Unresectable esophageal cancer treated with multiple chemotherapies in combination with chemoradiotherapy: A case report
Masahiro Yura, Kazuo Koyanagi, Asuka Hara, Keita Hayashi, Yuki Tajima, Yasushi Kaneko, Hiroto Fujisaki,Akira Hirata, Kiminori Takano, Kumiko Hongo, Kikuo Yo, Kimiyasu Yoneyama, Yoshifumi Tamai, Reiko Dehari, Motohito Nakagawa
Masahiro Yura, Asuka Hara, Keita Hayashi, Yuki Tajima, Yasushi Kaneko, Hiroto Fujisaki, Akira Hirata, Kiminori Takano, Kumiko Hongo, Kikuo Yo, Kimiyasu Yoneyama, Motohito Nakagawa,Department of Surgery, Hiratsuka City Hospital, Kanagawa 2540065, Japan
Kazuo Koyanagi, Department of Gastroenterological Surgery, Tokai University School of Medicine, Isehara 2591193, Japan
Yoshifumi Tamai, Department of Radiology, Hiratsuka City Hospital, Kanagawa 2540065,Japan
Reiko Dehari, Department of Surgical Pathology, Hiratsuka City Hospital, Kanagawa 2540065,Japan
Abstract BACKGROUND Definitive chemoradiotherapy (dCRT) using cisplatin plus 5fluorouracil (CF) with radiation is considered the standard treatment for unresectable locally advanced T4 esophageal squamous cell carcinoma (ESCC). Recently, induction chemotherapy has received attention as an effective treatment strategy.CASE SUMMARY We report a successful case of a 59-year-old female with unresectable locally advanced T4 ESCC treated by two additional courses of chemotherapy with CF after induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF)followed by dCRT. Initial esophagogastroduodenoscopy (EGD) detected a type 2 advanced lesion located on the middle part of the esophagus, with stenosis.Computed tomography detected the primary tumor with suspected invasion of the left bronchus and 90° of direct contact with the aorta, and upper mediastinal lymph node metastasis. Pathological findings from biopsy revealed squamous cell carcinoma. We initially performed induction chemotherapy using three courses of DCF, but the lesion was still evaluated unresectable after DCF chemotherapy.Therefore, we subsequently performed dCRT treatment (CF and radiation). After dCRT, prominent reduction of the primary tumor was recognized but a residual tumor with ulceration was detected by EGD. Since the patient had some surgical risk, we performed two additional courses of CF and achieved a clinically complete response. After 14 mo from last administration of CF chemotherapy,recurrence has not been detected by computed tomography and EGD, and biopsy from the scar formation has revealed no cancer cells.CONCLUSION We report successful case with tumor remnants even after DCF and subsequent dCRT, for whom a complete response was finally achieved with two additional courses of CF chemotherapy. Additional CF chemotherapy could be one radical treatment option for residual ESCC after treatment with induction DCF followed by dCRT to avoid salvage surgery, especially for high-risk patients.
Key Words: Unresectable esophageal cancer; Induction docetaxel, cisplatin and fluorouracil; Chemoradiotherapy; Complete response; Additional chemotherapy; Case report
INTRODUCTION
Esophageal cancer is the sixth most common cause of cancer deaths worldwide,according to statistics presented in 2018[1]. Esophageal squamous cell carcinoma(ESCC) often invades adjacent vital organs such as the trachea, bronchus, lungs and aorta. Approximately 15% of incidents of ESCC are clinically diagnosed at the T4 stage[2]. Standard treatment for unresectable ESCC without distant metastasis is definitive chemoradiotherapy (dCRT) with cisplatin (CDDP) plus 5fluorouracil [(5FU),combination denoted cisplatin plus 5-FU (CF)], using a total irradiation dose of 50.4 or 60 Gy[3,4]. A study by the Japan Clinical Oncology Group (JCOG9516), a multicenter phase II trial, demonstrated the efficacy of dCRT using CF for unresectable ESCC and reported a complete response rate (CR) of 15% and a 3-year survival rate of 23%[5]. On the other hand, among patients who fail to achieve CR, salvage esophagectomy is the only modality left that offers a chance for long-term survival[6,7]. However, previous studies have reported that salvage surgery after dCRT is associated with a high incidence of surgery-related death (8%-10.2%)[6,8,9]. In addition, not only is the R0 resection rate after dCRT not high but also the prognosis for patients who have failed R0 resection is unfavorable[6,7,10,11].
