Genome-wide alternative splicing variation and its potential contribution to maize immature-ear heterosis
Xiaojiao Hu, Hongwu Wang, Kun Li, Xiaogang Liu, Zhifang Liu, Yujin Wu, Shuqiang Li, Changling Huang
National Engineer Laboratory of Crop Molecular Breeding, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, China
Keywords:
ABSTRACT Heterosis is a well-known phenomenon widely applied in agriculture.Recent studies have suggested that differential gene and protein expression between hybrids and their parents play important roles in heterosis.Alternative splicing (AS) is an essential posttranscriptional mechanism that can greatly affect the transcriptome and proteome diversity in plants.However, genome-wide AS divergence in hybrids compared to their parents and its potential contribution to heterosis have not been comprehensively investigated.We report the direct profiling of the AS landscape using RNA sequencing data from immature ears of the maize hybrid ZD808 and its parents NG5 and CL11.Our results revealed a large number of significant differential AS (DAS) events in ZD808 relative to its parents, which can be further classified into parental-dominant and novel DAS patterns.Parental-dominant, especially NG5-dominant, events were prevalent in the hybrid,accounting for 42%of all analyzed DAS events.Functional enrichment analysis revealed that the NG5-dominant AS events were involved mainly in regulating the expression of genes associated with carbon/nitrogen metabolism and cell division processes and contributed greatly to maize ear heterosis.Among ZD808, CL11, and NG5, 32.5% of DAS contained or lacked binding sites of at least one annotated maize microRNA (miRNA) and may be involved in miRNA-mediated posttranscriptional regulation.Cis regulation was the predominant contributor to AS variation and participates in many important biological processes associated with immature ear development.This study provides a comprehensive view of genome-wide alternative splicing variation in a maize hybrid.
1.Introduction
Alternative splicing(AS)is a widespread mechanism in eukaryotes for creating multiple isoforms from a single gene by the regulated selection of splice sites during mRNA processing.In humans,approximately 98% of multiexonic genes are alternatively spliced[1].Recent studies [2–5]have revealed that more than 40%, 50%and 60% of intron-containing genes undergo AS in rice (Oryza sativa), maize (Zea mays), andArabidopsis thaliana, respectively.Alternative splicing can dramatically diversify the transcriptome and the proteome by producing transcript isoforms that can be translated into different proteins or by generating transcripts harboring premature termination codons (PTCs) that are recognized by nonsense-mediated decay(NMD)[6,7].AS events can be classified into five basic types: skipped exons (SEs), retained introns(RIs), mutually exclusive exons (MXEs), alternative 5′splice sites(A5SSs), and alternative 3′splice sites (A3SSs).The most common form of AS in animals is thought to be SEs, while RIs are the least common.In contrast, the most abundant AS events observed in plants are suggested to be RIs [8,9].Alternative splicing is performed by the spliceosome via complex networks involvingcisregulatory elements andtrans-acting factors [7,10].Thecisregulatory elements, which include the 5′splice site, the branchpoint, and the 3′splice site as well as exonic/intronic splicing enhancers or silencers, interact directly with components of the spliceosome,resulting in alternative transcripts[11,12].Thetransacting regulatory proteins, namely serine/arginine-rich (SR) proteins and heterogeneous nuclear ribonucleoproteins, function in AS, as general activators and repressors respectively, by recognizing splicing enhancers and silencers [13,14].As a major posttranscriptional regulatory event, alternative splicing is involved in most plant biological processes, including growth, development,circadian clock control, and biotic and abiotic stress responses[7,15].InArabidopsis,FLOWERING LOCUS M(FLM) belongs to the MADS-box proteins and is subject to temperature-dependent AS.Two splice variants, FLM-β and FLM-δ, play different roles in the control of flowering by ambient temperature [16].The splice variants of the rice transcription factorOsDREB2Bare differentially expressed in response to heat and drought stresses [17].A similar mechanism of AS has been described forZmDREB2Ain maize [18].AS of maizeZmrbohBgenerates two transcript isoforms, which carry a PTC that probably leads to NMD in response to several abiotic stresses, including cold, heat and salinity [19].
The years passed, and I finished college and took a job in another town. Once, while visiting my parents, I used the phone in their bedroom, and noticed that the pickle jar was gone. It had served its purpose and had been removed. A lump rose in my throat as I stared at the spot beside the dresser where the jar had always stood. My dad was a man of few words, and never lectured me on the values of determination, perseverance12, and faith. The pickle jar had taught me all these virtues13 far more eloquently14 than the most flowery of words could have done.
Hybridization is an important process in the evolution of plants that can lead to the emergence of transgressive phenotypes and heterosis [20].In interspecific hybrids, the combination of divergent genomes often induces immediate and extensive remodeling of gene expression levels and patterns of small RNA expression as well as epigenetic modifications.These phenomena are collectively termed transcriptome shock, which has been reported as a major cause of phenotype novelty [21].As a post-transcriptional regulation process modulating gene expression, alternative splicing has also been suggested to be a component of transcriptome shock and contribute greatly to hybrid vigor [22,23].Using reverse transcription PCR (RT-PCR), the AS patterns of 40 genes were investigated [23]in an interspecificPopulushybrid and its parents.Novel splice forms of SR genes were present in the hybrids and introduced a considerable impact on genetic novelty in them.InBrassica napus, parallel losses of many AS events after allopolyploidization were detected in two independently resynthesized lines [22].Using RNA sequencing (RNA-seq), an increased level of AS was detected in all vegetative tissues except fruit in tetraploid watermelon (Citrullus lanatus) throughout the growth period [24].
Maize is an example of one of the most successful uses of heterosis in crops.Genome-wide transcriptome and proteome profiling studies have revealed that changes in gene and protein expression among hybrids and their parents may contribute greatly to maize hybrid vigor[25–27].Although AS occurs in more than 50% of intron-containing genes in maize and is a major contributor to transcriptome and proteome diversity, the potential role of AS in maize heterosis has not been comprehensively studied.Genome-wide profiling of alternative splicing variations between maize hybrids and their parents will thus advance our understanding of how heterosis occurs.
The maize cultivar ZD808 is an excellent, high-yielding hybrid with strong heterosis in ear architectural traits.High midparent heterosis and best-parent heterosis have been detected in ZD808 immature ears at the spikelet and floret differentiation stages,which are stages that were strongly associated with maize grain yield[28].In that study,we investigated the transcriptome profiles of immature ears of the hybrid ZD808 and its parents CL11 and NG5, finding thatcis-regulated additive expression played a foundational role in maize ear heterosis.The object of the present study, using the same RNA-seq data, was to investigate systemically the contribution of AS to maize ear heterosis and identify the underlying regulatory mechanism.
