Paradoxical relationship between proton pump inhibitors and COVID-19: A systematic review and meta-analysis
Maddalena Zippi, Sirio Fiorino, Roberta Budriesi, Matteo Micucci, Ivan Corazza, Roberta Pica, Dario de Biase,Claudio Giuseppe Gallo, Wandong Hong
Maddalena Zippi, Roberta Pica, Unit of Gastroenterology and Digestive Endoscopy, Sandro Pertini Hospital, Rome 00157, Italy
Sirio Fiorino, Unit of Internal Medicine, Maggiore Hospital, Local Health Unit of Bologna,Bologna 40133, Italy
Roberta Budriesi, Matteo Micucci, Food Chemistry and Nutraceuticals Laboratory, Department of Pharmacy and Biotechnology (FaBiT), Alma Mater Studiorum, University of Bologna,Bologna 40133, Italy
Ivan Corazza, Experimental, Diagnostic and Speciality Medicine Department, University of Bologna, Bologna 40138, Italy
Dario de Biase, Department of Pharmacy and Biotechnology, University of Bologna, Bologna 40138, Italy
Claudio Giuseppe Gallo, Internal Medicine Unit, Budrio Hospital Azienda USL, Bologna 40138,Italy
Wandong Hong, Department of Gastroenterology and Hepatology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, China
Abstract BACKGROUND The proton pump inhibitors (PPIs), used to reduce gastric acid secretion, represent one of the most widely used pharmaceutical classes in the world. Their consumption as a risk factor for the evolution of severe forms of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been investigated as well as the mortality of these patients. These risks also appear to be linked to the duration and the dosage. On the other hand, several studies have emerged with regard to the protective or therapeutic effects of these drugs. More and more evidence underlines the immunomodulatory and anti-fibrotic role of PPIs. In addition, their ability to alkalize the contents of endosomes and lysosomes serves as an obstacle to the entry of the virus into the host cells.AIM To identify studies on the relationship between the intake of PPIs and coronavirus disease 2019 (COVID-19) in patients affected by SARS-CoV-2 infection, with the main objective of evaluating the outcomes related to severity and mortality.METHODS A literature review was performed in November 2020. The MEDLINE/PubMed,Cochrane Library, EMBASE and Google Scholar databases were searched for all relevant articles published in English on this topic. The search terms were identified by means of controlled vocabularies, such as the National Library of Medicine’s MESH (Medical Subject Headings) and keywords. The MESH terms and keywords used were as follows: “COVID-19”, “proton pump inhibitors”,”PPIs”, “SARS-CoV-2”, “outcomes”, “severity” and “mortality”. The inclusion criteria regarding the studies considered in our analysis were: meta-analysis, casecontrol, hospital-based case-control, population-based case-control, retrospective studies, online survey, as well as cohort-studies, while articles not published as full reports, such as conference abstracts, case reports and editorials were excluded. We tried to summarize and pool all the data if available.RESULTS A total of 9 studies were found that described the use of PPIs, of which only 5 clearly reported the severity and mortality data in SARS-CoV-2 patients. Our pooled incidence analysis of severe events did not differ between patients with and without PPIs (odds ratio 1.65, 95% confidence interval: 0.62-4.35) (P = 0.314),or for mortality (odds ratio 1.77, 95% confidence interval: 0.62-5.03) (P = 0.286).CONCLUSION Detailed and larger case studies are needed to accurately understand the role of PPIs in this viral infection.