Recently, there has been hope that chemotherapy combined with docetaxel (DTX) is a useful treatment for ESCC. Neoadjuvant chemotherapy with DTX, CDDP and 5FU(DCF) has demonstrated potential, with a pathological CR rate of 17% for resectable stage II/III ESCC[12]. Furthermore, a prospective multicenter phase I/II study revealed that induction chemotherapy with DCF followed by chemoradiotherapy showed efficacy for unresectable locally advanced ESCC[13]. In the present case, induction chemotherapy with DCF followed by dCRT did not result in a CR for an initially unresectable locally advanced T4 ESCC, but a CR was finally achieved with two additional courses of CF chemotherapy. No recurrence has been observed for more than 14 mo while maintaining the quality of life after the last chemotherapy.Combination of these treatment could be a useful treatment option for unresectable T4 ESCC.
CASE PRESENTATION
Chief complaints
A 59-year-old female had symptoms of dysphagia.
History of present illness
The patient complained of dysphagia for a month and visited a former doctor.Esophagogastroduodenoscopy (EGD) was performed and a tumor suspected of esophageal cancer was found in the middle part of the esophagus. After that, she was introduced to our hospital for the purpose of detailed medical examination and treatment.
History of past illness
The patient had a history of hypertension, was a hepatitis B virus carrier, and had experienced a brain stem hemorrhage with sensory paralysis. Her surgical history included total abdominal hysterectomy, bilateral salpingo-oophorectomy, and periarterial lymph node dissection to treat endometrial cancer (endometrial adenocarcinoma, pT1bN0M0) 7 years previously. Six courses of adjuvant chemotherapy(paclitaxel + carboplatin) were prescribed after surgery. Six years ago, a squamous cell carcinoma of the oral tongue (pT1, ly0, v0) was partially resected. She is also a smoker(Brinkman index 800; 20 cigarettes/day × 40 years).
Personal and family history
No family history to note.
Physical examination
There were no notable abnormal findings related to esophageal cancer on physical examination, and surgical scars during total hysterectomy were found in the lower abdomen.
Laboratory examinations
Laboratory data revealed a hemoglobin level of 12.9 g/dL, white blood cell count of 5700 cells/µL and platelet count of 339 × 103/μL. The creatinine level was 0.54 mg/dL,total bilirubin level 0.4 mg/dL, direct bilirubin level 0.1 mg/dL, glutamic oxaloacetic transaminase level 15 IU/L, glutamic pyruvate transaminase level 7 IU/L, albumin level 3.8 g/dL, and hemoglobin A1c level 6.1%. Tumor marker levels of the carcinoembryonic antigen was 9.2 ng/mL and squamous cell carcinoma antigen was 7.5 ng/mL.
Echocardiography showed almost normal cardiac function with moderate tricuspid regurgitation. Pulmonary function examination revealed vital capacity, 102.0% of predicted; forced expiratory volume in 1 s, 79.1% of predicted; forced expiratory volume in 1 s, 1.78 L.
Imaging examinations
EGD was performed and detected an advanced type 2 lesion on the middle part of the esophagus, with stenosis (Figure 1A). Computed tomography (CT) detected the primary tumor (40.7 mm × 30.7 mm) with suspected invasion of the main left bronchus(Figure 2A), 90° of direct contact with the aorta (Figure 3A). The upper mediastinal lymph node was swollen (10.16 mm × 8.02 mm) and was suspected to be metastasis to the right recurrent nerve lymph node (#106recR, Figure 4A), and no distant metastasis was detected.
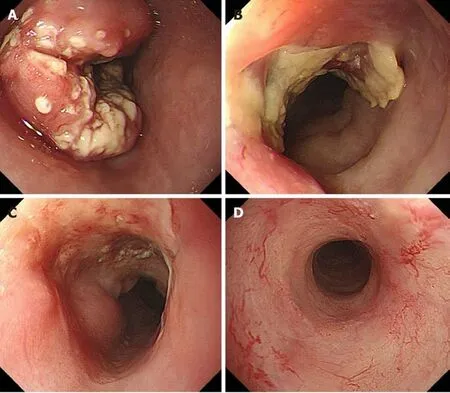
Figure 1 Endoscopic findings. A: Initial endoscopic findings detected type 2 tumor located on the middle part of the esophagus; B: After 3 courses of induction docetaxel, cisplatin and fluorouracil chemotherapy. Primary tumor volume decreased; C: After subsequent definitive chemoradiotherapy.Esophagogastroduodenoscopy findings showed a prominent reduction of the primary tumor, with ulceration; D: Fourteen months after additional 2 courses cisplatin plus 5fluorouracil chemotherapy. Primary lesion maintained clinically and pathologically complete response.
