Aloin attenuates chronic constriction injury-induced neuropathic pain in rats by inhibiting inflammatory cytokines and oxidative stress
Aarti S. Kale, Avinash R. Wadkar, Umesh B. Mahajan, Lalit A. Birari, Sateesh Belemkar, Sameer N. Goyal, Shreesh Ojha, Sanjay J. Surana, Chandragouda R. Patil✉, Kalpesh R. Patil✉
1Department of Pharmacology, R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur- 425405, Dist. Dhule, Maharashtra, India
2School of Pharmacy & Technology Management, SVKM's NMIMS, MPTP, Shirpur-425405, Dist-Dhule, Maharashtra, India
3Shri Vile Parle Kelavani Mandal's Institute of Pharmacy, Dhule -424001, Maharashtra, India
4Department of Pharmacology and Therapeutics, College of Medicine and Health Sciences, United Arab Emirates University, P.O. Box 17666, Al Ain, Abu Dhabi, UAE
5Department of Pharmacognosy, R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur- 425405, Dist. Dhule, Maharashtra, India
ABSTRACT
KEYWORDS: Aloin; Chronic constriction injury; Antioxidant; Neuropathic pain
1. Introduction
Neuropathic pain (NP) is an important global chronic health issue. NP is characterized by injury to the somatosensory neurons followed by the peripheral and central nervous systems. Central nervous system sensitization develops allodynia, hyperalgesia, and poor proprioception[1]. According to an exploratory clinical study, the prevalence of NP in the United States was 9.8%, whereas, the selfreporting prevalence of NP was found to be 12.4%[2]. Epidemiological studies from several regions of India stated that the prevalence of NP ranged from 5 (0.05%) to 2 400 (24%) per 10 000 population[3].
The peripheral and central NP is associated with multiple mechanisms including activation of inflammatory pathways, increased release of excitatory neurotransmitters, modulation of descending inhibitory pathways, and induction of oxidative stress[4,5]. The pro-inflammatory cytokines, interleukins, and oxidative stress produce nerve injury-induced NP and sensitization of nociceptive receptors[5]. Pharmacotherapy of NP consists of gabapentin, calcium channel modulators, tricyclic antidepressants, serotonin noradrenalin reuptake inhibitors, opioids, and local anesthetics. Among these drugs, gabapentin is a mostly prescribed anticonvulsant drug for the management of NP[5,6]. Existing therapies for NP are inadequate and provide limited relief from the NP. These treatments are often associated with dose-dependent adversities[4,7]. These disadvantages make it necessary to explore novel, effective, and safe anti-neuropathic therapies[8].
Aloin or barbaloin is a C-glycoside anthraquinone derivative. It has anti-inflammatory[9], antioxidant[10], anticancer and antiproliferative activities[11]. Aloin blocks the inducible nitric oxide synthase enzyme and reduces the cyclooxygenase-2 (COX-2) mRNA expression in murine macrophages leading to suppression of the inflammatory response[9]. A recent study reported that aloin suppresses lipopolysaccharide-induced reactive oxygen species (ROS) production and JAK1-STAT1/3 activation in RAW 264.7 macrophages[12].
Extensive involvement of inflammatory signaling and oxidative stress in the initiation and promotion of NP suggests the possibility of aloin in treating NP. Preclinical and clinical findings reported that several drugs including minocycline and atorvastatin diminished the chronic constriction injury (CCI)-induced NP[13,14]. Such attenuation of neuropathy has been attributed to the antioxidant property and antiinflammatory actions of these drugs. Even a proton pump inhibitor, omeprazole, is reported to exert antineuropathic effects through its anti-inflammatory and antioxidant effects[5]. Considering the antiinflammatory and antioxidant effects of aloin, we studied its effects on the oxidative stress parameters [malondiadehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), and catalase] and proinflammatory cytokines [tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β] in the compressed sciatic nerve of neuropathic rats. The CCI-induced NP is extensively used to establish preclinical models of NP. It involves a variety of biochemical, molecular, and histological alterations in the sensory neurons that causes pain similar to the NP observed in humans. Moreover, sciatic nerve ligation leads to symptoms like pain, allodynia, and hyperalgesia[15-17]. Therefore, we investigated the ameliorative efficacy of aloin against the CCI-induced NP in rats.
