Partial response to Chinese patent medicine Kangliu pill for adult glioblastoma: A case report and review of the literature
Ge Sun, Wei Zhuang, Qing-Tang Lin, Lei-Ming Wang, Yu-Hang Zhen, Sheng-Yan Xi, Xiao-Lan Lin
Ge Sun, Wei Zhuang, Lei-Ming Wang, Xiao-Lan Lin, Department of Pharmacy, Xuanwu Hospital of Capital Medical University, Beijing 100053, China
Qing-Tang Lin, Yu-Hang Zhen, Department of Neurosurgery, Xuanwu Hospital of Capital Medical University, Beijing 100053, China
Sheng-Yan Xi, Department of Traditional Chinese Medicine, School of Medicine and Cancer Research Center, Xiamen University, Xiamen 361102, Fujian Province, China
Abstract BACKGROUND Glioblastoma is the most common type of brain tumor and is invariably fatal, with a mean survival time of 8-15 mo for recently diagnosed tumors, and a 5-year survival rate of only 7.2%. The standard treatment for newly diagnosed glioblastoma includes surgery followed by concurrent chemoradiotherapy and further adjuvant temozolomide. However, the prognosis remains poor and longterm survival is rare. This report aimed to demonstrate a new therapeutic strategy for the treatment of glioblastoma.CASE SUMMARY A patient was referred to the Department of Neurosurgery with an intracranial space-occupying lesion with a maximum diameter of approximately 5 cm. The tumor was compressing functional areas, and the patient accordingly underwent partial resection and concurrent chemoradiotherapy. The imaging and pathological findings were consistent with a diagnosis of glioblastoma with oligodendroglioma differentiation (World Health Organization IV). The patient was finally diagnosed with glioblastoma. However, the patient discontinued treatment due to intolerable side effects, and was prescribed Kangliu pill (KLP)7.5 g three times/d, which he has continued to date. Significant shrinkage of the tumor (maximum diameter reduced from about 3.5 to about 2 cm) was found after 3 mo of KLP therapy, and the tumor was further reduced to about 1 cm after 3 years. The patient’s symptoms of headache, limb weakness, and left hemiplegia were relieved, with no side effects.CONCLUSION KLP has been a successful intervention for glioblastoma, and the current case indicates that traditional Chinese medicine may offer effective alternative therapies for glioblastoma.
Key Words: Glioblastoma; Kangliu pill; Traditional Chinese medicine; Therapeutic effect;Adjunct therapy; Chinese patent medicine; Case report
INTRODUCTION
Glioblastoma (GBM) is the most common type of malignant primary brain cancer with the lowest survival rate, representing approximately 48% of all primary malignant central nervous system tumors[1,2]. GBM is one of the most devastating brain tumors,and is highly heterogeneous and invasive, with a high incidence rate. The overall annual incidence rate of glioma is high, at 9.23 cases per 100000 individuals in the United States, with GBM accounting for about half of all these cases[2]. Patients diagnosed with GBM usually have a dismal prognosis and poor quality of life.Currently, the best outcome is a modest 14.6-mo median survival following surgical resection, radiotherapy, and/or chemotherapy with temozolomide (TMZ)[3]. However,the survival outcomes are generally very poor, with a mean survival rate of recurring GBM of just 3-9 mo[4]and a 5-year survival rate of 7.2%[5]. This poor prognosis is mostly due to the tumor’s therapeutic resistance and to tumor relapse after surgical removal[6]. Despite extensive efforts, little progress has been made in prolonging the survival of patients with GBM[7]. Thus, there is an urgent need to explore effective treatment strategies and drugs to improve the prognosis of patients with GBM.Traditional Chinese medicine (TCM) has recently gained wide attention due to its therapeutic effects and few side effects in patients with GBM, with increasing numbers of TCMs demonstrating antitumor effects against GBM. For example, toosendanin inhibited GBM cell proliferation and induced apoptosisin vitroandin vivo[8]. Celastrol sensitized glioma cells to the apoptosis-induced ligand TRAILviathe death receptor pathway, indicating a promising tumor-killing therapeutic strategy with high efficacy and low toxicity[9]. The aqueous extract of Pingliu Keli strongly inhibited cell proliferation and induced apoptosis in SHG-44 cells[10], and Jinlong capsules partly regulated the mTOR/S6 pathway to inhibit glioblastoma cell migration and invasionin vitro[11]. Kangliu pill (KLP) is a Chinese patent medicine consisting of 18 types of traditional Chinese medicine:Smilax glabraRoxb. (Tufuling) (Voucher Number:140105),Panax notoginseng(Burkill) F. H. Chen (Sanqi) (Voucher Number: 140815),Scleromitrion diffusum(Willd.) R. J. Wang (Baihuasheshecao) (Voucher Number:140702 ), Scutellaria barbataD. Don (Banzhilian) (Voucher Number: 140912), calcined Alumen (Kufan) (Voucher Number: 140201),Reynoutria multiflora(Thunb.) Moldenke(Zhiheshouwu) (Voucher Number: 140705),Aquilaria sinensis(Lour.) Spreng.