They turned to the east and fell to bowing till their bills touched the ground, but, oh horror--the magic word was quite forgotten, and however often the Caliph bowed and however touchingly46 his Vizier cried Mu
2.Materials and methods
2.1.Sample preparation and transcriptome sequencing
The RNA-seq data used for AS analysis were retrieved from the NCBI Sequence Read Archive, accession SRP066518 [28].The sample preparation and transcriptome sequencing methods were as in the previous study.Immature ears of the maize hybrid ZD808 and its parents CL11 and NG5 were collected at the spikelet and floret differentiation stages,which were identified by leaf age index and scanning electron microscopy.Total RNA was extracted from each sample using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and 3 μg of high-quality RNA was used for library preparation.RNAseq libraries were generated using the NEBNext Ultra RNA Library Prep Kit for Illumina (NEB, Beverley, MA, USA) and sequenced on an Illumina HiSeq 2000 platform.After low-quality reads were filtered out, between 43.9 and 63.5 million 100-bp paired-end reads were obtained for each of the replicates and genotypes.All sequencing was performed by Beijing Novogene Bioinformatics Technology Co., Ltd.
Presently the gingerbread boy said, Oh dear! I m quarter gone! 18 And then, Oh, I m half gone! And soon, I m three-quarters gone! And at last, I m all gone! 19 and never spoke1 again
2.2.Read alignment and alternative splicing isoform detection
To increase AS detection power,the RNA-seq data from the spikelet and floret differentiation stages of each genotype were combined into one sample, each of which was represented by two biological replicates.High-quality reads were mapped to the maize B73 RefGen_v4 genome obtained from the Gramene database(ftp://ftp.gramene.org/pub/gramene/release-62/gff3/zea_mays),and splicing junctions (SJs) were identified with TopHat2 2.0.13 software (http://tophat.cbcb.umd.edu/) [29]using the default parameters.Expressed genes or isoforms were identified with Cufflinks 2.2.1 (http://cole-trapnell-lab.github.io/cufflinks/) [30].To reduce false positives, the discovery of novel junctions required at least 10 spanning reads, and any new isoforms were required to represent at least 10% of total gene abundance in at least one genotype.A detailed flowchart of the data merging and mapping process is presented in Fig.S1a.
2.3.Identification of differential alternative splicing (DAS) events and classification of DAS patterns
Replicate multivariate analysis of transcript splicing (rMATS)v4.0.1 [31]was used to identify differential AS events among the hybrid ZD808 and its parents.The TopHat2 mapping results in BAM (Binary Alignment/Map format) format were submitted to rMATS, and annotation of the maize B73 RefGen_v4 genome was used to identify known splicing sites.The exon inclusion level(ψ) values for splicing events were calculated using both the SJ counts and the exon body counts, and Fisher’s exact tests were used to identify significant pairwise differences between ψ values.Finally, instances of the five main AS types, SEs, RIs, A5SSs, A3SSs,and MXEs,that satisfied the criteria|Δψ|>10%and a false discovery rate (FDR) <0.05 were assigned as DAS events.
To characterize DAS patterns in the hybrid ZD808 and its parental lines, pairwise comparisons of the DAS events among CL11,NG5, and ZD808 were performed using the criteria |Δψ| ≥10%and FDR <0.05.The AS events in ZD808 with ψ values not significantly different from one parent and significantly larger (smaller)than the other parent were said to exhibit parental dominance patterns.The AS events in ZD808 with ψ values significantly different from both parents were classified as novel AS events.
Mrs. Conroy watched the bus disappear down the road. She looked around and tried to figure out what part of the city she was in. Suddenly the sun seemed awfully5 bright.
2.4.Gene ontology (GO) enrichment analysis
We systematically investigated the genome-wide AS status of maize ears from the hybrid ZD808 and its parents and identified extensive AS changes among ZD808 and its parents.The NG5-dominant and novel DAS events in the hybrid were involved mainly in regulation of genes involved in protein metabolism,energy metabolism, and cell division processes and contributed greatly to the transgressive phenotype.DAS events may regulate functional genes posttranscriptionally by coupling with miRNAmediated mechanisms.The AS divergence among ZD808 and its parents was regulated predominantly byciseffects.Our findings shed light on AS variation and its potential contribution to maize heterosis.
2.5.Allele-specific alignment and filtering with a mock F1 hybrid
Systematic reference bias may occur when F1reads are aligned to a single reference genome to identify allele-specific events,because reads containing the reference allele are more likely to be mapped than reads with nonreference alleles [32].To address this read-mapping bias and eliminate most of these errors, allelespecific pseudogenomes were constructed.The Burrows-Wheeler Aligner (BWA 0.7.12, Wellcome Trust Sanger Institute, Cambridge,CB10 1SA, UK) program was used to map reads against the maize B73 RefGen_v4 genome.The BAMalignments from each sample were then merged and sorted using SAMtools version 1.4, and duplicates were then identified with Picard tools 1.57.The identification of SNPs was carried out using the SAMtools mpileup command.Unreliable SNPs between CL11 and NG5 were filtered out according to the following criteria: 1) all reads must be uniquely mapped,and the read quality value must be at least 20;2)all reads from one parent produce a base with consensus at the SNP position but different from that of the other parent.Filtered SNPs were converted to the genomic coordinates of the maize B73 RefGen_v4 genome using BCFtools 0.1.19.The‘‘FastaAlternateReferenceMaker” function of GATK 3.7.0 was used to generate a pseudogenome (allele-specific genome) for CL11 and NG5 using the reference genome backbone/coordinates,excluding the SNP variants specified in the VCF file.For the RNAseq samples of parental lines, reads were aligned to the corresponding pseudogenome.For the mixed (mock F1hybrid) and the F1hybrid samples, reads were aligned sequentially to each pseudogenome to identify allele-specific alignments that were mapped uniquely to a single species without mismatches,and only allele-specific reads were retained for further analysis.The workflow of the pseudogenome construction and allele-specific alignment processes is presented in Fig.S1b.
To characterize the divergence in AS among the maize hybrid ZD808 and its parents CL11 and NG5,rMATS was applied for quantification and comparison of splicing differences for the five major types of AS: SEs, RIs, A5SSs, A3SSs, and MXEs.A total, of 3961 (in 2057 genes) significant DAS events were identified between the two parental lines CL11 and NG5.Relatively few DAS events were discovered between ZD808 and its parents, with 2605 (in 1486 genes)between CL11 and ZD808 and 1256(in 818 genes)between NG5 and ZD808 (Table 2).These DAS events included all five AS types.RI accounted for 38.1%–50.2% of all DAS events and was the most abundant AS type in the three comparisons, followed by ES (20.7%–29.1%), A3SS (15.2%–15.9%), A5SS (9.9%–12.3%), and finally MXE events (3.8%–5.2%) (Fig.2; Tables S5–S7).All the AS types of DAS events between NG5 and ZD808 were decreased significantly between the two parents,and between CL11 and ZD808(Table 2).The Venn diagram analysis further identified 5152 nonredundant DAS events, whereas only 186 were detected in all comparisons (Fig.S2).These results indicate wide changes in AS among the maize hybrid ZD808 and its parents.