Key Words: COVID-19; Proton pump inhibitors; SARS-CoV-2, Severity; Mortality
INTRODUCTION
A global pandemic was declared in February 2020 by the World Health Organization.The causative agent for this pandemic, which initiated in the city of Wuhan in Hubei Province, China, is a novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which leads to coronavirus disease 2019 (COVID-19)[1].This pathogen shows similarities to two other previously identified coronaviruses,viz.,Severe Acute Respiratory Syndrome (SARS-CoV) in 2003 and Middle Eastern Respiratory Syndrome (MERS-CoV) in 2012[2]. Although the main post-infection manifestations due to this virus include respiratory symptoms and pneumonia, it has been found to attack and to affect almost all organs and organ systems, including the gastrointestinal tract[3]. Recently, Tianet al[4]highlighted the presence of gastrointestinal symptoms in patients infected with SARS-CoV-2, with an incidence ranging from 3% up to 79%. This virus binds to the host cells through the spike (S)glycoprotein present on its envelope. Various studies have shown how this protein binds to the angiotensin converting enzyme 2 (ACE-2) receptors expressed on the cell membrane in human cells[5,6]. In the digestive system, ACE-2 receptors are expressed in the intestine, liver and pancreas[7-10]. In fact, the same SARS-CoV-2 RNA has also been found in the stool[7-10].
Proton pump inhibitors (PPIs) represent a class of drugs which suppress gastric acid secretion[11]. The United States Food and Drug Administration (FDA) has approved these molecules as long-term therapy for several gastrointestinal diseases, including peptic ulcer, Barrett esophagus, gastroesophageal reflux disease (GERD), Zollinger-Ellison syndrome and as a preventive treatment for gastrointestinal bleeding due to nonsteroidal anti-inflammatory drugs[12]. The first PPI approved by the FDA in the United States in 1989 was omeprazole, followed by lansoprazole in 1995, rabeprazole in 1999, pantoprazole in 2000 and esomeprazole in 2001[13]. From the first launch on the market of the first PPI, these molecules have become one of the most commonly used drugs worldwide, being prescribed to outpatients and during hospitalizations, and self-prescriptions as over-the-counter[11]. In a study carried out by Rotman and Bishop[14]considering only outpatient visits, it was shown that the prescription rate of PPIs increased from 4% in the year 2002 to 9.2% in 2009. A Spanish study analyzed the frequency of PPIs use before, during hospitalization and at the time of discharge and found that the percentage of patients in each category was 28.65%, 82.62% and 54.75%,respectively[15]. It is noteworthy that once the use of PPI is started by hospitalized patients, more than 50% of them continue it for nearly 3-6 mo after their discharge,without an appropriate indication[16].
Pharmacological mechanism of PPIs
Acid secretion is regulated by the activity of the gastric parietal cells, also named oxyntic cells. These are a type of epithelial cell and are responsible for the production of hydrochloric acid (HCl), which results in an acidic pH with values between 1.5 and 3.5, and affects other intrinsic factors[17]. Their localization is predominantly at the level of the gastric glands of the fundus and in the body of the stomach[17]. This secretion of acid is regulatedviavarious pathways, such as paracrine, endocrine and neural pathways. These cells are considered “dynamic”, as they are able to modify their morphology between the resting state and activity[17,18]. The production of HCl occurs through stimulating molecules, such as gastrin, acetylcholine and histamine when they bind to their respective receptorsviz., CCK2or Cholecystokinin2, Muscarinic M3and histamine H2at the level of the basolateral cellular membranes[17]. The basic pharmacophore of all the available PPIs is 2-pyridylmethylsulfinylbenzimidazole,i.e.,as they have the same basic structural framework and differ only in the nature of substituents present in the rings of pyridine and benzimidazole[19,20]. These PPIs interact with the gastric H,K-ATPase or “proton pump”, an α,β-heterodimeric enzyme located internally with respect to the cytoplasmic membrane of the resting parietal cells[19]. When the parietal cell is activated, the H,K-ATPase enzyme moves from the cytoplasm to the apical portion of its membrane[21]. Of the two subunits of the gastric H,K-ATPase, the α sub-unit has catalytic function, while the β sub-unit is fundamentally responsible for the proper functioning of this enzyme. It binds to the membrane, thereby preventing this enzyme from going back into the cytoplasm[22]. The function of this protein is to exchange cytoplasmic hydronium with potassium present in the gastric lumen, which is associated with the secretion of chlorine leading to the formation of HCl[23]. It is due to this activity that the PPIs come into play, irreversibly blocking the gastric H,K-ATPase and thus preventing acid secretion (Figure 1).