FINAL DIAGNOSIS
Biopsies were taken, and the histological examination led to a diagnosis of a squamous cell carcinoma. The clinical diagnosis was esophageal cancer Mt circ cType2 cT4bN1(#106recR) M0 cStageIVA according to the Union for International Cancer Control TNM Classification of Malignant Tumors, eighth edition[14]. The lymph node station was defined according to the Japanese Classification of Esophageal Cancer, 11thEdition[15].
TREATMENT
First, a gastrostomy was created using an endoscope for the possibility of total occlusion when the tumor grew.
Induction chemotherapy with DCF
Five days after creating the gastrostomy, induction chemotherapy consisted of intravenous administration of DTX on day 1; infusion of CDDP on day 1; and continuous intravenous administration of 5FU on days 1-5. Oral prophylactic antibiotics with ciprofloxacin (200 mg × 3/d) were administered on days 5-15. This regimen was repeated for three courses. The first course was administered with a full dose of DCF (DTX: 70 mg/m2, CDDP: 70 mg/m2, 5FU: 750 mg/m2); the patient experienced grade 4 neutropenia, for which we used granulocyte-colony stimulating factor. The second and third courses of DCF were administered with the −1 Level of dose (DTX: 55 mg/m2, CDDP: 55 mg/m2, 5FU: 600 mg/m2). After three courses of DCF, the CT findings showed shrinkage of the tumor size (36.6 mm × 30.5 mm) but invasion of the left bronchus (Figure 2B) and aorta (Figure 3B) was still suspected.Thus, the lesion was still evaluated as unresectable. The #106recR lymph node had slightly shrunk (8.01 mm × 6.69 mm; Figure 4B). EGD findings also showed a decrease in the primary tumor volume (Figure 1B), and evaluation of the clinical efficacy of the chemotherapy was defined to be stable disease according to the Response Evaluation Criteria in Solid Tumors criteria version 1.0.
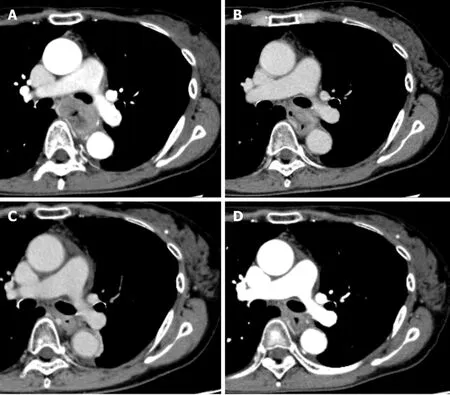
Figure 2 Computed tomography findings showing level of left bronchus. A: Initial computed tomography findings suspected to invasion to the left bronchus; B: After 3 courses of induction docetaxel, cisplatin and fluorouracil. Shrinkage of the tumor size was recognized but invasion of the left bronchus was still suspected; C: After subsequent definitive chemoradiotherapy. Primary tumor volume decreased; D: Fourteen months after additional 2 courses of cisplatin plus 5fluorouracil chemotherapy. Primary tumor has maintained shrinkage.
Definitive chemoradiotherapy with CF
After induction DCF therapy, dCRT with CF was performed, consisting of concurrently administered CDDP on days 1 and 28, and 5FU on days 1-4 and 28-31.The first and second courses of CF were administered with 55 mg/m2of CDDP and 550 mg/m2of 5FU. Radiotherapy consisted of 60 Gy with a daily dose of 2 Gy delivered with 10 MV Xrays. Three-dimensional treatment planning with a CT stimulator was used. The pretreatment gross tumor volume was determinedviaCT and EGD. The clinical target volume included the gross tumor volume and a craniocaudal margin of 2.4 cm around the primary tumor. Swollen lymph node(#106recR) was designated as gross tumor volume node. Prophylactic irradiation of mediastinal lymph node areas was performed (Figure 5). The maximum irradiation of the spinal cord was 43 Gy, and the volume of lungs exposed to 20 Gy was set at 17.1%.In the second course of CF, grade 4 neutropenia and grade 3 anorexia were observed.After dCRT, EGD findings showed a prominent reduction of the primary tumor, with ulceration (Figure 1C). The lesion was not stained by iodine staining, and the pink color sign was positive. CT findings showed shrinkage of the primary tumor (23.5 mm× 25.1 mm) (Figure 2C and Figure 3C), which was evaluated to be a partial response.The #106recR lymph node did not change in size (8.31 mm × 7.59 mm; Figure 4C). CT also revealed pulmonary embolism and deep vein thrombosis of the left popliteal vein.We began administering direct oral anticoagulant.