2.6.Cis- and trans-regulatory effects
Estimation ofcis-andtrans-regulation in splicing followed McManus et al.[11].Comparisons of the allele-specific ψ values in the hybrid were performed using Fisher’s exact test to identifycis-acting differences in splicing.The standard error of the difference between the parents and allelic ψ ratios was calculated and used to derive Z-scores andP-values.Q-values were further calculated, and an FDR cutoff of 0.05 was applied to tests ofcis- andtrans-acting splicing divergence.
2.7.RNA isolation and semi-quantitative RT-PCR validation analysis
Variation in the sequence of AS isoforms can affect transcript stability by the gain or loss of microRNA (miRNA) binding sites.As a family of small, noncoding RNAs, miRNAs negatively regulate gene expression in numerous cellular processes and regulate plant growth and response to environmental stress [43,44].To assess how miRNAs interact with the AS isoforms, DAS event pairs were examined for gain or loss of miRNA targets with the psRNATarget web server (http://plantgrn.noble.org/psRNATarget/), using a miRNA target pairing score cutoff of 3.0.In total,2541(32.5%)DAS pairs among ZD808,CL11,and NG5 were predicted for gain or loss of binding sites of at least one annotated maize miRNA, in which 462 are likely to change miRNA targets that affect only translation,and the remaining 2079 directly affect the cleavage of mRNAs(Table S13).To determine whether the miRNA targets were significantly enriched in the DAS events, Fisher’s exact test was calculated for the predicted miRNA targets, revealing that respectively 59.3% and 50.0% of the miRNA targets were significantly(P<0.05) enriched in DAS events between CL11 and ZD808 and between NG5 and ZD808, and that 31.1% of the miRNA targets were significantly enriched in the DAS events shared by both comparisons (Table S14).These enriched targets in the DAS events were more likely to interact with miRNAs and regulate the gene expression in hybrids.
Oh, no, they said, the conditions are too hard. You must not speak or laugh for six years,29 and must make in that time six shirts for us out of star-flowers.30 If a single word comes out of your mouth, all your labour is vain. And when the brothers had said this the quarter of an hour came to an end, and they flew away out of the window as swans.
3.Results
3.1.Identification of AS events in hybrid ZD808 and its parents
Clean reads from the hybrid ZD808 and its parents CL11 and NG5 were mapped to the maize B73 reference genome(Table S1),and splice junctions(SJs)were identified with TopHat2.The expressed genes or transcripts were identified with Cufflinks software.CL11,NG5,and the hybrid ZD808 contained respectively 322,143, 369,126, and 375,046 SJs.On average, 349,199 (98.2%)junctions had been previously annotated, and the remaining 6239 (1.8%) were novel junctions that had not been annotated.Respectively 21,180, 21,423, and 21,870 genes were expressed with a fragments per kilobase of transcript per million mapped reads (FPKM) value ≥1 in CL11, NG5, and ZD808, and 18,673(86.9%) genes on average were annotated with at least two exons(multiexonic genes) (Table 1).In CL11 and NG5, respectively 98,832 and 100,488 isoforms were identified in 12,919 and 13,215 genes, accounting for 70.4% and 70.9% of the multiexonic genes.In ZD808,102,673 isoforms were identified in 13,417 genes,accounting for 70.5%of the multiexonic genes(Table 1).Thus,both expressed gene numbers and AS frequency were increased in the hybrid relative to its parents.A Venn diagram revealed that 94,456 isoforms in 16,967 genes were shared among the three genotypes (Fig.1; Tables S2–S4).After removal of the same isoforms of different genotypes,105,546 nonredundant isoforms were detected in 20,126 genes, with a mean of 5.2 isoforms per gene.
He reproached her faintly with being the cause of his distress40, and at the same moment a stately lady appeared, and said very gravely: Ah! Beauty, you are only just in time to save his life
3.2.Divergence in AS among the hybrid ZD808 and its parents
To further avoid potential read-mapping biases, adjustment methods similar to those of previous studies [2,33,34]were adopted.A mock F1hybrid was created by mixing equal amounts of RNA-seq reads derived from CL11 and NG5.The ψ values from the parents’ samples were then compared with allele-specific ψ values from the mock F1hybrid using Fisher’s exact test.The FDR-adjustedP-value <0.05 was applied to filter out events with inconsistent ψ values.
3.3.Prevalence of parental dominance AS patterns in the hybrid ZD808
In previous studies [35–37], variation in non-additive gene expression has been suggested as an important factor underlying phenotypic variation and heterosis.To identify the AS patterns in hybrid ZD808, we conducted pairwise comparisons of the 5152 nonredundant DAS events among CL11,NG5,and ZD808.AS events in ZD808 with no significant splicing divergence from one parent but significant differential splicing with the other parent were considered to exhibit parental dominance patterns.The 748 (14.5%)events in 524 genes and 2162 (42%) events in 1281 genes were classified as CL11-dominant and NG5-dominant, respectively.A set of 330 (6.4%) events in 250 genes in the hybrid showed significant splicing divergence from both parents and were classified as novel AS events.The novel events can be further assigned to two classes: 141 events in ZD808 with ψ values significantly higher or lower than in both parents (class I) and 189 events with ψ values between those of the two parents (class II) (Tables 3, S8–S10).
These results show that the parent-dominant AS, especially NG5-dominant,accounted for the vast majority of DAS events,suggesting that ZD808 tends to maintain the AS patterns of superior alleles.The NG5-dominant AS events may influence maize ear heterosis.For the novel AS, class I events can affect gene function by creating new protein isoforms that differ from those of both parents or regulate gene expression in a specific way with transgressive phenotypic consequences.In contrast, class II events may result in a complementary effect on splicing variations among ZD808 and its parents.These widely changed AS patterns in ZD808 may influence the expression and protein production of many functional genes,leading to phenotypic variations between hybrid and its parents.
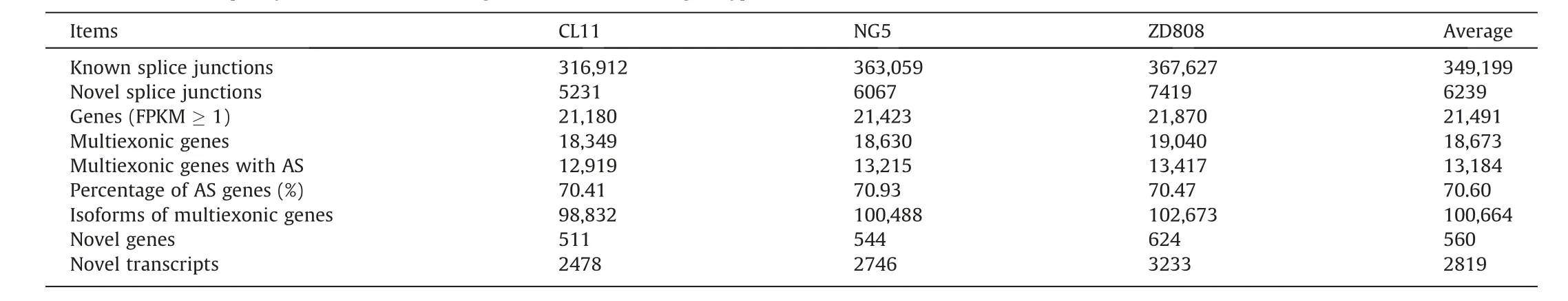
Table 1 Numbers of splice junctions, isoforms and genes with AS in three genotypes.