PPIs as a risk factor for COVID-19
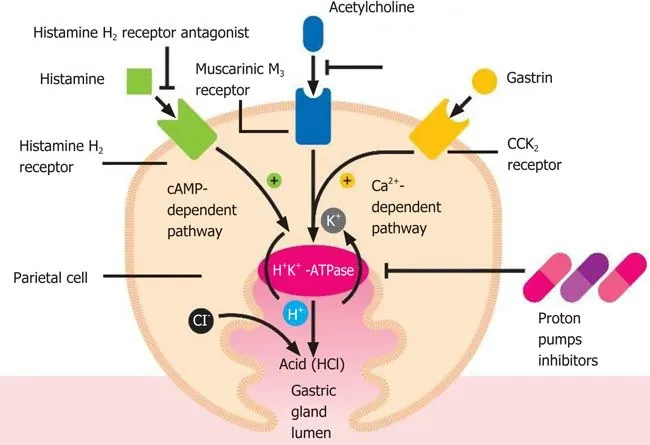
Figure 1 Parietal cell activation by histamine is followed by an intracellular increase in the cAMP-dependent protein kinase cascade(adenosine 3',5'-cyclic monophosphate), while gastrin and acetylcholine result in an intracellular increase in the calcium (Ca2+)-dependent cascade, all leading to insertion into the apical membrane and to activation of the H-K-ATPase. HCl: Hydrochloric acid.
In the last decade various reports have suggested that PPIs can favor the more severe course and mortality of illnesses in patients suffering from conditions, such as virosis,due to their side effects[24-26]. They include (1) inhibition of the enzymatic activity of DDAH (dimethylarginine dimethylaminohydrolase), with consequent cascade inhibition of nitric oxide synthase and a relative increase in inflammatory and thrombotic phenomena, with mainly cardiovascular sequelae; and (2) damage mainly to the renal tubules with the appearance of recurrent acute interstitial nephritis[24,27].PPIs are also able to inhibit neutrophil function, thereby compromising the immune response to infections, as neutrophils are at the initial forefront against external agents[28]. This dampened neutrophilic immune response can make the individual more prone to infections and the disease more severe, also in the context of SARSCoV-2 infection. Supporting this fact, several studies have shown that some viral infections (influenza virus, rotavirus, norovirus and, Middle East respiratory syndrome coronavirus infections) are more prevalent in PPIs users[29]. In a metaanalysis conducted by Eomet al[30],the use of these drugs was described to increase the risk of developing pneumonia, initially due to their gastric acid suppression followed by high digestive tract bacterial overgrowth. Ultimately the bacteria are translocated and colonize the lungs by microaspirations. However, this finding has only been partially confirmed by two other studies. A study led by Luxenburgeret al[31]found that GERD in 9 (14.5%) of 62 patients included in their study was an indication for PPIs treatment and another study by Almarioet al[32]found similar results in 109 (3.2%)of 3386 patients. The hypothesis that treatment with PPIs may increase the risk of secondary infections in hospitalized patients has not yet been fully confirmed.Luxenburgeret al[31]also demonstrated how the use of this therapy could increase the risk of contracting secondary infections and, subsequently, of developing acute respiratory distress syndrome. In a previous study carried out on SARS-CoV-1,Darnellet al[33]observed that a gastric pH ≤ 3 has the capacity to alter the infectivity of this virus, while further lower values of pH resulting from acid suppression by PPIs could not inactivate the virus. This could represent fundamental information, as SARSCoV-2 is very similar to SARS-CoV-1 and it has been proven that SARS-CoV-2 can cause infection in the gastrointestinal tract and in the airways[34]. Another opposite view on the use of PPIs during COVID-19 disease is that their administration may result in decreased absorption of several vitamins[35]. Moreover, PPIs seem to decrease vitamin C bioavailability and as a result leads to a reduction of vitamin C and its antioxidant form, ascorbic acid concentration in gastric juices. These effects were clinically reported by several researchers[36,37]. The above-mentioned observation could be correlated to recent findings demonstrating the efficacy of this vitamin both in prophylaxis and in severe forms of COVID-19. It has been shown by Feyaertset al[38]that low doses (0.5-2 g/d) of vitamin C could benefit patients if taken early in severe forms of COVID-19 and in severe later stages of the infection, while high dosages are able to reduce some mediators of inflammation, such as interleukin-6 and endothelin-1[38]. The benefits of using high-dose vitamin C in severe cases have also been shown by Hoanget al[39]. With regard to the role of magnesium and vitamin D in this infection and how are they related to PPIs, several