Organic perovskites
Chuantian Zuo and Liming Ding
Center for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China
The family of perovskite materials derived from the crystal structure of the mineral CaTiO3, which was first reported in 1839[1]. Then a series of materials with perovskite structure were discovered. Most perovskite materials have the ABX3stoichiometry, in which A is monovalent or divalent cation, B is divalent or tetravalent cation, and X is monovalent or divalent anion. Perovskite materials play important roles in various functional devices due to their unique optical,electronic and optoelectronic properties. Inorganic perovskites such as BaTiO3and PbTiO3have been widely used in ferroelectric and piezoelectric devices, actuators, capacitors,and nonlinear optical devices[2]. The emergence of organicinorganic hybrid perovskites such as CH3NH3PbI3triggers a new wave of research about perovskite-based devices, such as solar cells, light-emitting diodes (LEDs), photodetectors,lasers, and field-effect transistors[3,4]. Motivated by the outstanding performance of perovskite materials, a variety of perovskite derivatives were developed, such as two-dimensional perovskites, double perovskites, and vacancy-ordered perovskites[5,6].
Though there are many different perovskites, almost all of them contain metal cations, such as Pb2+, Ba2+, and Ti4+.The metal-containing perovskites can not fully degrade, making it hard to dispose of the waste. Moreover, some metal cations are highly toxic, limiting their application, especially the application in wearable devices, which are in fast-growing demand. Metal-free perovskites, composed of organic components, can solve the above issue[7]. Organic perovskites were thought to be inexistent before the report of C4N2H12·NH4Cl3·H2O in 2002[8]. However, no new organic perovskites was found in the next 15 years.
Xiong et al. reported a series of metal-free perovskites,which have a formula of A(NH4)X3(A: divalent organic cation;X: Cl-, Br-, or I-)[2]. The metal-free perovskites have similar crystal structure with the traditional perovskites, but there is clear difference about the relative position of the ions in the lattice. The standard perovskites take the structure shown in Fig. 1(a), in which six anions (X) form an octahedron, the highly-charged cation locates at the center of the octahedron. In another type of perovskites, the octahedron forms by six cations (Fig. 1(b)), and the A and B sites are occupied by anions, e.g. Li3OBr. This type of perovskites is called “antiperovskite”[6]. For the metal-free perovskites reported by Xiong et al., the A-site cation has more charges than the B-site cation (Fig. 1(c)), which are different from both the standard perovskites and the antiperovskites. The metal-free perovskites show similar structure with the inorganic perovskite BaLiF3, called “inverse perovskite”[6].
Organic perovskites can be developed due to the chemical diversity of the organic cations. Xiong et al. studied the ferroelectric property of MDABCO-NH4I3, which showed a ferroelectric phase at 293 K (Fig. 1(d)). The ferroelectric phase transformed into paraelectric phase at 448 K, and this phase-transition temperature is higher than that of the inorganic perovskite BaTiO3(390 K). What’s more, MDABCO-NH4I3has a large spontaneous polarization (Ps= 22 μC/cm2), close to that of BaTiO3(Ps= 26 μC/cm2). These results suggest that organic perovskites have potential use in ferroelectric devices instead of inorganic perovskites. The organic perovskites can also be used in X-ray imaging. Zhao et al. made X-ray detectors by using large single crystals of DABCO-NH4Br3[9]. The detectors presented a good sensitivity of 173 μC Gyair-1cm-2. Organic perovskites used in solar cells and LEDs were not reported due to their large bandgap (> 5 eV).
Compared with the inorganic perovskites, the organic perovskites have the advantage of low-temperature processing,which avoids the fracture caused by high-temperature annealing. The organic perovskites can decompose to non-toxic products, making them suitable for biocompatible and implantable devices like biosensors and bioelectronics. More organic perovskites and new applications may be developed in the future due to the chemical diversity of organic materials.