Fig.1.Venn diagrams of the expressed multiexonic genes (a) and isoforms (b) in ZD808 and its parents CL11 and NG5.
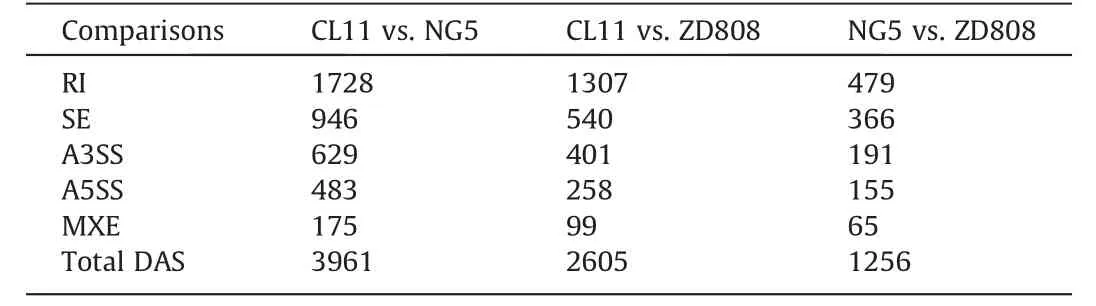
Table 2 Numbers of DAS types discovered among three comparisons.
3.4.Functional enrichment analysis of divergence in alternative splicing

Fig.2.Divergence in AS among the maize hybrid ZD808 and its parents.Five major types of AS(a).SEs,skipped exons;RIs,retained introns;MXEs,mutually exclusive exons; A5SSs, alternative 5′ splice sites; A3SSs, alternative 3′ splice sites.The quantity and percentage of each AS type in DAS events between CL11 and NG5(b),CL11 and ZD808 (c) and NG5 and ZD808 (d).
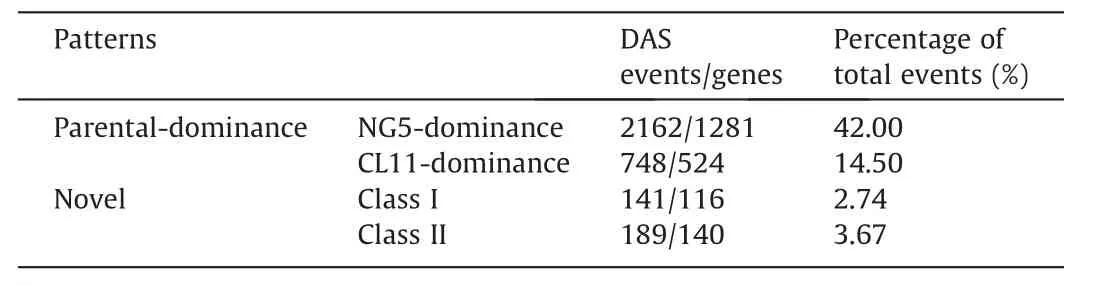
Table 3 Patterns of DAS events in maize hybrid ZD808.
To investigate the molecular and biological functions of genes with different AS patterns,GO enrichment using single enrichment analysis was performed with AgriGO.A total of 1281 genes with NG5-dominant AS patterns were enriched (FDR <0.01, Yekutieli FDR dependency) in 53 GO terms from the biological process(BP), molecular function (MF) and cellular component (CC) categories.In the BP category, the most overrepresented terms were‘‘primary metabolic process” (89.2%), ‘‘nitrogen compound metabolic process”(62.5%),‘‘regulation of cellular process”(55.4%),‘‘cellular component organization” (42.6%), ‘‘reproduction” (19.2%),and ‘‘cell cycle” (11.9%).Under the ‘‘primary metabolic process”term, 551 (43.0%) and 183 (14.3%) genes were significantly enriched in the ‘‘protein metabolic process” and ‘‘carbohydrate metabolism process” subcategories, respectively.In the MF category, ‘‘catalytic activity” (67.4%) and ‘‘nucleotide binding” (24.5%)were the most overrepresented terms (Fig.3a; Table S11).The 748 genes with CL11-dominant AS patterns were significantly enriched in 26 GO terms.The most overrepresented BP terms were‘‘reproduction”(21.2%)and‘‘cell cycle”(12.2%)(Fig.3b;Table S11).For the 250 genes with a novel splicing pattern,15 GO terms were significantly overrepresented, including 4 BP terms and 11 CC terms.In the BP category, ‘‘protein metabolic process” (46.8%),‘‘macromolecule modification” (38%), and ‘‘regulation of gene expression,epigenetic”(7.6%)were the most overrepresented subcategories (Fig.3c; Table S11).
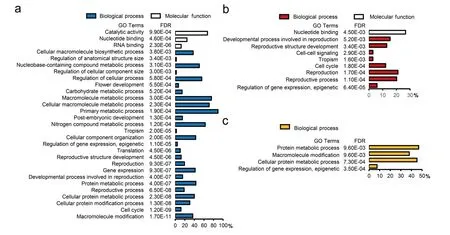
Fig.3.GO enrichment of genes with NG5-dominant (a), CL11-dominant (b) and novel (c) AS patterns in ZD808.The abscissa represents the percentage of genes annotated with that GO term in the input list.
3.5.RT-PCR validation of the DAS events
To functionally validate the AS divergence between ZD808 and its parents, 28 DAS events of 25 genes with the NG5-dominant or the novel AS patterns were chosen for semi-quantitative RT-PCR validation.cDNAs from immature ears of CL11, NG5, and ZD808 at spikelet and floret stages were used as the templates for amplification.RT-PCR experiments confirmed 24 DAS events in 23 genes,accounting for 86%of all tested events(Tables S3–S4,S12).The 10 NG5-dominant events of genes involved in ‘‘carbohydrate metabolism” (e.g.,Zm00001d014150,Zm00001d021119, andZm00001d047253), ‘‘nitrogen compound metabolic” (e.g.,Zm00001d005151andZm00001d013831), and ‘‘cell cycle” (e.g.,Zm00001d052072,Zm00001d028365, andZm00001d017392) were confirmed as having splicing patterns in hybrid ZD808 more like those in NG5 than those in CL11.Fourteen novel events of genes participated in ‘‘protein metabolic process” (e.g.,Zm00001d032889,Zm00001d038805andZm00001d021473) and‘‘macromolecule modification” (e.g.,Zm00001d017392,Zm00001d012295, andZm00001d052248) were verified with splicing patterns in ZD808 differing from those of either parent(Figs.S3–S4; Table S12).