studies have shown that hypomagnesemia is one of the side effects reported in patients with long-term use of PPIs[40]. Low levels of serum magnesium may be associated with a wide spectrum of symptoms, including fatigue, tetany, delirium, convulsions and arrhythmias[40]. Chronic use of PPIs has been shown to increase the risk of bone fractures, whose incidence is decreased by the use of vitamin D in low doses[40]. Magnesium (Mg2+) is a cation present in the human body,ranking second among the intracellular cations and fourth among extracellular cations,whose intestinal absorption occurs through two proteins located on the apical membrane of enterocytes, TRPM6 and TRMP7 (transient receptor potential melastatin family)[41,42]. These optimally work in an acidic intraluminal environment[43]. PPIs can decrease the activity of TRPM6, resulting in a reduction in magnesium absorption and in the onset of hypomagnesemia[44]. Several functions are attributed to this element,including the regulation of calcium and potassium ion channels[41]. The lipid-soluble vitamin D needs magnesium to assume its active form (1,25[OH]2D)[45]. More and more studies have shown a link between low levels of vitamin D and increased susceptibility to contracting the SARS-CoV-2 infection, and in the severity of the clinical course[46,47]. In a recent meta-analysis, which included six studies[31,48-52]with a total of 5884 hospitalized COVID-19 patients, Hariyantoet al[53]showed how the use of PPIs was significantly associated with an increased risk of severity and mortality in COVID-19 cases. Abyet al[54]also underlined the high rate of mortality in such patients(COVID-19 positive and PPIs users). This was a retrospective study conducted in the American population, but details of the data have not been published. The authors also highlighted how the association between mortality and PPIs intake tends to decrease by considering and by correcting some factors, such as age, cardiovascular,pulmonary and renal comorbidities. They argued that other causes associated with the use of PPIs may affect mortality[54].
MATERIALS AND METHODS
Literature search
A systematic computer-based search of available published articles was carried out in order to identify relevant studies on the association between PPIs and COVID-19(primary outcome) and the consequent related severity and mortality (secondary outcomes). Four electronic databases, including the MEDLINE/PubMed, the Cochrane Library, EMBASE, and Google Scholar were searched up to November 2020, for all relevant articles published in English on this topic. Also, relevant articles, published in other languages were taken into account, and information was taken from their English abstracts. The search terms were identified by means of controlled vocabularies, such as the National Library of Medicine’s MESH (Medical Subject Headings) and keywords. The MESH terms and keywords used were: “COVID-19”,“proton pump inhibitors”, ”PPIs”, “SARS-CoV-2”, “outcomes”, “severity” and“mortality”. All studies that contained material on this specific topic were considered.The inclusion criteria regarding the studies considered in our analysis were: metaanalyses, case-control, hospital-based case-control, population-based case-control as well as cohort-studies, while papers not published as full reports, such as conference abstracts, case reports, and editorials were excluded. Data extraction was performed by two authors (de Biase D and Hong W), in parallel and in independent mode. The other two authors (Pica R and Gallo CG), independently extracted and tabulated all relevant data from the selected studies. Lastly, the accuracy of data extrapolated was checked by Fiorino S, and when discrepancies concerning the results were found among the selected works, a consensus between all authors was mandatory. In order to avoid possible duplicates, we searched the first author’s name of the research, as well as the place and the period of the subjects’ enrolment. In the case of different versions of the same study, only the most recent was considered (Figure 2).
