGC2016B05 and CXGC2018E06).
- The Crop Journal的其它文章
- Brief Guide for Authors
- Short Communication Seed-specific overexpression of cotton GhDGAT1 gene leads to increased oil accumulation in cottonseed
- Dominant early heading without yield drag in a sister-line BC breeding progeny DEH_229 is controlled by multiple genetic factors with maineffect loci
- Genome-wide alternative splicing variation and its potential contribution to maize immature-ear heterosis
- Identification of microRNAs involved in crosstalk between nitrogen,phosphorus and potassiumunder multiple nutrient deficiency in sorghum
- RNA interference targeting ω-secalin genesdifferentially affects the processing quality in a wheat T1BL·1RS translocation line