Declaration of competing interest
The AS patterns of some cloned genes controlling maize ear differentiation,includingRA1,TSH4,FEA2,TD1,andIDS1,which affect the determinacy and identity of meristems,andZAG1,ZAG2,ZAG3,andIFA1, which control the floral meristem identities and floral organ specification [41,42]were also investigated.However, onlyIDS1(Zm00001d034629) was differentially spliced between ZD808 and its parents in our RNA-seq data,and this was confirmed by RT-PCR(Fig.4c).The results suggest that these genes are critical in maize ear differentiation and that their splicing patterns are relatively well conserved among genotypes.
3.6.Effect of alternative splicing in potential microRNA targets
Total RNA was isolated from immature ears of CL11, NG5 and ZD808 at the spikelet and floret development stages using a RNA Easy Fast Plant Tissue Kit (DP452, Tiangen).Reverse transcription was performed using the FastKing First Strand cDNA Synthesis kit (KR118, Tiangen) to synthesize cDNA as the template for semi-quantitative RT-PCR.Primers were designed to specifically amplify DAS events via placement in unique exons or spanning unique splice junctions.The primer sequences are listed in Table S12.In the semi-quantitative RT-PCR, cDNA was amplified for 28–33 cycles and the fragments were separated on 1.5% agarose.Tubulin(Zm00001d013367) was used as an internal reference.
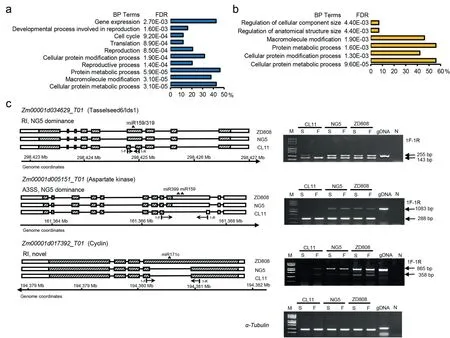
Fig.4.Functional analysis of NG5-dominant and novel AS genes with divergent miRNA targets.GO enrichment of NG5-dominant AS genes (a) and novel AS genes (b) with gain or loss of miRNA target sites among ZD808 and its parents.DAS patterns were validated by semi-quantitative RT-PCR with unique sets of primers(c).Lanes from left to right are Trans2K plus DNA ladder (M), cDNAs of immature ears of CL11, NG5, and ZD808 at spikelet and floret stages, genomic DNA (gDNA) and negative control (N).α-Tubulin (Zm00001d013367) was used as internal control.Arrows indicate length of the PCR amplification products.
Further inspection of the AS patterns of these DAS pairs with divergent miRNA targets revealed that 129 DAS pairs (in 102 genes) showed novel AS patterns in the hybrid and 224 pairs(within 163 genes) and 718 pairs (within 485 genes) showed the CL11- and NG5-dominant AS patterns, respectively (Tables S15–S17).The GO enrichment analysis revealed that 485 genes with the NG5-dominant AS patterns were enriched mainly in the BP terms‘‘protein metabolic process”(46.2%),‘‘macromolecule modification” (37.7%), ‘‘reproduction” (20.6%) and ‘‘cell cycle” (11.8%)(Fig.4a).Genes with novel AS patterns were enriched mainly in the BP terms‘‘protein metabolic process”(55.9%),‘‘macromolecule modification” (46.1%), and ‘‘regulation of anatomical structure size”(6.9%)(Fig.4b).No significant BP terms were overrepresented in genes with the CL11-dominant AS patterns.The divergent miRNA targets in the AS events of these genes may lead to large transcriptional changes among ZD808 and its parents, resulting in a novel phenotype.For example,Zm00001d034629encodes TASSELSEED6/IDS1, which is a member ofthe APETALA2(AP2) gene family of transcription factors functioning in spikelet meristem determinacy [45].The AS events ofAP2 in ZD808 and NG5 were predicted to contain miRNA binding sites for zma-miR159h/i/g-3p and miR319a/b/c/d-3p, whereas AS events of CL11 lost the miRNA binding sites (Fig.4c).Zm00001d005151encodes an aspartate kinase (ASK2) and participates in amino acid synthesis and nitrogen metabolism [46].AS events ofASK2in ZD808 and NG5 were predicted to contain miRNA binding sites for miR159a/b/e/f/g/k-3p and miR399j-5p, whereas the AS events of CL11 lost the miRNA binding sites (Fig.4c).Zm00001d017392encodes a cyclin protein, which has a key role in orderly progression of the events of cell division [47].The AS events ofCYCLINin ZD808 were predicted as more likely to contain miRNA binding sites for miR171c than the parents NG5 and CL11 (Fig.4c).Several SR-rich splicing factors (e.g.,Zm00001d029329,Zm00001d029256), hnRNP (e.g.,Zm00001d049680) and AGO protein (argonaute4a,Zm00001d008249) with the NG5-dominant AS patterns in the hybrid were discovered to have gained or lost miRNA targets.
The king-lion came out to meet him; he took the negro chief s daughter---whose name was also Gul--in lawful174 marriage, and then marched with her and her possessions and her attendants to the Place of Gifts
3.7.The predominant role of cis-regulation on AS divergence
Bothcis-acting andtrans-acting factors have the potential to regulate AS divergence between species.To estimate the relative contributions ofcis- andtrans-acting differences underlying splicing regulation, we compared the AS divergence between the two parents and the allele-specific AS levels in the hybrid ZD808.To eliminate allelic mapping bias of RNA-seq reads, a mock F1hybrid was created by mixing equal amounts of RNA-seq reads derived from the two parental lines.The ψ values of splicing events in both lines estimated based on the separate RNA-seq data was then compared to the allelic ψ values using the allele-specific reads from the mock F1hybrid.The AS events with significant differences between the two ψ values for either allele were filtered out.After filtering,83.7%(2,062)of identified events with both the ψ and the Δψ values of the parental lines correlated well with the allele-specific ψ from the mock F1and were retained for further analysis(Table S18).
If an event showed different AS between the parents but the same allelic AS in the hybrid,AS divergence of the event in parents was considered to have been caused only bytranseffects.An event was considered to have onlyciseffects if the AS divergence in the parents was equal to the allelic AS in the hybrid.Otherwise, if the AS divergence of the event in parents was significantly different from the allelic AS in the hybrid, bothcis-andtrans-effects (cis_-trans) were inferred.Of 2062 retained events, 812 showed divergent regulation between parental lines, with 290, 163, and 120 events identified ascis-only,trans-only andcis_transdivergences,respectively.A total of 612 events showing no significant evidence of AS divergence with either parent or significantcis- ortransregulatory divergence were classified as ‘‘conserved”.The remaining 877 events had an ambiguous AS pattern with no clear biological interpretation(Fig.5a;Table 4).Further analysis of the results revealed thatcis-regulatory divergence was predominant in all the five types of AS (Fig.5b; Table S19).The absolute exon inclusion level of parental divergence resulting from different regulatory categories showed that a vast majority of the AS divergence between parents was regulated byciseffects (Fig.5c).Further GO enrichment analysis was performed for thecis-,trans-andcis_transregulated DAS genes.Thecis-regulated DAS genes were significantly enriched in many important biological processes,including mainly‘‘nitrogen compound metabolic process” (67.1%), ‘‘cellular biosynthetic process” (58.1%), ‘‘reproductive” (24.7%), ‘‘carbohydrate metabolic process” (17.1%), ‘‘response to external stimulus”(18.0%) and ‘‘cell growth” (12.4%) (Fig.5d).For thetransregulated DAS genes, ‘‘cellular component organization” (53.5%)and ‘‘cell cycle” (18.3%) were the most overrepresented BP terms.In contrast, no significant BP terms were enriched for thecis- andtrans-regulated DAS genes (Fig.5e).These results suggest that thecis-regulatory mechanism contributes greatly to the AS divergence among ZD808 and its parents and plays an important role in ear-development heterosis.