2. Materials and methods
2.1. Animals
Male adult Sprague-Dawley rats (180-250 g) were obtained from the animal house facility of R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, District. Dhule, Maharashtra, India registered under Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA; Registration No.: 651/PO/ReBi/S/02/CPCSEA), Government of India, New Delhi. Animals were kept under standard environmental conditions of temperature [(20 ± 2) ℃] and humidity [(55 ± 5)%]. Food supplied by Nutrivet Life Sciences, Pune, India and water were provided ad libitum.
2.2. Drugs and chemicals
Aloin was procured from AK Scientific, Inc. India. Gabapentin was obtained from JohnLee Pharmaceuticals Pvt. Ltd. Mumbai, India. Hydroxylamine, nitroblue tetrazolium, Triton-X and 5, 5-dithio-bis-(2-nitrobenzoic acid) were purchased from Sigma-Aldrich, India. ELISA kits for cytokines were purchased from e-Biosciences, USA. TNF-α (Cat No: 837324-22), IL-1β (Cat No: 887013-22), and IL-6 (Cat No: 837064-22) ELISA kits were used. All chemicals and reagents were of analytical grade.
2.3. Peripheral neuropathy (PN) induction by CCI
PN was induced by CCI of the sciatic nerve in rats as method of Chanchal et al[5]. Animals were anesthetized with xylazine and ketamine. The thigh region was depilated followed by skin sterilization using 70% alcohol and povidone-iodine (Betadine). Blunt dissection was performed through biceps of the right hind limb and common sciatic nerve was exposed in the middle thigh region. Adhering tissues were carefully removed from the nerve about 5-7 mm proximal to the sciatic trifurcation. Four silk ligatures (4-0) were loosely placed around the sciatic nerve at 1 mm distance. Skin and muscle layers were immediately sutured and followed by the external application of a povidone-iodine solution. Animals were kept individually in separate cages with soft bedding to reduce the pain sensation induced by surgical procedures. The workflow and methodology used in the present study are represented as Supplementary Figure 1.
2.4. Drug treatments
After recovery period, rats were randomly divided into 7 groups with 10 rats per group. Rats in GroupⅠ(normal control) did not undergo sciatic nerve ligation and received only vehicle treatment (1.0 mL of 0.5% carboxymethylcellulose solution in water, once daily, orally). The sciatic nerve of rats in Group Ⅱ (sham-operated) was exposed and kept unligated, and rats only received a vehicle. Rats in Group Ⅲ (CCI control) underwent CCI surgery and received vehicle treatment. Group Ⅳ, Ⅴ, Ⅵ, and Ⅶ underwent CCI surgery and then administered with aloin (5 mg/kg, p.o.; 25 mg/kg, p.o.; 125 mg/kg, p.o.) and gabapentin (50 mg/kg, p.o.), respectively for 14 d. Tested doses of aloin were selected based on the biological efficacy of aloin and previous reports[18,19]. Drug solutions (aloin and gabapentin) were recently prepared before the administration. Nociceptive thresholds of the right hind paw (i.e. compressed sciatic nerve) at plantar region were determined at Day-3, 7, 11, and 14. Cytokines were estimated at Day-7 in four rats per group. At the end of treatment, animals were euthanatized by overdose anesthetics and sciatic nerve was instantly isolated. Nerve tissue homogenate was prepared in ice-cold PBS or KCl solution for biochemical estimations.
2.5. Apparatus
The CCI-induced mechanical allodynia was evaluated by electronic Von-Frey apparatus (IITC Life sciences, USA) using super tips probes. Motor nerve conduction velocity (MNCV) was recorded using LabChart 5.0 software and Power Lab data acquisition system (AD Instruments, Australia).
2.6. Measurement of behavioral parameters
2.6.1. Cold and warm allodynia
Cold allodynia was estimated using the method of Naik et al[20]. Concisely, the hind paw was smoothly immersed in a beaker containing cold water [(12 ± 1) ℃]. In warm allodynia assay, the hind paw was smoothly immersed in a beaker containing warm water [(40 ± 1) ℃]. Paw withdrawal latency (PWL) was noted within 20 s as the cut off time.