Statistical analysis
We summarized and collated all the available information from the final analysis of the studies, identifying five works from which the data were extracted and collected where possible the two secondary outcomes: severe events, defined as the incidence of patients needing organ support or intensive care, and mortality, namely death occurring during hospitalization. Studies were excluded from the analysis if either the outcomes of interest were not mentioned or it was impossible to calculate them from the published results. Similarly, other studies were ruled out if the sample size was less than ten and if focused on animals. We used forest plots to estimate the pooled effect sizes of each study (Table 1), with 95%CI, to provide a visual summary of the data. Heterogeneity among studies was evaluated by the CochranQ-test and I-squared index, withI2values above 75% as thresholds for high heterogeneity. When heterogeneity was present, we used a random-effects model (DerSimonian-Laird model). Otherwise, a fixed-effects model (Mantel-Haenszel) was used to compute the overall effects.

Table 1 Studies considering proton pump inhibitors use in patients with severe acute respiratory syndrome coronavirus 2
RESULTS
Available studies on this topic
To date, according to the available data in the literature, we identified 9 papers describing the use of PPIs in SARS-CoV-2 patients. The results are summarized in Table 1.
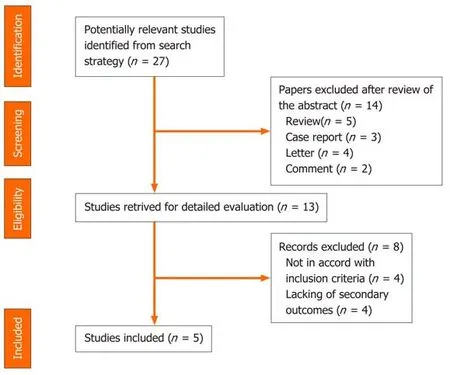
Figure 2 Summary of study identification and selection.
Taking into account the study by Hariyantoet al[53], we included data on the severity of the disease, specifically for 16 patients and 77 patients with shock, respectively, in the PPI-group and in the non-PPI-group[50], 18 patients and 30 patients had secondary infection, respectively, in the PPI-group and in the non-PPI-group[31]. A study by García-Menayaet al[55]included data related to severity and mortality of the disease,but they were calculated for patients with and without allergic conditions and for overall patients. It was observed that a history of PPIs intake was related to an increased risk of admission to the intensive care unit for all patients in the cohort (P=0.017)[55]. In some of these studies, it was possible to extrapolate the type of PPI used,the dosage and the posology. As in the study conducted by Luxenburgeret al[31], the percentage of patients using PPIs was calculated to be 83.9% (52 patients) for pantoprazole, 9.7% (6 patients) for esomeprazole and 6.5% (4 patients) for omeprazole.Almarioet al[32]found that people taking PPIs once daily [adjusted odds ratio (aOR)2.15; 95% confidence interval (CI): 1.90-2.44] or twice daily (aOR 3.67; 95%CI: 2.93-4.60)showed a statistically significant risk of testing positive compared to those who did not use such drugs. On the contrary, it emerged that the use of histamine-2 receptor antagonist (H2RA) was not associated with the risk of contracting the infection. In particular, 415 individuals (5.6%) were using H2RA once daily (aOR 0.85; 95%CI: 0.74-0.99) and 143 (12.4%) twice daily (aOR 0.86; 95%CI: 0.66-1.11)[32]. Leeet al[51]noted that people who had taken PPIs for less than 30 d before contracting the virus showed an increased risk of severity compared to those who had never taken these drugs. This outcome was further confirmed by a study on a subgroup of Korean individuals who were at that time under PPIs therapy[56,57].
Statistical analysis
A random-effect model was used both for disease severity and for mortality, due to the extensive heterogeneity occurring between studies (I2= 95.7% andI2= 89.0%respectively). The pooled incidence of severe events did not differ between patients with and without PPIs (OR 1.65, 95%CI: 0.62-4.35) (P= 0.314), or for mortality (OR 1.77, 95%CI: 0.62-5.03) (P= 0.286) (Figure 3 and 4).
