Polycystic ovary syndrome: Pathways and mechanisms for possible increased susceptibility to COVID-19
Ioannis Ilias, Spyridon Goulas, Lina Zabuliene
Ioannis Ilias, Department of Endocrinology, Elena Venizelou Hospital, Athens GR-11521,Greece
Spyridon Goulas, Department of Gastroenterology Unit, Elena Venizelou Hospital, Athens GR-11521, Greece
Lina Zabuliene, Institute of Clinical Medicine, Faculty of Medicine, Vilnius University, Vilnius LT-03101, Lithuania
Abstract In 75% of women with polycystic ovary syndrome (PCOS), insulin action is impaired. In obesity, visceral adipose tissue becomes dysfunctional: Chronic inflammation is favored over storage, contributing to the development of metabolic complications. PCOS, metabolic syndrome (MetSy) and non-alcoholic fatty liver disease (NAFLD) apparently share common pathogenic factors; these include abdominal adiposity, excess body weight and insulin resistance.Alterations in the gut microbiome have been noted in women with PCOS compared to controls; these may lead to deterioration of the intestinal barrier,increased gut mucosal permeability and immune system activation,hyperinsulinemia and glucose intolerance, which hamper normal ovarian function and follicular development (all being hallmarks of PCOS). It has been proposed that PCOS may entail higher susceptibility to coronavirus disease 2019(COVID-19) via its associated comorbidities (NAFLD, obesity, MetSy and alterations in the gut microbiome). Studies have found an association between acute respiratory distress syndrome (seen in severe cases of COVID-19) and the intestinal microbiome. Furthermore, apparently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can gain entry to the gastrointestinal tract via locally-expressed angiotensin converting enzyme type 2 receptors. Excess body weight is associated with more severe COVID-19 and increased mortality.Although robust links between SARS-CoV-2 infection and PCOS/NAFLD/gut microbiome/metabolic consequences are yet to be confirmed, it seems that strategies for adapting the intestinal microbiome could help reduce the severity of COVID-19 in women with PCOS with or without NAFLD, MetSy or obesity.
Key Words: Adipose tissue; Obesity; Polycystic ovaries; SARS-CoV-2; COVID-19;Human
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common and heterogeneous endocrine disorder that becomes symptomatic in adolescence and occurs in 5%-10% of women of reproductive age[1]; it is frequently associated with metabolic abnormalities. It is the most common cause of androgen hypersecretion and accounts for more than a third of menstrual disorders. PCOS is also the most common hormonal disorder that leads to hair loss, appearance of acne, seborrhoea and male pattern baldness (symptoms may be absent in patients with moderate hyperandrogenemia, as in most cases of PCOS[1]).
The definition of PCOS has shifted over the years, according to different diagnostic criteria, which define an array of disease phenotypes (Figure 1), taking into account signs of hyperandrogenemia and ovarian dysfunction and of ovarian morphology[1-4].Currently, experts suggest the use of the Rotterdam criteria, as refined in 2018[5-7].
The current understanding of the pathogenesis of PCOS suggests that it is a complex polygenic disorder[8]. Various studies have studied candidate genes that can regulate the hypothalamic-pituitary-ovary axis as well as genes responsible for insulin resistance[9]. There is a familial predisposition for high levels of dehydroepiandrosterone sulphate in siblings of women with PCOS, suggesting that this is a genetic characteristic[10]. Additionally, first-degree relatives of patients with PCOS carry an increased risk of cardiovascular disease, as do patients with PCOS[11-13]. Whether the molecules involved in low-grade inflammation are involved in the pathogenesis of hyperandrogenemia or, conversely, if excess androgens may-in some way-lead to the promotion of inflammation, is still a controversial issue. Although direct involvement of androgens in low-grade inflammation has not been shown, available evidence suggests that androgens may be indirectly involved in the development of low-grade inflammation,viaan effect on adipose tissue and on resistance to insulin[14,15]. In fact,androgens stimulate adipocyte hypertrophy, affecting the expression of enzymes and proteins involved in lipid and carbohydrate metabolism, oxidative stress and differentiation of pre-adipocytes into mature adipocytes. Androgen excess can impair insulin action, either directly at the insulin receptor level or indirectlyviachanges effected at various tissues. In addition, androgens increase lipolysis, resulting in increased release of free fatty acids[16,17].