2.6.2. Mechanical allodynia
Von Frey anesthesiometer and filaments (IITC Life Science, USA) were used to assess the mechanical allodynia with some modifications[21]. Rats were placed in the plastic box (18 cm×8 cm×8 cm) on the mesh floor. The box was open at the bottom to enable Von Frey monofilament to apply on the animal hind paw at the plantar surface. Sudden jerk, paw licking, excessive grooming, body movement, and paw lifting were recorded as a positive response[22].
2.6.3. MNCV
Rats were anesthetized by urethane for the electrophysiological recording. Rat paw was shaved at the dorsal side and cleansed with ethanol. On the 14th day after treatment, MNCV was determined through sciatic and tibial nerve stimulation using monopolar needle electrodes (1.0-1.5, 182 mA, 2.0 mV/D) and stimulator. The MNCV was recorded from the plantar region using Power Lab System (AD Instruments, Australia)[13].
2.7. Biochemical estimations
Four rats from each group were euthanatized on Day-7 using an overdose of anesthesia after the determination of behavioral parameters. The levels of cytokines were estimated on the 7th day of treatment considering that the level of cytokines reaches the peak by 7th day. The sciatic nerve was isolated and homogenized in icecold PBS solution for the determination of cytokine concentrations. Other animals were sacrificed on the 14th day post-treatment. The sciatic nerve at the injury site was promptly isolated along with distal portions and tissue beneath the nerve. The PBS (pH 7.4) or KCl was used for the preparation of sciatic nerve homogenate (10%, w/v). The homogenate was further used for the assessment of lipid peroxidation, GSH, catalase, and SOD.
2.7.1. Total protein estimation
Sciatic nerve homogenate was evaluated for total protein content according to methods of Babu et al. and Lowry et al[23,24]. The homogenate was incubated with an alkaline copper reagent for 10 min at room temperature. Folin-Phenol reagent (0.5 mL) was added to this mixture and kept for 30 min in the dark. Absorbance against blank was recorded at 750 nm and total protein content was estimated as mg/100 mg of tissue.
2.7.2. Lipid peroxidation
The MDA content was evaluated as an index of lipid peroxidation according to the method of Ohkawa et al[25]. A total of 3 mL 1% w/v glacial acetic acid and 1 mL of 0.6% w/v thiobarbituric acid were added to tissue homogenate (0.5 mL) and the mixture was heated at 90 ℃ for 45 min. It was followed by the addition of n-butanol (4 mL), vortexing and centrifugation for 10 min at 5 000 rpm. The optical density of the organic layer was recorded at 535 nm and MDA content was stated as nmol of MDA/mg of protein.
2.7.3. GSH
GSH was measured as reported previously[26]. Precisely, trichloroacetic acid (10%) was mixed with tissue homogenate. The mixture was subsequently vortexed and centrifuged. The supernatant was thoroughly mixed with 5, 5-dithio-bis-(2-nitrobenzoic acid) and phosphate buffer (0.3 M; pH 8.4). The developed yellow colour was read at 412 nm (endpoint). The GSH concentration in sciatic nerve tissue was expressed as µg/mg of protein[26].
2.7.4. SOD
The SOD activity was evaluated following the previous method[27]. Tissue supernatant was mixed with the cocktail consisting of 100 µL of each of the NaCO(500 mM), EDTA (1 mM), nitroblue tetrazolium (240 µM), distilled water (640 µL), Triton-X 100 (0.3%; 10 µL), and 10 mM hydroxylamine (25 µL). The absorbance was read spectrophotometrically in kinetic mode at 560 nm up to 3 min at an interval of 1 min. SOD enzyme activity was stated as U/mg protein.
2.7.5. Catalase
The method reported by Aebi was used for the catalase estimation[28]. The cocktail of phosphate buffer (50 mM; pH 7; 1 mL) and hydrogen peroxide (30 mM; 0.1 mL) was added to the tissue supernatant (50 µL). The decline in the optical density was recorded in kinetic mode spectrophotometrically at 240 nm at every 5 s up to 30 s. The catalase enzyme activity was stated as U/mg protein.
2.8. Cytokine estimation by ELISA
All the samples, standard dilutions, and reagents were prepared as per the instructions of the manufacturer. The cytokine concentrations were determined by sandwich enzyme immunoassay protocol using ELISA Ready SET-Go immunoassay kits (e-Biosciences, USA). The concentrations of TNF-α, IL-6, IL-1β were represented as pg/mL[29].