DISCUSSION
Evidence suggesting PPIs do not worsen COVID-19
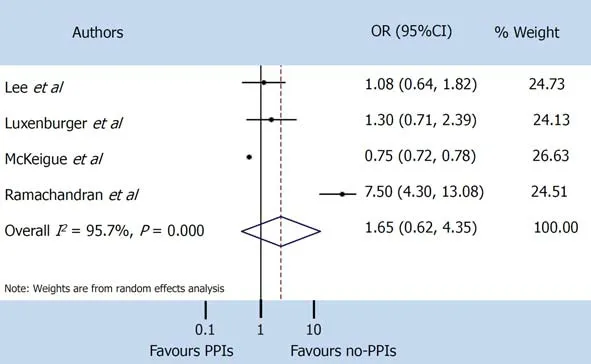
Figure 3 Severe events. PPIs: Proton pump inhibitors; CI: Confidence interval; OR: Odds ratio.
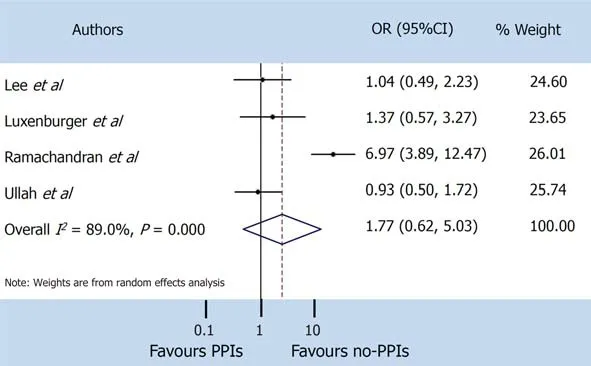
Figure 4 Mortality. PPIs: Proton pump inhibitors; CI: Confidence interval; OR: Odds ratio.
The literature, on the other hand, also provided a few studies supporting the beneficial effect of PPIs and their explicit action to counteract infections, including viral infections. It has been shown that the lowering of pH due to the gastric acid antisecretory action of PPIs can kill ingested microorganisms and avoid gastric bacterial overgrowth[58]. These two activities are useful both in preventing infections caused byClostridium difficileand in the development of spontaneous bacterial peritonitis[58,59].Taştemuret al[60]hypothesized that PPIs might play a role, both in prophylaxis and in the treatment of COVID-19. Rayet al[61], based on available scientific evidence,suggested the use of PPIs for therapeutic purposes in this virosis. It has been demonstrated that both pantoprazole and omeprazole can show a modulatory and an inhibitory effect on autophagy. This physiological mechanism occurs in almost all cells and is capable of eliminating damaged or malfunctioning proteins, thereby ensuring cytoplasmic homeostasis[62-64]. Autophagy may not work properly in various diseases,such as neurodegenerative disorders, metabolic diseases, cancer and infectious diseases. Host autophagy can help to maintain homeostasis of intracellular organelles through the elimination of cellular waste, and affect the activities of some viruses to promote their proliferation and spread, and this modality is also true for SARS-CoV-2[65]. The mechanisms involved in these events are not fully understood, nevertheless it has become apparent that as the intracellular pH rises, autophagy is also stimulated[66].For this reason, researchers are now considering particularly the use of pantoprazole as a possible molecule in the course of COVID-19 pharmacologic treatment[67]. PPIs are also able to regulate fibrogenesis, showing anti-fibrotic effects through the inhibition of molecules, such as fibronectin, collagen and matrix metalloproteinase enzymes[68].Pulmonary fibrosis, thought to be a long-term consequence of this viral infection is still debatable, many studies have correlated the use of PPIs with clinical improvement in patients suffering from idiopathic pulmonary fibrosis[69-72]. During the previous SARS pandemic, SARS-CoV-1 virus inactivation based on intracellular pH was analyzed in alkaline conditions[73]. Pagatet al[73]in their experiments, using Vero cells and sodium hydroxide (NaOH) as an alkalizing agent, observed that the virus was active at a pH of 11, while at pH 13-13.5 it was immediately inactivated[73]. Viruses can directly enter host cells by binding their specific surface proteins to precise receptors followed by endocytosis, as in the case of SARS-CoV-2, in which the protein is a spike (S)glycoprotein and the receptor is an ACE-2[74]. This protein is organized in two functional domains: S1 subunit containing the receptor-binding domain (RBD) to bind the target cell by adhering to the ACE-2 receptor and S2 subunit, which is a second phase allowing the virus to enter into the host cell by fusing the respective membranes[75]. The fusion between S2 and the ACE-2 receptor is preceded by the action of a specific cellular protease, serine protease transmembrane 2 (TMPRSS2)[76]. In this way, the entire virus can enter into cells after formation of the