(Baimuxiang) (Voucher Number: 140615),Monetaria annulus(Linnaeus) (Baibeichi)(Voucher Number: 140216),Solanum lyratumThunb. (Baiying) (Voucher Number:140708),Fritillaria cirrhosaD. Don (Chuanbeimu) (Voucher Number: 140207),Stemona japonica(Blume) Miq. (Baibu) (Voucher Number: 140211),Eriobotrya japonica(Thunb.)Lindl. (Pipaye) (Voucher Number: 140416),Aster tataricusL.f. (Ziwan) (Voucher Number: 140409), stir-bakedCrataegus pinnatifidaBunge (Jiaoshanzha) (Voucher Number: 140809), stir-bakedHordeum vulgareL. (Jiaomaiya) (Voucher Number:140512), stir-baked Massa Medicata Fermentata (Jiaoshenqu) (Voucher Number:140608),Platycodon grandiflorus(Jacq.) A. DC. (Jiegeng) (Voucher Number: 140713), andSuccinium(Hupo) (Voucher Number: 140914). KLP has been used for 40 years and has been shown to reduce temperature and achieve detoxification, as well as promote blood circulation and resolve blood stasis[12-15]. Preclinical studies showed that KLP promoted GBM cell apoptosis by adjusting the ratio of Bcl-2/Bax, suggesting that its inhibitory effect could be related to the induction of glioma cell apoptosis[12,13]. In addition, researchers have identified the main components of KLP and determined quality standards[14,15].
A clinical study also found that KLP exerted antitumor effects against malignant gliomas[16]. Here we report a patient with GBM who experienced a remarkable response to KLP.
CASE PRESENTATION
Chief complaints
The protocols in this case were conducted in strict accordance with the clinical trial guidelines of the Ministry of Science and Technology of the People’s Republic of China and the study was approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University. The specimens of Chinese herbal medicine have been stored in the Pharmacy Department of Xuanwu Hospital of Capital Medical University for future reference, and were identified and independently verified by Professor Jing-Xia Wang from the Chinese Medicine Department of Beijing University of Chinese Medicine.
A 27-year-old man was referred to the Department of Neurosurgery with an intracranial space-occupying lesion on February 21, 2016.
History of present illness
Eight months before the current referral, the patient had experienced left upper limb convulsions, urinary incontinence, nausea, vomiting, and loss of consciousness with no apparent cause, which were relieved after a few minutes. He did not receive any treatment at that time. However, his headache, limb weakness, and left hemiplegia returned 4 mo later. The symptoms were partially relieved by symptomatic supportive treatment, including carbamazepine 400 mg twice/d orally; however, the patient stopped carbamazepine of his own accord due to aggravated limb weakness.
History of past illness
The patient had no previous medical history.
Personal and family history
The patient had no personal and family history.
Physical examination
The patient’s temperature was 36.7°C, his heart rate was 70 bpm, his respiratory rate was 18 breaths/min, and his blood pressure was 110/70 mmHg. Clinical neurological examination revealed normal right limb muscle strength, but his left limbs were slightly weaker than his right limbs. His mental functions were normal, with no other pathological signs.
Imaging examinations
Computed tomography examination in a local hospital showed no obvious abnormalities, but magnetic resonance imaging indicated a space-occupying lesion in the right frontal lobe. The patient did not receive any treatment at that time. However,headache, limb weakness, and left hemiplegia returned 4 mo later, and repeat computed tomography suggested a right frontal parietal lobe cerebral hemorrhage. He presented to the hospital for re-examination on February 18, 2016. Cranial magnetic resonance imaging showed that the right frontal-parietal lobe was occupied by a mass,with a maximum diameter of about 5 cm, irregular enhancement of the parenchyma ring, and the necrotic center was not enhanced, indicating a possible high-grade glioma (Figure 1A).