2.9. Histopathological evaluation
The sciatic nerve samples were fixed in formalin (10%) and cut into sections in 4 µm. The hematoxylin and eosin protocol was used for staining. The longitudinal sections of nerve tissues were examined under a light microscope (Leica DM1000, Leica Microsystems, Germany) for histological alterations like nerve fiber derangement, fiber swelling, and the presence of Schwann cells and satellite cells[30,31].
2.10. Statistical analysis
Results are represented as mean ± SEM. The behavioral data were recorded across multiple time points (3rd, 7th, 11th, and 14th day) and analyzed using two-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test. The biochemical data were recorded at only one time point (for cytokines on 7th day and for other parameters on 14th day) and analyzed using one-way ANOVA followed by Bonferroni’s multiple comparisons test. Statistical analysis was performed using GraphPad Prism software. The results with P<0.05 were considered statistically significant.
2.11. Ethical statement
Experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC/RCPIPER/2018-19/01) and executed following the guidelines of CPCSEA under the provisions of the Prevention of Cruelty to Animal (PCA) Act, 1960, India.
3. Results
3.1. Effect of aloin on CCI-induced hyperalgesia in rats
3.1.1.Cold allodynia
The result of cold allodynia assay showed that the CCI control group exhibited a significant (P<0.001) decrease in PWL as compared with the normal group (Figure 1). Aloin at 5, 25, and 125 mg/kg significantly prolonged PWL in a dose-dependent manner as compared with the CCI control (P<0.001). A similar increase (P<0.001) in the PWL was also recorded in gabapentin (50 mg/kg, p.o.) treated rats.
3.1.2. Warm allodynia
Similar to the result of cold allodynia assay, the CCI control displayed a significant (P<0.001) reduction in the PWL compared with that of normal group rats (Figure 2). Aloin and gabapentin treatment significantly increased PWL (P<0.001), and the increase by aloin was dose-independent.
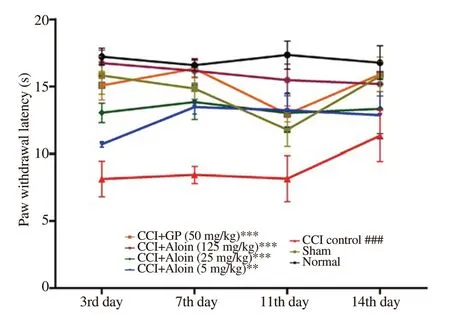
Figure 1. Effect of aloin on paw withdrawal latency in chronic constriction injury (CCI)-induced cold allodynia. Data are expressed as mean ± SEM (n= 6/group). Two-way ANOVA followed by Tukey’s multiple comparisons test, F (6, 35) = 17, P<0.001; ###P<0.001 as compared with the normal group; **P<0.01, ***P<0.001 as compared with the CCI control group. GP: gabapentin.

Figure 2. Effect of aloin on paw withdrawal latency in CCI-induced warm allodynia. Data are expressed as mean ± SEM (n= 6/group). Two-way ANOVA followed by Tukey’s multiple comparisons test, F (6, 35) = 25, P<0.001; ###P<0.001 as compared with the normal group; ***P<0.001 as compared with the CCI control group.
3.1.3. Mechanical allodynia
CCI surgery induced severe mechanical allodynia in rats with a significant (P<0.001) decline in PWT. Treatment with aloin (5, 25, 125 mg/kg, p.o.) and gabapentin (50 mg/kg, p.o.) significantly (P<0.001) prolonged PWL, which was comparable to the results of the normal group (Figure 3).

Figure 3. Effect of aloin on paw withdrawal threshold in CCI-induced mechanical allodynia. Data are expressed as mean ± SEM (n= 6/group). Two-way ANOVA followed by Tukey’s multiple comparisons test, F (6, 35) = 123, P<0.001; ###P<0.001 as compared with the normal group; ***P<0.001 as compared with the CCI control group.

Figure 4. Effect of aloin on motor nerve conduction velocity (MNCV) in CCI-induced neuropathic pain. Data are expressed as mean ± SEM (n= 6/group). One-way ANOVA followed by Bonferroni’s multiple comparisons test, F (6, 35) = 12, P<0.001; ###P<0.001 as compared with the normal group; ***P<0.001 as compared with the CCI control group.