At last the young man gave in, and threw the fish back into the sea; and both brothers went supperless to bed, and wondered what fortune the next day would bring
The busy cook was just going to refuse her demand and order her out of the kitchen, but the words died on his lips when he turned and beheld54 the beautiful Hyacinthia, and he answered politely, You have just come in the nick of time, fair maiden25
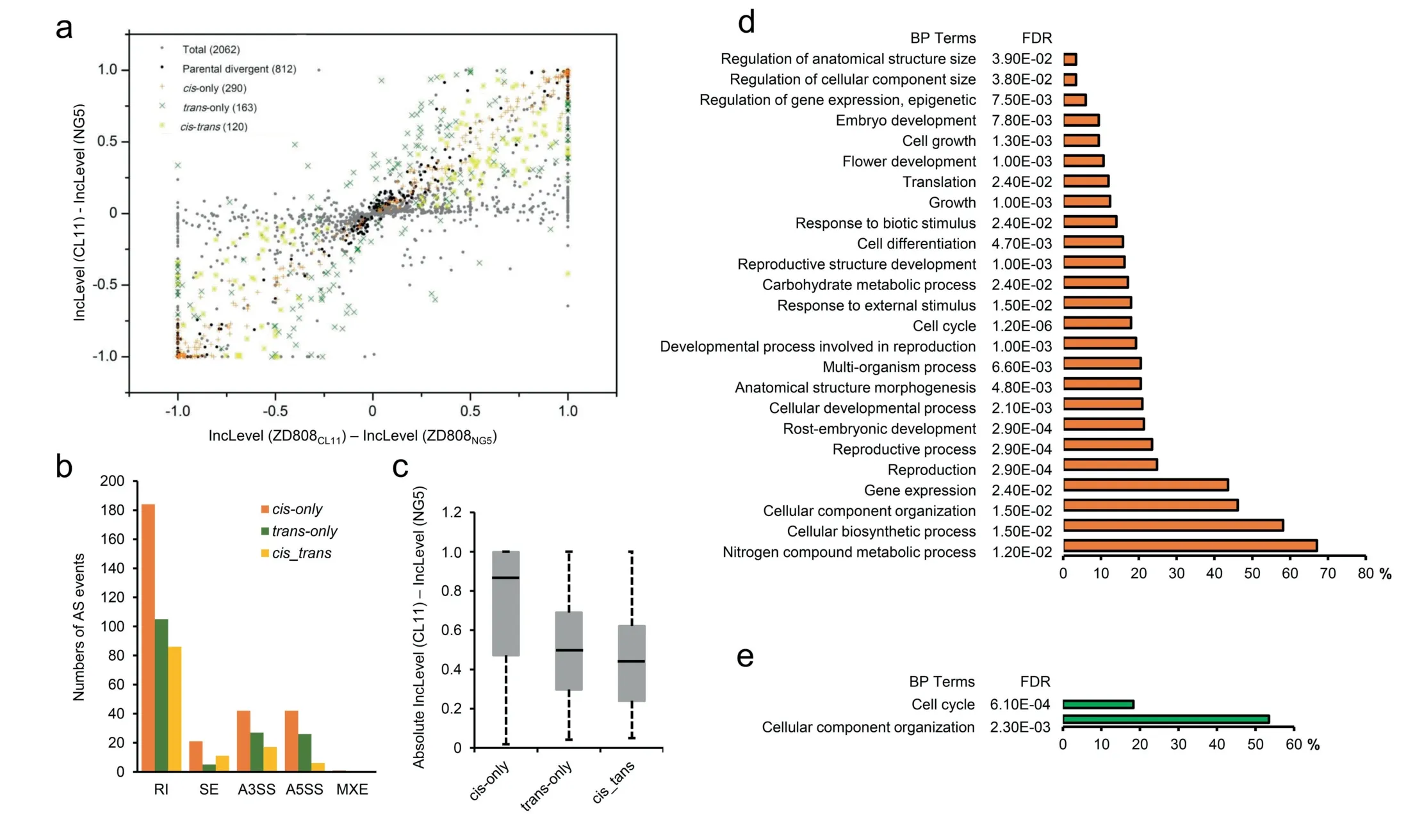
Fig.5. Cis-and trans-regulation of AS in the maize hybrid ZD808.The plot summarizes the relative AS levels in the parents and F1 hybrids.Each symbol represents a single AS event based on the mechanism of regulatory evolution inferred from statistical tests(a).Number of AS events regulated by cis-only,trans-only,and cis_trans effects for the five major AS types(b).Absolute exon inclusion level(IncLevel)divergence of the parents resulting from cis-only,trans-only,and cis_trans effects(c).GO enrichment of genes with AS events regulated by cis-only (d) and trans-only (e).
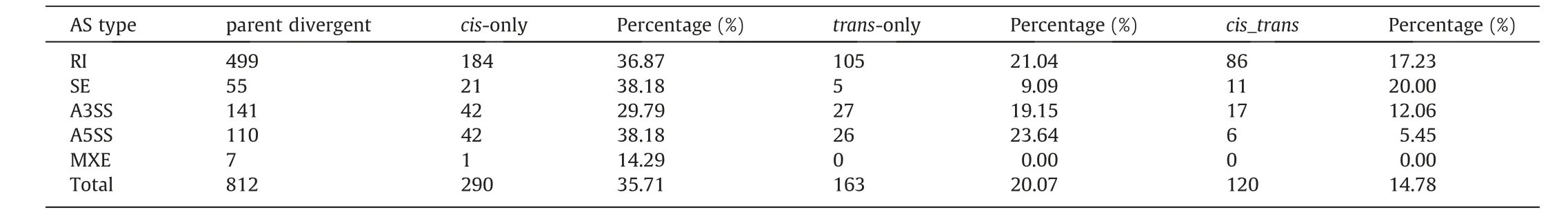
Table 4 The relative contributions of cis- and trans-acting differences underlying splicing regulation.