Research on the relationship between adiponectin and testosterone has given conflicting results. Similar results have been found for leptin. A strong negative relationship between circulating levels of ghrelin with androgens, especially androstenedione, has been found in women with PCOS[18]. The production of the latter steroid results from endogenously malfunctioning ovaries and adrenal glands. The high intra-ovarian concentration of androgens inhibits follicular maturation, leading to the appearance of polycystic ovaries. The usual source of excess androgens is due to functional ovarian hyperandrogenism, which is characterized by increased 17-hydroxyprogesterone after stimulation with luteinizing hormone releasing hormone or human chorionic gonadotropin. Endogenous dysfunction of ovarian cells appears to contribute to 50%-75% of cases of PCOS[19].
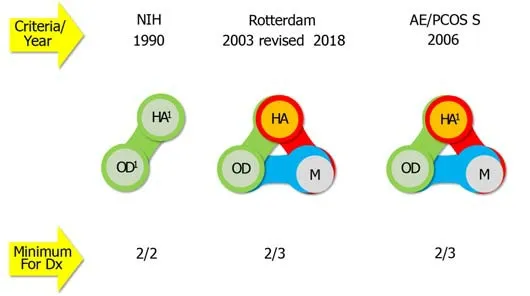
Figure 1 Criteria for defining polycystic ovary syndrome and describing its phenotypes (summarized from[5-7]). 1Sine qua non for diagnosis,when excluding all similar/mimicking disorders after thorough laboratory and instrumental investigations. NIH: National Institutes of Health (United States) criteria;Rotterdam: European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine criteria; AE/PCOS S: Androgen Excess and Polycystic Ovary Syndrome Society criteria; HA: Hyperandrogenism; OD: Ovulatory dysfunction; M: Polycystic ovary morphology: At least one ovary with volume > 10 cm3 or at least 12-20 antral follicles (with a diameter of 5-9 mm) per ovary; Dx: Diagnosis.
PCOS, OBESITY AND INSULIN RESISTANCE
Obesity is present in about 50%-80% of women with PCOS, but this relationship also depends on environmental factors[20,21]. A lower prevalence of obesity in women with PCOS has been reported in populations where severe obesity is less prevalent, such as in Asians and in some Europeans[22,23]. Some argue that in the community there may not be more obese women with PCOS than obese women without PCOS[24]. With age,there is also increase in body mass index (BMI), waist circumference and of the waist to hip ratio[25,26]. The annualized conversion rate from normal glucose tolerance to impaired is higher in obese women with PCOS (16%) compared to the general obese population (1%-5%); the annualized conversion rate of obese women with PCOS from impaired glucose tolerance to diabetes at 2% is not different from the rate of the general obese population[27]. There is a synergistic effect of obesity on worsening glucose intolerance in women with PCOS. We have to note that regarding obesity,women with PCOS present a challenge for researchers, since BMI may not characterize them adequately[28,29]. Studies have evaluated the distribution of fat (subcutaneous and visceral fat) in obese women with PCOS using magnetic resonance imaging and dual energy X-ray with conflicting results. Women with PCOS have more central body fat distribution and increased waist/hip circumference compared to women without PCOS and similar BMI[11](central obesity is a risk factor for pre-diabetes and cardiovascular disease). It is estimated that in about 75% of normal weight and overweight women with PCOS insulin action is impaired. Hyperinsulinemia and insulin resistance can induce both the endocrine and reproductive traits of PCOS, but the mechanisms that underlie this remain elusive[30]. Furthermore, in obesity, visceral adipose tissue becomes dysfunctional (with an increase in inflammatory molecules and a decrease in the expression of lipogenic enzymes); in this way-viavarious signaling pathways-chronic inflammation is favored over storage, contributing to the development of metabolic complications[31,32]. These obesity-associated signaling pathways and mechanisms are not fully delineated. Various adipose tissue genes are differentially expressed in subjects with obesity/insulin resistance; more in detail, in these subjects genes which are associated with lipid uptake and processing are less expressed compared to lean individuals[33,34]. Recent research indicates that in obesity,adipokine imbalance (low adiponectin and high leptin) modulates the activation of inflammasomes (receptors/sensors of the innate immune system that regulate caspase-1 activation and promote inflammation)[35]; thus the latter may be the connectors between excess adiposity and obesity-associated complications.