3.1.4. MNCV
Rats in the CCI control showed a significant (P<0.001) reduction in nerve conduction velocity compared with conduction velocity in the normal group animals (Figure 4). Aloin treatment (25 and 125 mg/kg, p.o.) significantly elevated nerve conduction velocity in CCIinduced NP rats (P<0.001). Animals administered with aloin (125 mg/kg) and gabapentin (50 mg/kg) showed the MNCV comparable to the normal group rats.
3.2. Effect of aloin on cytokine expression in rats with CCIinduced NP
The level of TNF-α in the CCI control group was significantly increased in comparison with the normal animals (P<0.01). Aloin treatment at all doses reduced the TNF-α level, which was similar to the effect of gabapentin (50 mg/kg, p.o.) (Figure 5A). However, the effect of aloin on TNF-α level was not in a dose-dependent manner. The aloin at 5 & 25 mg/kg (P<0.01) demonstrated more significant decreases in the TNF-α level as compared with the CCI control group. Increases in the IL-6 and IL-1β levels of the sciatic nerve samples were noted in the CCI control rats as compared with normal group rats. Aloin (5, 25 and 125 mg/kg, p.o.) and gabapentin (50 mg/kg, p.o.) significantly declined the levels of both IL-6 (P<0.01) and IL-1β (P<0.001) (Figure 5B & C).
3.3. Effect of aloin on oxidative parameters
A significant (P<0.05) rise in MDA of sciatic nerve tissue in the CCI control was noted as compared with normal rats. Aloin at 5 & 25 mg/kg dosage reduced the level of MDA, but the decreases were not significant. Gabapentin significantly decreased MDA content (P<0.05) (Figure 6A). Chronic constriction of sciatic nerve resulted in the depletion of GSH content in nerve tissues. Whereas, the treatment with aloin and gabapentin reversed such depletion of GSH content (Figure 6B). The restorative effect of aloin (125 mg/kg) on the GSH level was statistically significant (P<0.001). Activities of SOD (P<0.01) and catalase (P<0.001) were significantly reduced in the CCI control rats. Levels of antioxidant enzymes were increased after aloin administration. However, the aloin was unable to restore the activities of SOD and catalase up to normal levels. The effects of the highest dose of aloin (125 mg/kg) were comparable to the effects of gabapentin (50 mg/kg) (Figure 6C and 6D).
3.4. Histopathological examination of sciatic nerve in the CCI-induced NP model
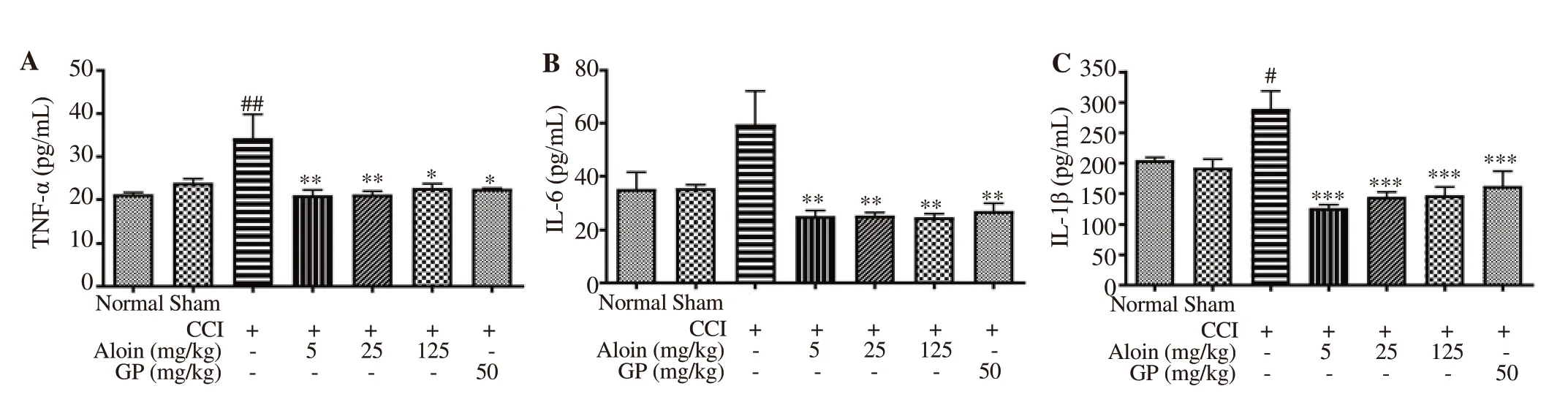
Figure 5. Effect of aloin on the release of pro-inflammatory cytokines in the sciatic nerve. Data are expressed as mean ± SEM (n = 4/group); One-way ANOVA followed by Bonferroni’s multiple comparisons test; For tumor necrosis factor-α (TNF-α), F (6, 21) = 4.5, P=0.004; For interleukin (IL)-6, F (6, 21) = 5.1, P=0.002; For IL-1β, F (6, 21) = 11.0, P<0.001. #P<0.05, ##P<0.01 as compared with the normal group; *P<0.05, **P<0.01, ***P<0.001 as compared with the CCI-control group.