4.Discussion
To identify patterns of AS in a maize hybrid and their potential contribution to heterosis,we performed a genome-wide investigation of AS in the maize hybrid ZD808 and its parents CL11 and NG5 by RNA-seq.About 70% of intron-containing genes (RPKM ≥1)were alternatively spliced, with a mean of 5.2 isoforms per gene(Table 1).These numbers are slightly higher than those reported from recent studies [5,48]in maize, which found that 50–60% of expressed multiexon genes showed evidence of AS.More isoforms were discovered in ZD808 (102,673)than in its parents (98,832 in CL111 and 100,488 in NG5), reflecting the direct impacts of hybridization on AS.These could be the result of complementation expression of parental isoforms(Tables 1,S2–S4).The increased AS levels in the hybrid may affect gene expression patterns and result in phenotypic variation.
Genome-wide analysis revealed extensive AS changes among ZD808 and its parents.Between CL11 and NG5, 3961 AS events were differently spliced, while 2605 and 1256 were discovered between CL11 and ZD808 and between NG5 and ZD808, respectively (Table 2).A few studies have investigated AS changes after interspecific hybridization and polyploidization.The first study was conducted with an interspecificPopulushybrid and its parents by measuring the expression of 40 genes using RT-PCR.The results suggested that novel AS patterns were present in only a small percentage of genes in hybrids and that new AS variants present in two SR genes led to alterations in the splicing patterns of other genes [23].In the resynthesized allopolyploidBrassica napus, 82 AS events were identified by RT-PCR and sequencing assays, of which 20% showed likely AS change in the polyploid compared to its progenitors[22].RNA-seq analysis of the AS between diploid and tetraploid watermelon revealed that the AS events of 22 genes were determined by ploidy across different tissues [24].Although these studies highlighted the importance of AS in plant evolution,relatively few AS events were investigated, and the studies were limited by the RT-PCR technique used.Our study has provided a comprehensive overview of AS variation in a maize hybrid compared to its parents.All the five AS types (RIs, SEs, A3SSs, A5SSs and MXEs) were included in the DAS events, while RIs was the most prevalent type, followed by SEs (Fig.2; Tables S5–S7).RIs has been reported to be the most frequent type of AS in plants.A recent genome-wide study of AS in maize also revealed that RIs accounted for the majority of AS forms, followed by SEs, alternate acceptors, alternate donors and alternate positions [49].We confirmed 16 RI events in ZD808, CL11 and NG5 using RT-PCR methods.For example, the DAS events ofZm00001d021119(βglucosidase)andZm00001d052072(anaphase-promoting complex)both have an intron retained in hybrid and NG5, leading to amino acid sequence changes in the functional domains(Figs.S3 and S4).The RI type DAS events in hybrids may result in large changes in gene expression or protein function.
The DAS events in the hybrid were further classified into parental-dominant and novel AS patterns.Most(56.5%)DAS events exhibited a parental dominance pattern in the hybrid, suggesting that the hybrid ZD808 tends to maintain the favorable AS patterns of both parents,which may have resulted in conserved expression of superior protein isoforms in the hybrid.Given that the NG5-dominant pattern was predominant and accounted for 74.3% of parental-dominant AS events, NG5 may contribute greatly to conferring beneficial alleles with favorable transcriptome isoforms(Tables 3, S8-S9).For the novel pattern, the class I events were more likely to create new protein isoforms that differ from those of both parents or regulate gene expression by PTC formation followed by NMD (Tables 3 and S10).Four class I events with novel intron retention or exon skipping in hybrid ZD808 were confirmed by semi-quantitative RT-PCR.For instance,retention of intron 2 in the AS events ofZm00001d021473(ATP binding protein) in ZD808 results in a premature stop codon and a truncated protein losing a complete serine-threonine/tyrosine-protein kinase domain, and could be degraded by the NMD pathway.In contrast, for the class II events, 10 were selected and validated with AS levels between the two parents (Fig.S4).This type of events may play finetuning roles in regulating the expression of isoforms from the two parents in hybrids.Thus,as a critical posttranscriptional regulatory mechanism, parent-dominant and novel AS events in hybrids may increase diversity of gene products and affect gene expression, ultimately leading to heterosis.
AS has been well documented [8,15]for its strong influence on plant growth, flowering, reproduction, and stress resistance.Our GO enrichment analysis showed that genes with NG5-dominant AS were overrepresented mainly in ‘‘macromolecule metabolic process”, ‘‘protein metabolic process”, ‘‘nitrogen compound metabolic process”, ‘‘carbohydrate metabolism process”and ‘‘cell cycle” (Fig.3a; Table S11).The hybrid ZD808 showed larger ears than its parents.Rapid cell division and growth may be highly correlated with this superior phenotype.Under the ‘‘cell cycle” term, many genes involved in meiosis were found, including cyclin (Zm00001d028365), anaphase-promoting complex (Zm00001d052072), serine/threonine-protein kinase(Zm00001d008321), and crossover junction endonuclease(Zm00001d042128).Carbon and nitrogen are two essential nutrients affecting plant growth and development.Active and balanced C and N metabolism is required for maize cell division,ear growth, kernel setting, and dry matter accumulation, which strongly affect yield [50–52].Under the ‘‘nitrogen compound metabolic process” term, key enzymes involved in amino acid metabolism and glutamine and asparagine synthesis, including glutamate synthase (Zm00001d043845), glutamine synthetase(Zm00001d026501andZm00001d005157), glutamate dehydrogenase (Zm00001d034420), and aspartokinase (Zm00001d042264andZm00001d013831), were differentially alternatively spliced in the hybrid with the NG5 dominance pattern.Under ‘‘carbohydrate metabolism process”, genes involved in the citric acid(TCA) cycle, glycolysis, and starch and sucrose synthesis, such as citrate synthase (Zm00001d053685andZm00001d053684),beta-glucosidase (Zm00001d033650,Zm00001d021119, andZm00001d008236), pyrophosphate-fructose 6-phosphate 1-phosphotransferase (Zm00001d017830), starch synthase(Zm00001d051976,Zm00001d014150, andZm00001d027242) and sucrose synthase (Zm00001d047253), exhibited the NG5-dominant AS pattern in the hybrid.Given that the NG5-dominant AS accounts for the majority of the DAS events in the hybrid, we considered NG5 to provide more superior AS isoforms associated with C and N partitioning during immature ear development compared to CL11.It has been suggested [53]that hybrids achieve greater cellular energy efficiency and more rapid cell division by selectively regulating transcription and translation of their more stable, beneficial alleles.We speculate that NG5-dominant AS events play important roles in coordinating the expression levels of protein metabolism, energy metabolism and cell division genes and contribute greatly to maize ear heterosis.Novel AS genes were enriched mainly in ‘‘protein metabolic process” (e.g.,Zm00001d032889,Zm00001d038805andZm00001d021473) (Fig.3b; Table S11).The protein metabolic process has been reported to be inversely correlated with heterosis.Inbred crops are considered to be less efficient at growth than hybrids because of higher energy loss in the metabolism of specific unstable proteins.It has been suggested [53]that hybrids have more allelic choices to optimize the allelespecific gene expression to conserve energy and promote growth.A decrease in the expression of protein metabolism genes in the hybrid relative to the inbred parental lines has been observed in many transcriptome and proteome studies [54,55].AS can regulate gene expression posttranscriptionally by coupling with NMD (AS-NMD) and miRNA-mediated mechanisms[7].We speculate that the novel AS patterns in ZD808 negatively regulate the expression of protein metabolic genes through NMD or miRNA strategies to reduce energy dissipation and promote the cell proliferation and ear growth.