SARS-CoV-2/ACE-2 complex and the relative fusion between the viral membrane and the luminal membrane of the endosome. This favors the transfer of the relative RNA to the cytosol[77-79]. The entry process also requires the intervention of other factors, such as endosomal pH acidification[80]. Bayatiet al[81]found that the pathway of endocytic entry is represented by clathrin-mediated endocytosis (CME) (Figure 5). This discovery is very important, as one of the molecules used in treatment of patients affected by COVID-19 is chloroquine (CQ), which blocks CME by reducing the expression of the phosphatidylinositol binding clathrin assembly protein (PICALM)[82,83]. Aprotinin, a serine protease inhibitor, is used to treat pancreatitis and to reduce bleeding during surgery[84,85]. Its use was also assessed against influenza viruses, so much so that in Russia a formulation of this molecule in the form of an aerosol has been approved for the treatment of these viruses[86]. As previously observed by Shenet al[87], omeprazole can interfere with the acidification of lysosomes, a necessary requirement for viral replication. In a recent SARS-CoV-2-infected cell culture study, Bojkovaet al[88]discovered that the administration of omeprazole, at a therapeutic plasma concentration of 8 μmol/L, has been shown to increase the efficacy of aprotinin by 2.7 times and remdesivir by 10 times. The findings showed that the combination of these two molecules (aprotinin and remdesivir) or single use, supported by the intake of omeprazole, may represent one of the possible emerging treatments for COVID-19[89].A common feature of both endosomes and lysosomes is a low intraluminal pH[90].Vacuolar ATPase (V-ATPase), functionally identical to H+-ATPase, expressed on the surface of lysosomes and endosomes, is fundamental for the acidification of these two organelles[91,92](Figure 5). An increase in the pH of these organelles could inhibit viral replication[93]. Omeprazole and lansoprazole might inhibit V-ATPase in gastric mucosa and omeprazole has been shown to block this pump in rat models[94,95]. It has also been observed that both omeprazole and esomeprazole can change the location of the VATPase in cells[96]. The inhibition of V-ATPase by PPIs results in loss of the pH gradient across the membranes of lysosomes and endosomes, resulting in cytosolic acidification and in endolysosomal alkalinization, thus hindering viral replication and the resulting spread[97-99]. Further studies are needed to clarify these findings.
CONCLUSION
The role of PPIs in the progression of infection by SARS-CoV-2 is still not fully understood. Most of the published works underline how their use, both before and during hospitalization, can represent a risk factor in the course of the disease in terms of severity and mortality. Our review, based on the available studies, has some limitations: (1) most of the studies are observational or case-control studies; (2) there are no randomized controlled trials; (3) confounding aspects are present, such as statistically underpowered; and (4) statistically powered longitudinal studies in order to explore causality are lacking. These drugs possess well known anti-inflammatory and anti-fibrotic properties. If viral activity is regulated by intracellular pH, then what role can PPIs play? Thus, a paradoxical relationship between SARS-CoV-2 infection and PPIs has emerged. This review analyzed the possible effects of PPIs in COVID-19 patients with the aim of trying to understand if their administration may be deleterious or may interfere with the clinical course of the disease.