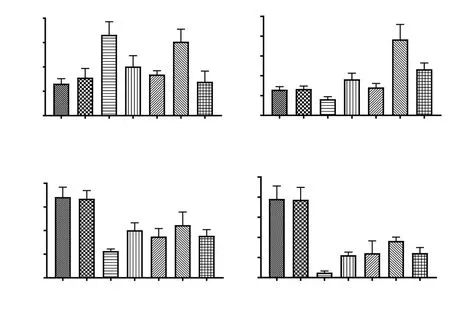
Figure 6. Effect of aloin on oxidative stress in CCI-induced neuropathic pain in rats. Data are expressed as mean ± SEM (n = 6/group). One-way ANOVA followed by Bonferroni’s multiple comparisons test. For malondiadehyde (MDA), F (6, 33) = 4.1, P=0.003; For glutathione (GSH), F (6, 35) = 8.1, P<0.001; For superoxide dismutase (SOD), F (6, 35) = 5.6, P<0.001; For catalase, F (6, 35) = 11, P<0.001. #P<0.05, ##P<0.01, ###P<0.001, as compared with the normal group; *P<0.05, ***P<0.001, as compared with the CCI control.
Histopathological examination indicated alterations in the normal architecture of the sciatic nerve. Axonal swelling was denoted by green arrow, whereas, fiber derangement and CCI-induced changes in Schwann and satellite cells were denoted by red arrow. Sciatic nerve ligation-induced CCI resulted in the nerve fibre derangement and axonal swelling along with the expression of Schwann and satellite cells (neuroglial cells) as shown in Figure 7C. Treatment with aloin (5, 25, 125 mg/kg, p.o.), and gabapentin (50 mg/kg, p.o.) exhibited protective effects against histopathological alterations of the constricted sciatic nerve due to CCI surgery (Figure 7D-G).
4. Discussion
Previous study on the effect of crude ethanolic extract of Aloe vera against NP highlighted the need of further studies and exploration of mechanism of actions[32]. In this study, we tested three oral doses of aloin (5, 25 & 125 mg/kg) that is the main constituent of Aloe vera in the CCI-induced NP model. Tested doses of aloin (5, 25 & 125 mg/kg, p.o.) were selected based on the biological efficacy of aloin and previous reports[18,19]. Aloin significantly reduced CCI-induced mechanical and thermal allodynia. It also inhibited oxidative stress and reduced pro-inflammatory cytokine concentrations. These results support the efficacy of aloin as an antineuropathic agent.
Dysfunction of somatosensory system maladapts the nociceptor neuron and causes NP[33]. CCI-induced NP animal model is a reproducible, widely used and reliable model of neuropathy associated hyperalgesia and allodynia[34]. In this model, NP is characterized as primary lesion and somatosensory dysfunction[7]. Rats in our study showed NP symptoms similar to clinical NP symptoms in human. The PWT and PWL in response to mechanical and thermal stimuli were evident in the CCI-induced NP model. Aloin treatment effectively increased the PWT and PWL in CCIinduced NP rats, which implies alleviating effect of aloin on NP sensation. Deteriorated nerve fibers with reduced nerve energy have diminished MNCV, which was improved by aloin.