MULTIDISCIPLINARY EXPERT CONSULTATION
Qing-Tang Lin, MD, PhD, Associate Professor and Deputy Chief, Department of Neurosurgery, Xuanwu Hospital of Capital Medical University
The patient should undergo surgical treatment with right frontal craniotomy and resection of the brain tumor.
Lei-Ming Wang, MD, PhD, Attending Physician, Department of Pathology, Xuanwu Hospital of Capital Medical University
Under the microscope, the specimens submitted for examination showed diffuse growth of tumor cells, with short spindle-shaped nuclei in some areas and roundshaped nuclei in some areas. The cytoplasm was empty and bright, with vascular endothelial proliferation and palisade necrosis (Figure 2). Combined with the immunohistochemical results, these findings were consistent with the diagnosis of glioblastoma with oligodendroglioma differentiation (World Health Organization IV).
Xiao-Lan Lin, Associate Professor and Deputy Chief, Department of Pharmacy,Xuanwu Hospital of Capital Medical University; Qing-Tang Lin, MD, PhD, Associate Professor and Deputy Chief, Department of Neurosurgery, Xuanwu Hospital of Capital Medical University
The patient discontinued concurrent chemoradiation due to intolerable side effects,and he should therefore be prescribed KLP 7.5 g three times/d.
FINAL DIAGNOSIS
The final diagnosis of the presented case was glioblastoma.
TREATMENT
There was a clear indication for surgery, with no contraindications in the preoperative examination, and the patient underwent partial resection on February 23, 2016. The postoperative pathological report confirmed GBM. After surgery, the patient started concurrent chemoradiation therapy until April 2016, with 75 mg/m2TMZ once daily for 45 d combined with focal radiotherapy of 60 Gy administered in 30 fractions.However, he opted to discontinue the chemoradiotherapy due to intolerable adverse reactions, including nausea, vomiting, and loss of appetite. Two months later, the patient attended the hospital for postoperative review (Figure 1B) and was prescribed KLP 7.5 g three times/d.