PCOS: NON-ALCOHOLIC FATTY LIVER DISEASE AND THE METABOLIC SYNDROME
Various definitions by different authorities have been proposed for the definition of the cluster of metabolic disturbances that comprise the metabolic syndrome (MetSy);invariably they include central obesity, dyslipidemia, insulin resistance, and hypertension[36]. A diet rich in saturated fat and fructose may lead to non-alcoholic fatty liver disease[37,38](NAFLD; indicating hepatic steatosis which is not attributed to alcohol or other specific etiologies and is found in at least 25% of the world population[39,40]), metabolic endotoxinemia and increased resistance to the action of insulin[36]. PCOS, MetSy and NAFLD apparently share common pathogenic factors;these include abdominal adiposity, excess body weight and insulin resistance[41,42].Women with PCOS-particularly with hyperadrogenemia-have a two-fold to four-fold higher probability of having NAFLD compared to non-PCOS women[43,44]; 35%-70% of women with PCOS have NAFLD[44-46]and 60% have insulin resistance[45]. Insulin resistance,viaactivation-among others-of the carbohydrate response element binding protein and sterol response element binding protein 1c (both act as transcription factors), leads to intra-hepatic lipid accumulation[44].
PCOS AND THE GUT MICROBIOME
Three main mechanisms have been put forth regarding the effect of intestinal microbiome on glucose intolerance/insulin resistance and type 2 diabetes: The promotion of metabolic inflammation, the modification of incretin secretion and the modification of hydroxybutyric acid production[47-49].Parabacteroides merdae, Bacteroides fragilis, CatenibacteriumandKandleriagenera and strains ofEscherichiaandShigellaare more abundant in women with PCOS compared to controls[50]; the presence of specific microbes in women with PCOS is positively correlated with BMI, high serum testosterone and elevated luteinizing hormone[51-54]. Women with PCOS have less hydroxybutyric acid-producing genera[55]. Low levels of interleukin 22 (IL-22) are noted in women with PCOS[56]. This interleukin helps to maintain the integrity of the gut epithelial barrier[57]. Thus, an altered gut microbiome may lead to deterioration of the intestinal barrier, increased gut mucosal permeability and passage into the circulation of lipopolysaccaride from Gram negative colonic bacteria. Lipopolysaccaride in the circulation (attached to the glycoproteinL. barbarum polysaccharides) binds to the CD14 toll-like receptor complex (TRL-4) on the surface of innate immune cells,leads to activation of a downstream signaling pathway and immune system activation[58]. The latter impedes insulin receptor function and leads to hyperinsulinemia and glucose intolerance, which hamper normal ovarian function and follicular development (all being hallmarks of PCOS)[58,59]. Modulation of the intestinal(and of the vaginal) microbiome has been put forth as holding therapeutic potential for PCOS[60].
PCOS VS SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 INFECTION
To gain cell entry, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)uses the host’s angiotensin-converting enzyme 2 (ACE2), in synergy with the host’s transmembrane protease, serine 2 (TMPRSS2); the latter’s expression is androgenregulated[61,62]. It has been proposed that PCOS, given this condition’s hyperadrogenemic environment, may entail higher susceptibility to coronavirus disease 2019(COVID-19)[63-65]. Furthermore, PCOS may also increase susceptibility to COVID-19viaits associated comorbidities (NAFLD, obesity, MetSy and alterations in the gut microbiome) (Figure 2). Obese patients with advanced NAFLD have been shown to have increased hepatic mRNA expression of ACE2 and TMPRSS2, the critical molecules for SARS-CoV-2 cellular entry (gender-specific differences may exist in the expression of these molecules)[66].