Additional CF chemotherapy
Considering of the patient’s status and of complication risks of salvage surgery after dCRT, we performed two additional courses of CF treatment, consisting of concurrent administration of CDDP on days 1 and 28, and of 5FU on days 1-5 and 28-32. The first course of CF was administered 4 wk after last irradiation with 50 mg/m2of CDDP and 500 mg/m2of 5FU, while the second course was administered with 40 mg/m2and 400 mg/m2, respectively. After this additional CF, CT findings revealed tumor shrinkage and wall thickening, which was considered to be the effect of radiotherapy.
#106recR lymph node had significantly shrunk and not be detected (Figure 4D).EGD findings revealed a scarred and flattened primary lesion and biopsy found no cancer cells. Positron emission tomography showed no accumulation and viability of the primary tumor or any metastasis were not indicated.

Figure 3 Computerized tomography findings showing level of aorta. A: Initial computed tomography findings suspected to 90° of direct contact with the aorta, which defined as invasion to the aorta; B: After 3 courses of induction docetaxel, cisplatin and fluorouracil. Shrinkage of the tumor size was recognized but invasion of the aorta was still suspected; C: After subsequent definitive chemoradiotherapy. Primary tumor volume decreased; D: Fourteen months after additional 2 courses of cisplatin plus 5fluorouracil chemotherapy. Primary tumor has maintained shrinkage.
Adverse events
Our present case experienced grade 4 neutropenia in the first course of full-dose induction DCF and the second course of dCRT and grade 3 thromboembolic event.Adverse events was defined according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0[16].
OUTCOME AND FOLLOW-UP
Six months later, CT showed no remarkable change, and gadolinium scintigraphy did not produce any findings suggestive of malignant accumulation. Fourteen months after the last chemotherapy, CT and EGD showed no recurrence and maintained clinical CR (Figures 1D, 2D and 3D).
Pathological examination and classification
Before treatment, biopsy from the primary tumor revealed squamous cell carcinoma(Figure 6A). Fourteen months after the last chemotherapy, biopsy from the scarred primary lesion revealed no cancer cells (Figure 6B).
DISCUSSION
In the present study, we have described the successful case of an unresectable locally advanced T4 ESCC treated by additional CF chemotherapy after induction DCF followed by dCRT. The most characteristic point of this case is that additional CF chemotherapy can be an alternative radical treatment of residual ESCC for salvage surgery after induction DCF followed by dCRT, especially in high-risk patients.
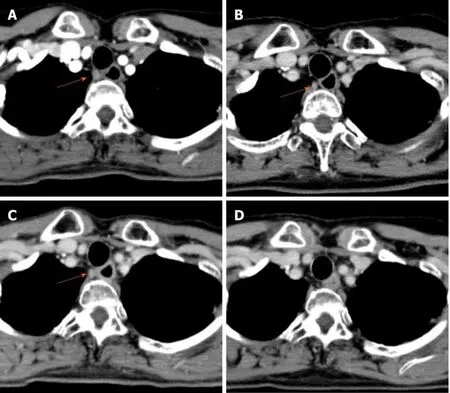
Figure 4 Computed tomography findings showing #106recR lymph node. A: Initial computed tomography findings suspected swollen lymph node(10.16 mm × 8.02 mm); B: After 3 courses of induction docetaxel, cisplatin and fluorouracil. Slightly shrinkage of the lymph node size (8.01 mm × 6.69 mm) was recognized; C: After subsequent definitive chemoradiotherapy. #106recR lymph node did not change in size (8.31 mm × 7.59 mm); D: After additional 2 courses of cisplatin plus 5fluorouracil chemotherapy. #106recR lymph node could not be detected (Orange arrow; 106recR lymph node).