OUTCOME AND FOLLOW-UP
After taking KLP for nearly 3 mo, the tumor had shrunk significantly (from about 3.5 to about 2 cm) (Figure 1C), and he therefore continued to take KLP as prescribed for the next 3 years. A subsequent examination showed that the tumor had shrunk to about 1 cm after 3 years (Figure 1D). No adverse reactions were detected throughout the course of KLP treatment, and the patient’s symptoms of headache, limb weakness,and left hemiplegia were relieved (Table 1).
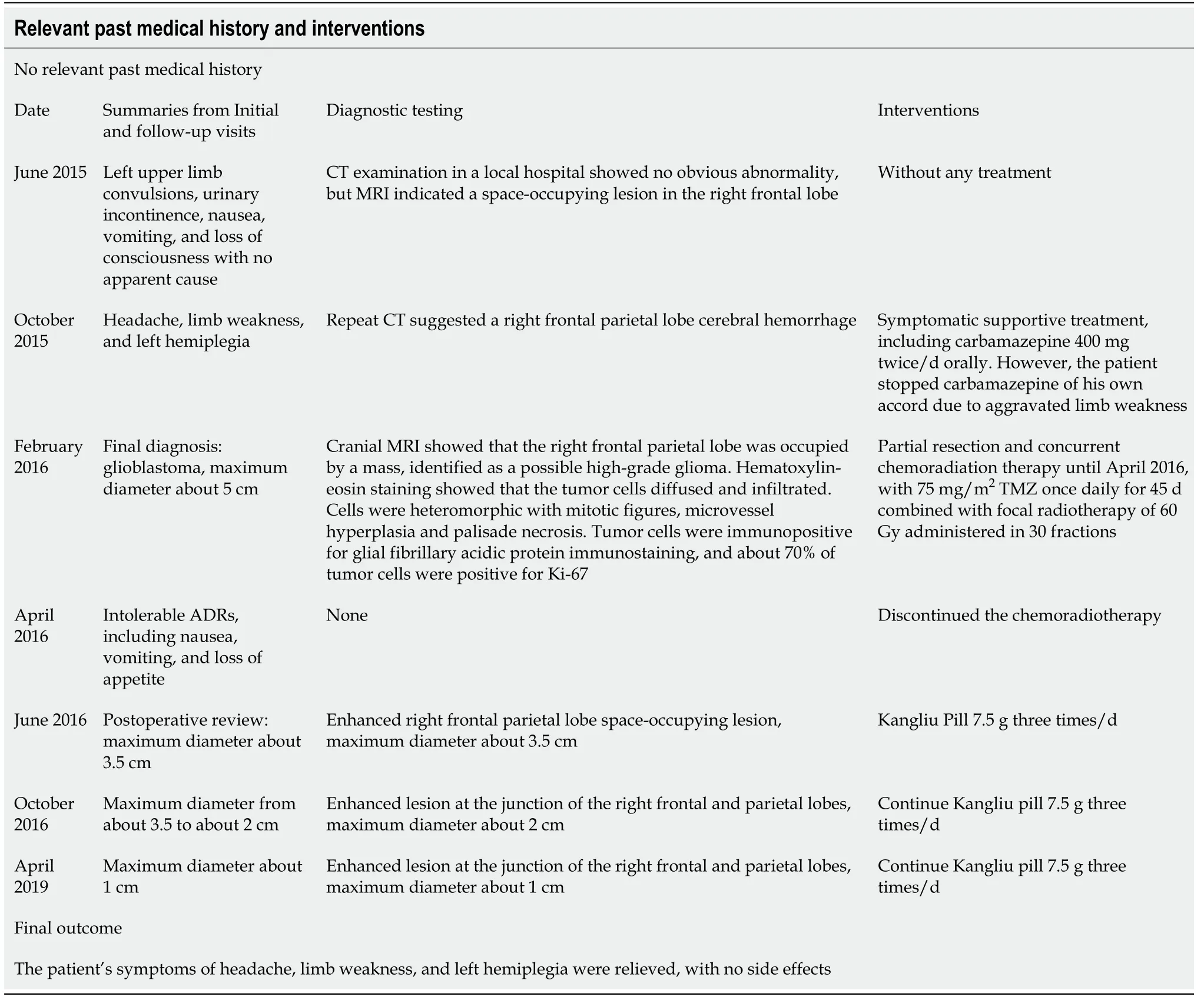
Table 1 Timeline
DISCUSSION
GBM, which accounts for the majority of gliomas (57.7%)[5], is characterized histologically by appreciable cellularity with mitotic activity, vascular proliferation,and necrosis. The standard treatment for newly diagnosed GBM includes surgery followed by concurrent chemoradiotherapy and further adjuvant TMZ. The benefits of TMZ may derive from O-6-methylguanine-deoxyribonucleic acid methyltransferase promoter methylation, which silences epigenetic genes[17]. TMZ is associated with toxicities including myelosuppression and nausea, and especially thrombocytopenia and neutropenia, particularly during adjuvant therapy. GBM usually recurs within 6 mo after standard concurrent chemoradiotherapy[18]. The humanized vascular endothelial growth factor monoclonal antibody bevacizumab has been shown to improve progression-free but not overall survival in patients with GBM[19]. Tumortreating fields, delivering low-intensity, is approved for adjuvant TMZ therapy based on the progression-free and overall survival benefits[20]. However, despite Food and Drug Administration approval, the evidence for and use of tumor-treating fields remain controversial. Extensive research efforts have also been made in the fields of immunotherapy and targeted therapy, but few encouraging outcomes have been observed[18]. Overall, despite advances in multimodality therapy, the prognosis of patients with GBM thus remains poor and long-term survival is rare. There is accordingly a need to identify more effective therapies for GBM.