On the next evening Tsarevitch Vasilii went into the garden to watch, and he, too, fell asleep at midnight, and next morning when his father summoned him, he, like his brother, being ashamed to tell the truth, answered: Gracious Sir, I watched throughout the night but the Fire Bird that steals the golden apples did not enter thy garden.
MicroRNAs are the key regulators of plant development.Studies[7,56]have suggested that AS can modulate miRNA-mediated regulation of gene expression by generating mRNA isoforms that either contain or lack target sites for miRNA.In our study,changing the AS patterns between hybrid ZD808 and its parents resulted in 2541(32.5%)DAS pairs with a gain or loss of miRNA targets.Statistical analysis showed that more than 50% of the predicted miRNA targets were significantly (P< 0.05) enriched in DAS events between hybrid ZD808 and its parents (Table S14).Of these DAS pairs, 129, 718 and 224 exhibited novel, NG5-dominant and CL11-dominant AS patterns, respectively (Tables S15–S17).The novel and NG5-dominant AS events with a gain or loss of miRNA binding sites in hybrids were enriched mainly in ‘‘protein metabolic” and ‘‘cellular protein metabolic process” (Fig.4a and b).These results further support our hypothesis that AS suppresses protein metabolic genes posttranscriptionally by coupling with miRNA-mediated mechanisms.
Changes in AS, one of the major driving forces that shape phenotypic novelty, could arise from divergence incis-regulatory elements and/ortrans-acting factors.Several studies have investigated the relative contributions of the two factors to AS regulation.InDrosophila, splicing regulatory networks were determined by comparing allele-specific splicing in F1interspecific hybrids,revealing that RIs,A3SSs,and A5SSs were attributable primarily to changes incis-regulatory elements, whereas SE differences were driven by bothcis- andtrans-regulatory divergence[11].In another study in mice[33],RNA-seq showed thatcis-regulatory changes contributed predominantly to all AS types and played important roles in the evolution of AS.A recent study inArabidopsiss [34]suggested that AS divergence caused bycisvariation in genomic sequences may account for difference in responses to environmental stimuli in different accessions.In maize, population-level transcriptome assembly and a genomewide association study [5]were conducted to identify splicing quantitative trait loci (sQTL) in developing maize kernels.The results suggested thatcis-sQTL explained more splicing variation thantrans-sQTL and that natural variation in AS and overall mRNA level was independently regulated with differentcis-sequences preferentially used.In our study, a higher frequency (35.7%) ofcis-regulatory (than that oftrans-regulatory) differences was discovered, and thecis-regulatory variants contributed predominantly to all the five AS types (Fig.5; Table S19).Thecisregulated DAS genes were also found to be significantly enriched in many biological processes associated with ear development,including carbon/nitrogen metabolic process, cell growth and response to stimulus (Fig.5d and e).The results suggest thatcisregulatory divergence results solely from sequence variants in pre-mRNA sequences, could directly affect canonical splicing sites or exonic/intronic regulatory elements,and may strongly influence heterosis in maize immature ears.
5.Conclusions
GO enrichment was evaluated by single-enrichment analysis in AgriGO (http://systemsbiology.cau.edu.cn/agriGOv2/index.php).The analysis was performed using the plant GO slim classification with Fisher’s exact test, Yekutieli multiple test adjustment, and a 0.05 significance level.A set of 39,324 genes from the maize RefGen_v4 genome were used as the background set.
For example, β-glucosidase (Zm00001d021119) is implicated in the hydrolyze cytokinin-conjugate and releases free cytokinin during plant growth and development [38].RT-PCR revealed that the intron 6 ofZm00001d021119was retained in ZD808 and NG5,resulting in amino acid insertion in the glycoside hydrolase family domain and possibly affecting protein activity (Fig.S3).Zm00001d032889encodes an F-box containing protein that may regulate diverse cellular processes, including hormonal signal transduction, floral development, secondary metabolism, and responses to stresses [39].The exon 4 ofZm00001d032889was skipped in ZD808.The novel AS form creates a premature stop codon, resulting in a truncated protein with 140 amino acids in ZD808, which would be a target for nonsense-mediated decay(Fig.S4).Zm00001d052072encodes a subunit of the anaphasepromoting complex, which governs cell cycle progression and influences organ size [40].Three DAS events ofZm00001d052072were detected among ZD808 and its parents, including one NG5-dominant event and two novel events.Analysis of RT-PCR confirmed that the intron 18 ofZm00001d052072was more likely retained in both hybrid ZD808 and NG5 compared to that in CL11.This AS event may change the anaphase-promoting complex subunit 4 domain in ZD808 and NG5,and result in different protein function form CL11.As for the other two novel events ofZm00001d052072, the RT-PCR analysis showed a complementary expression change in ratios of AS forms in ZD808 compared to its parents (Figs.S3 and S4).
Authors declare that there are no conflicts of interest.
CRediT authorship contribution statement
Changling Huang, Xiaojiao Hu, and Hongwu Wang conceived the project.Xiaogang Liu and Yujin Wu conducted the field experiments.Xiaojiao Hu and Hongwu Wang performed the laboratory work and data analysis.Xiaojiao Hu wrote the manuscript.Changling Huang, Zhifang Liu, and Kun Li revised the manuscript.Shuqiang Li conducted the additional RT-PCR verification experiments.All authors read and approved the final manuscript.
Acknowledgments
We thank Dr.Junjie Fu, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences (CAAS) for valuable comments and suggestions that improved the manuscript.This project was supported by the National Key Research and Development Program of China (2016YFD0101002 and 2017YFD0101201), the National Natural Science Foundation of China (31500984), the Agricultural Science and Technology Innovation Program of CAAS, and the National Engineering Laboratory of Crop Molecular Breeding.
Availability of data and materials
The data sets supporting the results of this article are included within the article and its supplementary files.The Illumina sequences were deposited in the NCBI Sequence Read Archive as accession SRP066518 (http://www.ncbi.nlm.nih.gov/sra/SRP066518).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2020.09.003.
I like to oblige, said the old man. Then customers come back, later in life, when they are better off, and want more expensive things. Here you are. You will find it very effective.
- The Crop Journal的其它文章
- Brief Guide for Authors
- Short Communication Seed-specific overexpression of cotton GhDGAT1 gene leads to increased oil accumulation in cottonseed
- Waterlogging stress in cotton:Damage,adaptability,alleviation strategies,and mechanisms
- Dominant early heading without yield drag in a sister-line BC breeding progeny DEH_229 is controlled by multiple genetic factors with maineffect loci
- Identification of microRNAs involved in crosstalk between nitrogen,phosphorus and potassiumunder multiple nutrient deficiency in sorghum
- RNA interference targeting ω-secalin genesdifferentially affects the processing quality in a wheat T1BL·1RS translocation line