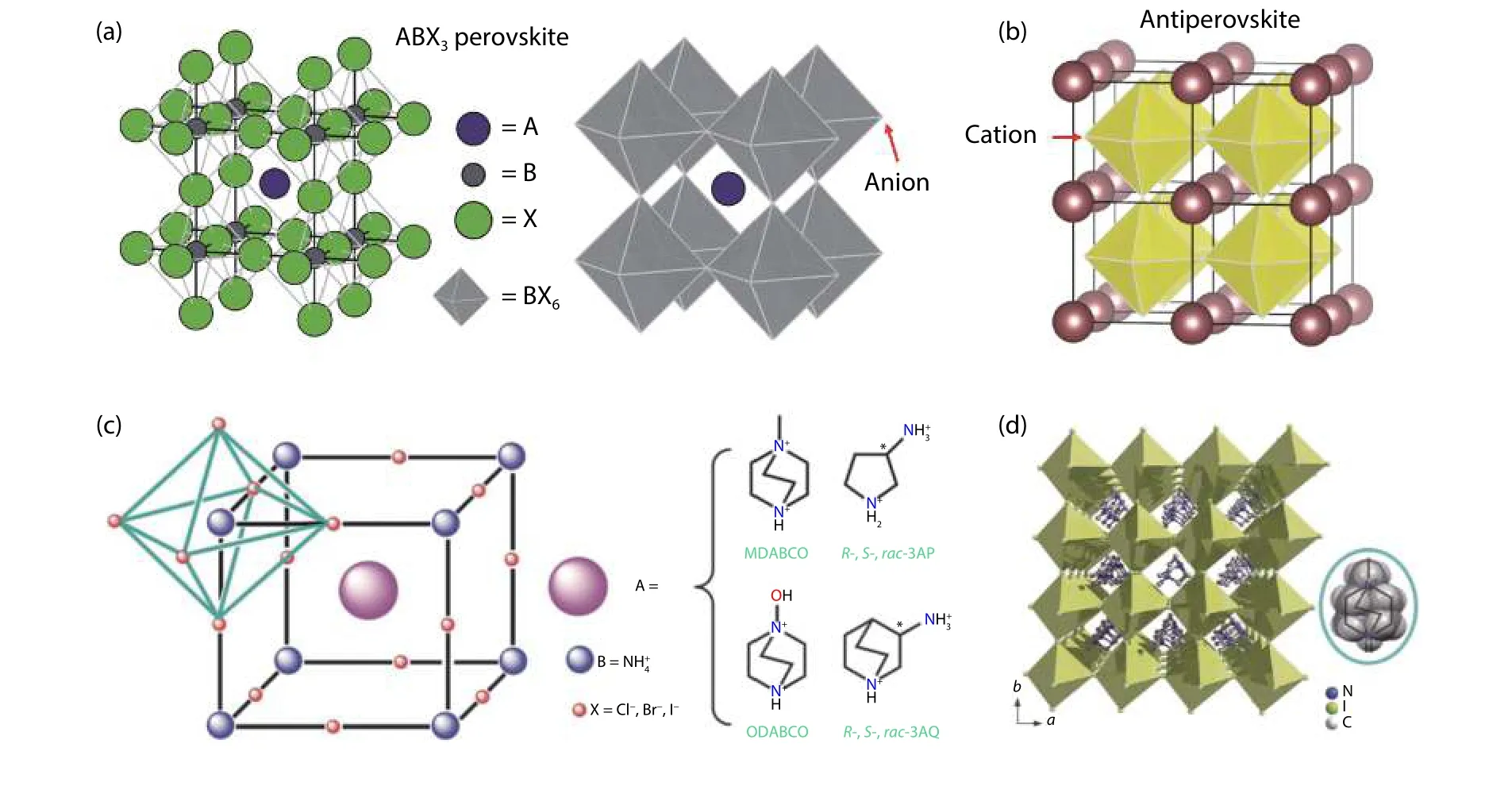
Fig. 1. (Color online) (a) Structure of the standard perovskite. (b) Antiperovskite. Reproduced with permission[6], Copyright 2020, American Chemical Society. (c) Structure of the organic perovskites A(NH4)X3. (d) Ferroelectric phase of MDABCO-NH4I3. Reproduced with permission[2], Copyright 2018, American Association for the Advancement of Science.
Acknowledgements
We thank the National Key Research and Development Program of China (2017YFA0206600) and the National Natural Science Foundation of China (51773045, 21772030, 51922032 and 21961160720) for financial support.
 Journal of Semiconductors2021年4期
Journal of Semiconductors2021年4期
- Journal of Semiconductors的其它文章
- Beyond the 100 Gbaud directly modulated laser for short reach applications
- Orthogonally polarized RF optical single sideband generation with integrated ring resonators
- P hotonic devices based on thin-film lithium niobate on insulator
- Recent progress in integrated electro-optic frequency comb generation
- Monolithic DWDM source with precise channel spacing
- L atest advances in high-performance light sources and optical amplifiers on silicon