The oxidative stress following CCI of sciatic nerve in rats is a consequence of secondary metabolite-associated ischemic hypoxic changes[35]. Inflammation and oxidative stress are connected and cause nerve injury and insistent pain[22,36]. The CCI-associated rise in lipid peroxidation and ROS generation causes oxidative stress in ligated sciatic nerve. The ROS scavengers are effective in the alleviation of CCI-induced allodynia and hyperalgesia[37,38]. Aloin could alleviate the nerve injury by reducing MDA in sciatic nerve tissue[39]. Aloin at 125 mg/kg dose exhibits less significant effect on lipid peroxidation. This indicates dose-limiting side effect of aloin and needs further investigation. The CCI surgery also reduced levels of GSH, catalase, and SOD, which were improved by aloin. These enzymes are main anti-oxidants, which scavenge the free radicals and protect tissues against oxidative stress[17]. Thus, the present study indicates the antioxidative effect of aloin.

Figure 7. Histopathological changes in hematoxylin and eosin stained longitudinal sections of the sciatic nerve. The green arrows show axonal swelling. Red arrow shows fiber derangement and CCI-induced changes in Schwann and satellite cells. D, F and G show attenuation of CCI-induced fiber derangement and reversal of Schwann and the satellite cell changes by aloin and gabapentin. Microscopic examinations were performed under 400× light microscopy, scale bar 100 µm.
The inflammatory changes associated with CCI surgery involve cytokines like TNF-α, IL-6, and IL-1β, which promote the development of NP[40]. Pro-inflammatory cytokines have important roles in the hyperalgesia. CCI upregulates the pro-inflammatory cytokines, TNF-α, IL-1β, and IL-6[41]. Peripheral nerve damage releases TNF-α from immune cells and Schwann cells. Pain threshold was lowered by TNF-α in NP and CCI-induced pain was relieved by anti-TNF-α treatments[42]. We noted a significant decline of TNF-α levels in sciatic nerve of rats by aloin as compared with the CCI-control rats. Similarly, IL-1β and IL-6 levels were elevated in the nociceptive neurons and these inflammatory cytokines have a vital role in the pain induction in many experimental models[43]. The cytokines were estimated on the 7th day of treatment considering that the level of cytokines reaches the peak by 7th day. Besides, exposing all the rats to repeated pain threshold testing was considered unnecessary and unethical[44].
Present study demonstrates the protective effect of aloin against CCI-induced peripheral NP in rats. Aloin could reduce thermal nociception and mechanical allodynia. Aloin especially at the doses of 5 & 25 mg/kg exhibited prominent anti-inflammatory and anti-oxidant effects. It is suggested that attenuated inflammatory response and reduced oxidative stress primarily contribute to the anti-neuropathic potential of aloin against the CCI-induced NP in rats. Although these findings indicate the antineuropathic efficacy of aloin, extensive investigations involving animal and human studies are needed to validate these effects.
Conflict of interest statement
We declare that there is no conflict of interest.
Authors’ contributions
ASK performed literature search, conducted experiments, data acquisition, statistical analysis and manuscript preparation. CRP designed the concept and study, literature search, conducted and supervised experimental studies, data and statistical analysis, manuscript preparation, editing and review. KRP performed literature search, study design and concept, experimental studies, data and statistical analysis, preparation, editing and review of manuscript. UBM contributed to biochemical estimations, data acquisition, prepared figures, and manuscript editing. SNG assisted in study design, performed data and statistical analysis, manuscript review. SO, SB, and SJS contributed to chemicals, data analysis, and manuscript review. LAB and ARW executed literature search, performed experiments, and data acquisition, assisted in preparing first manuscript draft. All authors have read, contributed and approved the final version of manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年5期
Asian Pacific Journal of Tropical Biomedicine2021年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Protective effects of rice bran hydrolysates on heart rate variability, cardiac oxidative stress, and cardiac remodeling in high fat and high fructose diet-fed rats
- Origanum vulgare L. leaf extract alleviates finasteride-induced oxidative stress in mouse liver and kidney
- Argemone mexicana extract alleviates gastrointestinal disorders by stimulating muscarinic receptors and blocking voltage-gated L-type calcium channels
- Honokiol attenuates oxidative stress-induced cytotoxicity in human keratinocytes via activating AMPK signaling