The standard treatment for unresectable ESCC without distant metastasis is dCRT with CF, using a total irradiation dose of 50.4 or 60 Gy[3,4]. Previous multicenter clinical trials of JCOG (JCOG9516, JCOG0303) with concurrent chemoradiotherapy with CF in patients with ESCC with cT4 tumor or M1 Lymph node showed a CR rate of 1.4%-15%[5,17]. Recently, two high-volume centers reported that the CR rate after dCRT was 22.4%-29%[7,11]. While, salvage esophagectomy may be chosen as the treatment of residual or recurrent tumors after dCRT if the tumor is resectable. However, salvage esophagectomy after dCRT is associated with a high incidence rate of severe postoperative complications (22.9%-29%) and surgery-related death (8.6%-10%)[6,11].Although some studies conversely described that the surgery-related mortality rate was zero[7,10], this discrepancy may be due to selection bias; thus, the indication for salvage surgery needs to be carefully determined. The Esophageal Cancer Practice Guidelines 2017, edited by the Japan Esophageal Society, weakly recommended against performing salvage surgery for T4 ESCC patients showing residual disease after dCRT[3,4].
Bookaet al[7]reported from their experience of salvage surgery for T4 ESCC that the 5-year overall survival (OS) following a CR after dCRT, salvage esophagectomy, and chemotherapy or best supportive care groups were 83.0%, 51.6%, and 1.3%,respectively. In addition, in the salvage group, R1/R2 resection was a significantly poor prognostic factor, for which the 3-year OS was 0%. Okamuraet al[6]reported their experience regarding the role of salvage surgery after dCRT in 35 patients; the 5-year OS was 5.7% and all patients with incomplete resection died within 2 years.Considering the results of these previous studies, incomplete resection in salvage surgery not only impairs quality of life but also fails to improve the prognosis.Therefore, we should select good candidates for salvage surgery after dCRT. Several studies have described the risk factors for incomplete resection: (1) Patients with a tumor in the upper thoracic esophagus were up to 50% more likely to undergo incomplete (R1/R2) resection[7]; and (2) Patients with a tumor evaluated T3 or deeper after dCRT were also up to 55.2% likely to undergo incomplete resection[6]. In our present case, the tumor was located on middle part of the esophagus and remnant tumor was estimated less than T2 after dCRT, therefore salvage surgery may have been the one option if the patient was adequately tolerated for surgery.
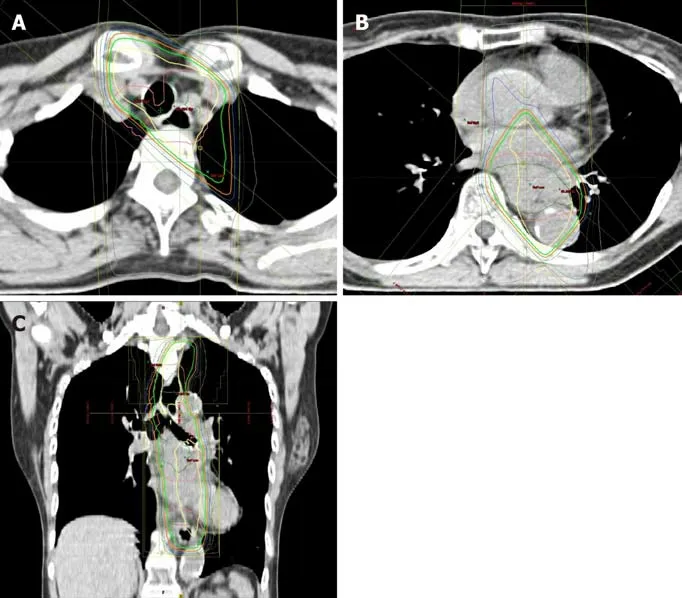
Figure 5 Chemoradiation planning. A: Swollen lymph node (#106recR) was designated as gross tumor volume node; B and C: The clinical target volume included the gross tumor volume and a craniocaudal margin of 2.4 cm around the primary tumor.
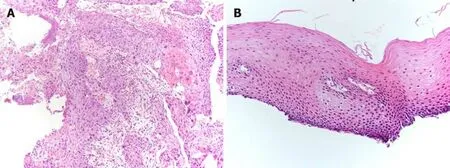
Figure 6 Pathological examination, biopsy from primary lesion. A: Initial biopsy from primary tumor revealed squamous cell carcinoma (× 10). A proliferation of large atypical cells with swollen deep-stained nuclei was observed. A squamous cell carcinoma with a tendency toward keratinization, intercellular bridges, necrosis, and large atypical cells with bizarre nuclei were evident; B: Fourteen months after additional 2 courses of cisplatin plus 5fluorouracil chemotherapy,primary lesion has maintained pathologically negative for cancer (× 20). There is a small infiltration of inflammatory cells, but no obvious evidence of malignant cells.