SARS-COV-2 AND THE GUT-LUNG AXIS
The term gut-lung axis describes the interaction between the intestinal microbiome and the lungs[67]. This communication is, in fact, two-way[68]. Endotoxins and metabolites produced by bacteria in the gut (due to systemic inflammation, IL-6-induced vascular damage and increased intestinal permeability that may facilitate bacterial translocation) can move through the bloodstream and reach the lungs[69].Similarly, pulmonary inflammation can have an effect on intestinal integrity. This raises the question of whether the SARS-CoV-2 virus can affect the intestinal microbiome[68]. In fact, several studies have shown that respiratory infections are associated with changes in the composition of the intestinal microbiome[70]. Some studies have found an association between acute respiratory distress syndrome (seen in severe cases of COVID-19) and the intestinal microbiome[67,69]. Furthermore,apparently, SARS-CoV-2 can gain entry to the gastrointestinal tractvialocallyexpressed ACE2 receptors[71].
SARS-COV-2 AND OBESITY/MET-SY/NAFLD
Regardless of the definition of obesity (in western countries it is defined as a BMI higher than 30.0 kg/m2or in China over 27.5 kg/m2), excess body weight is associated with more severe SARS-CoV-2 infection (COVID-19) and increased mortality[72,73]. The etiology for the latter is still obscure, although it is known that obesity is a state of lowgrade inflammation, which COVID-19 pushes to extremes (with a characteristic“cytokine storm”[74]). Of note, obesity may lead not only to more adipose tissue accumulation but also to larger abdominal organ size[75]; we have speculated that larger abdominal organs may provide a larger tissue reservoir for the pervasive SARSCoV-2 virus, since the latter has indeed been localized in abdominal organs[76].
The MetSy is characterized by hyperinsulinemia, which may be associated with facets of COVID-19[77], particularly regarding microvascular dysfunction[78-80], systemic hypercoagulability and extensive micro- and macrovascular thrombosis[81,82].
As indicated above, ACE2 offers entry to SARS-CoV-2 for cell infection[83]. This enzyme normally shows low expression in cholangiocytes and hepatocytes, but its expression increases-at least in cholangiocytes-in chronic liver disease and experimental diet-induced NAFLD[83]. The virus is pervasive and is localized in abdominal and extraabdominal organs, including the liver[84]. There are conflicting reports regarding NAFLD and COVID-19[85,86]. Some researchers have shown increased hospitalization[87], morbidity[88-91]and mortality from COVID-19 in patients with NAFLD, whereas other researchers have shown that NAFLDper sein hospitalized patients was not linked with worse prognosis[92](but NAFLD-associated inflammatory parameters were associated with prognosis[86]).
CONCLUSION
Although robust links between SARS-CoV-2 infection and the chain of PCOS/NAFLD/gut microbiome/metabolic consequences have not been confirmed,there is evidence that merits further investigation. No research to date has been able to answer whether there is a cause-and-effect relationship. In order to determine whether the intestinal microbiome-particularly in women with PCOS with or without NAFLD,MetSy or obesity-affects the risk of COVID-19 or if SARS-CoV-2 is the factor that changes the composition of the microbiome, more research will be needed.Nevertheless, strategies for adapting the intestinal microbiome (probably in all patients) could help reduce the severity of COVID-19 in women with PCOS with or without NAFLD, MetSy or obesity.
 World Journal of Clinical Cases2021年12期
World Journal of Clinical Cases2021年12期
- World Journal of Clinical Cases的其它文章
- Standardization of critical care management of non-critically ill patients with COVID-19
- Mediastinal lymphadenopathy in COVID-19: A review of literature
- Circulating tumor cells with epithelial-mesenchymal transition markers as potential biomarkers for the diagnosis of lung cancer
- Clinicopathological features of superficial CD34-positive fibroblastic tumor
- Application of a rapid exchange extension catheter technique in type B2/C nonocclusive coronary intervention via a transradial approach
- Paradoxical relationship between proton pump inhibitors and COVID-19: A systematic review and meta-analysis