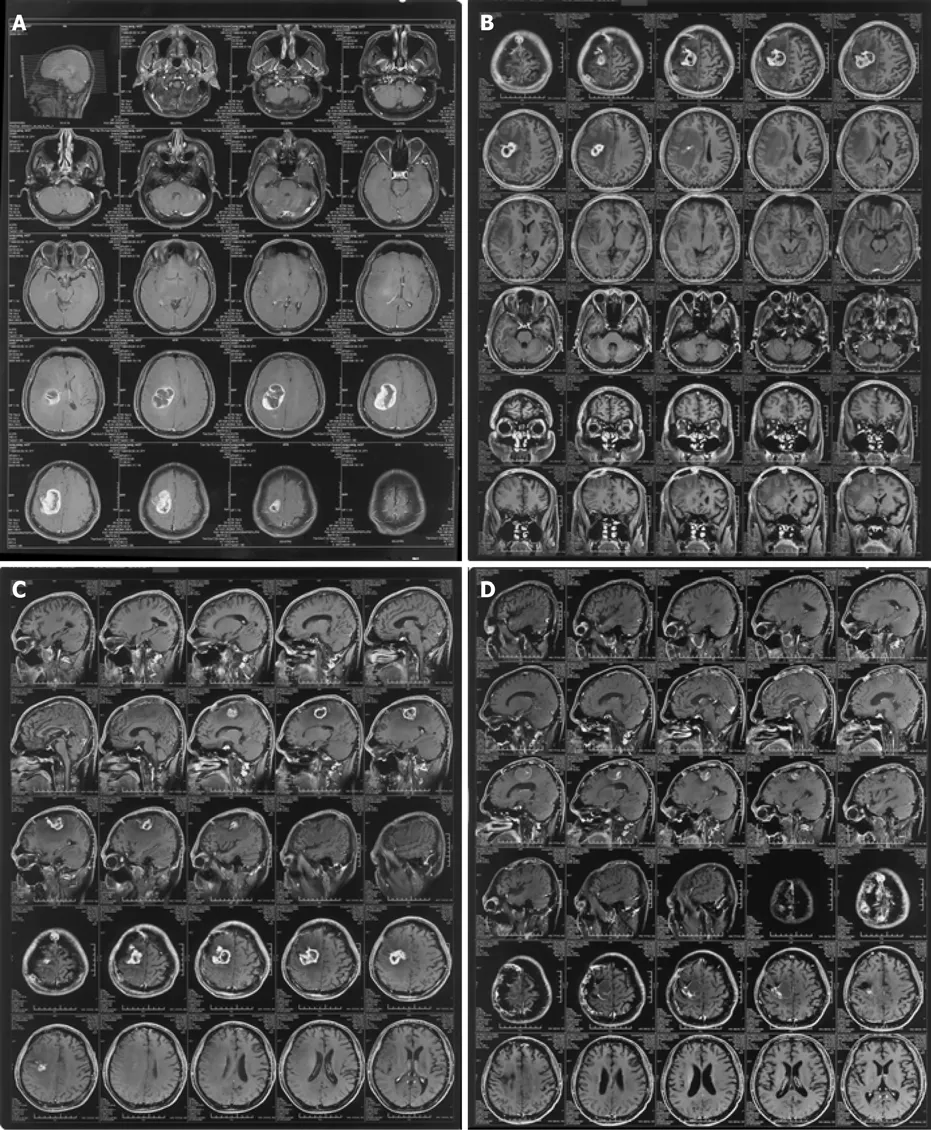
Figure 1 Neuroimaging findings. A: February 20, 2016 Preoperative image of enhanced right frontal parietal lobe space-occupying lesion, maximum diameter about 5 cm; B: June 3, 2016 End of chemoradiation. Enhanced right frontal parietal lobe space-occupying lesion, maximum diameter about 3.5 cm; C: September 14,2016 Enhanced lesion at the junction of the right frontal and parietal lobes, maximum diameter about 2 cm, after taking Kangliu pill for 3 mo; and D: April 29, 2019 Enhanced lesion at the junction of the right frontal and parietal lobes, maximum diameter about 1 cm, after taking Kangliu pill for nearly 3 years.
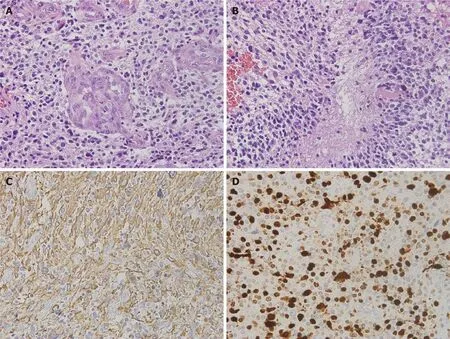
Figure 2 Pathological findings. A: Hematoxylin-eosin staining showed that the tumor cells diffused and infiltrated; B: Cells were heteromorphic with mitotic figures, microvessel hyperplasia, and palisade necrosis. Original magnification, × 40; C: Tumor cells were immunopositive for glial fibrillary acidic protein immunostaining. Original magnification, × 40; and D: About 70% of tumor cells were positive for Ki-67. Original magnification, × 40.