Recently, induction chemotherapy has become another important treatment strategy for unresectable ESCC. Satakeet al[13]conducted a phase I/II trial of induction chemotherapy with DCF followed by dCRT using CF with 60 Gy radiotherapy in patients with unresectable locally advanced ESCC. The CR rate was 39.4% and the 1-and 3-year OS rates were 78.8% and 40.4%, respectively, and 8 out of 33 patients underwent salvage surgery, with 5 patients achieving curative resection (62.5%; 5/8).Yokotaet al[18]conducted a phase II trial of induction chemotherapy using DCF for patients with unresectable locally advanced ESCC. After induction chemotherapy,patients whose cancer was converted to being resectable received conversion surgery,and patients whose cancer remained unresectable received dCRT. The overall rate of R0 resection was 39.6% and the 1-year OS rate was 67.9% across all patients. Four of 17 patients (23.5%) who completed dCRT achieved a CR without conversion surgery.
In our present case, the lesion was evaluated to be stable disease and unresectable after three courses of induction DCF. However, after dCRT, prominent tumor volume reduction was observed and evaluated to be partial response. Satakeet al[13]reported in their phase II study that of 13 patients who had no response after induction DCF, 2 achieved a clinical CR and 4 achieved a clinical partial response after subsequent dCRT. Thus, a shift in strategy to dCRT might also be effective for non-responders to induction DCF chemotherapy.
The most characteristic treatment strategy in this case is that we chose additional CF chemotherapy, not salvage surgery, for residual ESCC after induction DCF followed by dCRT, considering the patient’s status such as histories of endometrial cancer,tongue cancer, brainstem hemorrhage and hepatitis B virus. Although it is unknown whether additional CF is superior to salvage surgery, it was reported that salvage esophagectomy patients had a significantly worse OS rate than CR patients without surgery[7]. Thus, avoiding salvage surgery by combining additional chemotherapy after chemoradiation to achieve a CR may be useful treatment strategy. In fact, in this case, our patient has maintained a good quality of life and a CR for more than 14 mo.There is also no clear evidence of efficacy in additional chemotherapy after definitive chemoradiotherapy, however previous large-scale trials of dCRT for esophageal cancer included two courses of additional chemotherapy[19-22], therefore the Japanese guideline weakly recommend additional chemotherapy after dCRT[3]. We think that even after induction DCF and subsequent dCRT, additional CF could be useful treatment option to achieve definitive cure, especially for high-risk patients of salvage surgery.
Some limitations of this study should be discussed. First, we initially chose induction DCF chemotherapy, however, it is unclear whether this is superior to dCRT,because there is a lack of direct comparative trials. Currently, the JCOG is conducting a phase III trial (JCOG1510) comparing induction chemotherapy using DCF followed by conversion surgery with dCRT in patients with unresectable locally advanced ESCC(UMIN000031165). Second, the observation period after the additional CF chemotherapy was only 14 mo, thus, we should continue to check for recurrence during additional follow-up visits.
CONCLUSION
We report a successful case with tumor remnants even after docetaxel, cisplatin and fluorouracil followed by definitive chemoradiotherapy, for whom a complete response rate was finally achieved with two additional courses of CF chemotherapy. Especially for high-risk patients, additional CF chemotherapy could be one radical treatment option for residual esophageal squamous cell carcinoma after treatment with induction docetaxel, cisplatin and fluorouracil followed by definitive chemoradiotherapy to avoid salvage surgery.
 World Journal of Clinical Cases2021年12期
World Journal of Clinical Cases2021年12期
- World Journal of Clinical Cases的其它文章
- Standardization of critical care management of non-critically ill patients with COVID-19
- Mediastinal lymphadenopathy in COVID-19: A review of literature
- Polycystic ovary syndrome: Pathways and mechanisms for possible increased susceptibility to COVID-19
- Circulating tumor cells with epithelial-mesenchymal transition markers as potential biomarkers for the diagnosis of lung cancer
- Clinicopathological features of superficial CD34-positive fibroblastic tumor
- Application of a rapid exchange extension catheter technique in type B2/C nonocclusive coronary intervention via a transradial approach