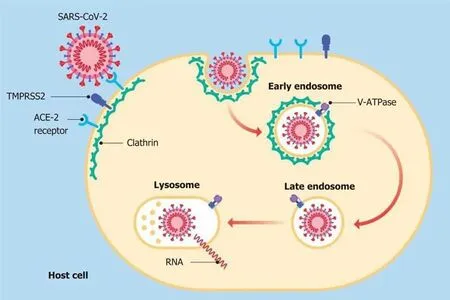
Figure 5 After the virus binds to its respective receptors, a "dimple" forms on the host cell membrane. This inward fold resulting from the initial endocytosis gives rise to the formation of a clathrin-coated vesicle (early endosome). Later, the vesicle loses this coating (late endosome) and merges with the lysosome for degradation. SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; ACE-2: Angiotensin converting enzyme 2; TMPRSS2: Serine protease transmembrane 2; V-ATPase: Vacuolar ATPase.
ARTICLE HIGHLIGHTS
Research background
Coronavirus disease 2019 (COVID-19) is an emerging infection increasingly found in clinical practice, and is now a pandemic. Conventional pharmacotherapies, including proton pump inhibitors, are commonly used in the treatment of such patients.However, their clinical efficacy, as well as the risk of severe adverse events, remain a problem in their clinical application. Based on the fact that the use of this class of drugs seems to show both negative, particularly in terms of severity and mortality,and positive effects on the course of this disease, it is neither realistic nor definitive to consider not using these medicinal products in these patients.
Research motivation
Several basic and clinical studies have reported a relationship between proton pump inhibitors (PPIs) use and COVID-19. As the results obtained so far regarding these drugs are both against their use and in favor of it, these discordant conclusions may create confusion among doctors regarding their treatment choices.
Research objectives
The main objectives of this review were to critically analyze the available data from studies accessible from the literature. Moreover, by following the extracted outcomes,future research is needed.
Research methods
We searched three electronic databases up to 30 November 2020, for studies that examined the safety and efficacy of the administration of PPIs in patients suffering from COVID-19. Two endpoints were considered: the severity and the mortality rates in this cohort. In order to calculate the estimated risks in this meta-analysis, fixed and random effects models were used.
Research results
The pooled incidence of severe events did not differ between patients with and without PPIs [odds ratio 1.65, 95% confidence interval (CI): 0.62-4.35] (P = 0.314), or for mortality (odds ratio 1.77, 95%CI: 0.62-5.03) (P = 0.286).
Research conclusions
This review analyzed the possible effects of PPIs in COVID-19 patients with the aim of trying to understand if their administration may be deleterious or may interfere with the clinical course of the disease. The role of PPIs in the progression of severe acute respiratory syndrome coronavirus 2 infection is still not fully understood.
Research perspectives
The paradoxical relationship between PPIs and COVID-19 should not be a confounding factor for doctors but should prompt them to proceed with further studies in this regard.
ACKNOWLEDGEMENTS
The Authors thank Dr. Varlotta A, Rome, Italy and Washington, United States, for her contribute in graphic illustration and Dr. de Vito S, Italian Medicines Agency, Rome,Italy, for the further English revision of the manuscript.
 World Journal of Clinical Cases2021年12期
World Journal of Clinical Cases2021年12期
- World Journal of Clinical Cases的其它文章
- Standardization of critical care management of non-critically ill patients with COVID-19
- Mediastinal lymphadenopathy in COVID-19: A review of literature
- Polycystic ovary syndrome: Pathways and mechanisms for possible increased susceptibility to COVID-19
- Circulating tumor cells with epithelial-mesenchymal transition markers as potential biomarkers for the diagnosis of lung cancer
- Clinicopathological features of superficial CD34-positive fibroblastic tumor
- Application of a rapid exchange extension catheter technique in type B2/C nonocclusive coronary intervention via a transradial approach