In the current case, the tumor location and the intense side effects meant that total resection with chemoradiotherapy was impossible, and KLP was therefore prescribed.Surprisingly, the patient experienced a remarkable response to KLP therapy, with a reduction in tumor size from about 3.5 cm to about 1 cm. This therapeutic effect might have been related to the anti-tumor effects of the main components of KLP, such asSmilax glabraRoxb. (Tufuling),Panax notoginseng(Burkill) F. H. Chen (Sanqi),Scleromitrion diffusum(Willd.) R. J. Wang (Baihuasheshecao), andScutellaria barbataD.Don (Banzhilian).Smilax glabraRoxb. (Tufuling) is a natural dietary supplement widely used in food-making and health care, based on its abilities to detoxify and reduce temperature[21]. Previous studies revealed that it also had an anti-tumor function in counteracting the invasiveness of a subset of cancer cells by suppressing the transforming growth factor-β1 pathway[22].Panax notoginseng(Burkill) F. H. Chen(Sanqi), which has been applied for medical uses for over four centuries, includes saponins that can be effective in the treatment of cancer, partly by modulating the Met/miR-222 axis[23].Scleromitrion diffusum(Willd.) R.J. Wang (Baihuasheshecao) was shown to suppress GBM growthin vitroby inducing mitochondria-mediated apoptosisviaAkt/ERK signaling[24].Scutellaria barbataD. Don (Banzhilian) is used as an immunomodulatory and anti-tumor agent in TCM, and its extracts have exhibited growth-inhibitory effects in various cancersin vitroand/orin vivo[25]. Recent studies have provided a preliminary understanding of the mechanism of traditional Chinese medicine in regulating the permeability of the blood-brain barrier. Some studies found that astilbin inSmilax glabraRoxb. (Tufuling) enters the brain tissue through the bloodbrain barrier, at a low concentration, but with a long duration[26].Notoginseng saponinsR1 and ginsenoside Rg1 inPanax notoginseng(Burkill) F. H. Chen (Sanqi) could penetrate the blood-brain barrier and enter the cerebrospinal fluid in rats[27]. Few studies have investigated the ability ofScleromitrion diffusum(Willd.) R. J. Wang andScutellaria barbataD. Don to affect blood-brain barrier permeability, but we speculate that other components in KLP may also play a role in assisting the main components to enter the blood-brain barrier. TCMs act on the human body through multiple channels and targets; however, the mechanism of KLP is still unclear.
TCMs have demonstrated visible effects in relieving clinical symptoms, prolonging survival, and preventing recurrence of GBM, and even reducing the toxicity of chemotherapy[28-31]. In addition, they have dual immunomodulatory effects of immune promotion and suppression, which can dramatically enhance the patient’s immune function and improve their quality of life. TCMs are thus widely used as adjuvant therapy in all cancer stages, especially for the palliative treatment of advanced and refractory tumors. Progress in related technologies has allowed researchers to investigate the mechanisms of TCMs in relation to glioma treatment at the molecular level, and increasing evidence has confirmed their effectiveness and reliability[31].TCMs may thus offer new therapeutic strategies for the treatment of glioma.
CONCLUSION
Western medicine is usually considered to be the first choice of drug therapy for GBM in clinical practice. However, TMZ treatment was not suitable for the current patient and the TCM KLP was administered as an alternative. Compared with other therapies,KLP has the advantages of lower cost, comprehensive efficacy, and few side effects,and should thus be considered as a recommended option for the treatment of GBM.However, although this report demonstrated the potential suitability of TCM as an alternative therapy for GBM, the detailed mechanisms responsible for its effects remain complex and unknown. Extensive further research and more clinical trials are therefore required to confirm the efficacy and mechanisms of TCMs.
 World Journal of Clinical Cases2021年12期
World Journal of Clinical Cases2021年12期
- World Journal of Clinical Cases的其它文章
- Standardization of critical care management of non-critically ill patients with COVID-19
- Mediastinal lymphadenopathy in COVID-19: A review of literature
- Polycystic ovary syndrome: Pathways and mechanisms for possible increased susceptibility to COVID-19
- Circulating tumor cells with epithelial-mesenchymal transition markers as potential biomarkers for the diagnosis of lung cancer
- Clinicopathological features of superficial CD34-positive fibroblastic tumor
- Application of a rapid exchange extension catheter technique in type B2/C nonocclusive coronary intervention via a transradial approach
