Protective effects of rice bran hydrolysates on heart rate variability, cardiac oxidative stress, and cardiac remodeling in high fat and high fructose diet-fed rats
Ketmanee Senaphan, Upa Kukongviriyapan, Pisit Suwannachot, Geerasak Thiratanaboon, Weerapon Sangartit, Supawan Thawornchinsombut, Akkasit Jongjareonrak
1Division of Physiology, Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
2Cardiovascular Research Group, Khon Kaen University, Khon Kaen 40002, Thailand
3Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen 40002, Thailand
4Department of Food Technology, Faculty of Technology, Khon Kaen University, Khon Kaen 40002, Thailand
5Faculty of Agro-Industry, Chiang Mai University, Chiang Mai 50100, Thailand
ABSTRACT
KEYWORDS: Rice bran hydrolysates; Heart rate variability; Cardiac remodeling; Metabolic syndrome; Oxidative stress
1. Introduction
Cardiovascular diseases (CVD) are major causes of morbidity and mortality worldwide[1]. The major risk factors consist of smoking, genetic predisposition, sedentary life style, diabetes mellitus (DM), and metabolic syndrome (MetS)[1,2]. MetS is a clustering of various metabolic changes that include obesity, insulin resistance, hypertension, dyslipidemia, proinflammatory, and prothrombic states[3]. CVD in MetS can be caused by one or more factors associated with this condition, and their combination carries additional risk[4].
Accumulating evidence indicates that CVD is closely linked to insulin resistance, and the evidence of insulin-resistant cardiomyopathy is increasing[5]. Moreover, insulin resistance has been found to affect the heart by altering the mechanical function and cardiac autonomic balance[6]. Heart rate variability (HRV), a key indicator of sympathetic and parasympathetic activity, has been used to indicate cardiac autonomic balance. Decreased HRV indicates an imbalance in autonomic tone[6,7]. The autonomic imbalance is often caused by increased sympathetic and/or reduced parasympathetic activities. Recent studies have demonstrated that autonomic imbalance, as characterized by increased sympathetic and decreased parasympathetic activity, is associated with cardiovascular morbidities and MetS[8,9]. Abnormal cardiovascular parameters in obesity are related to autonomic imbalance and are further complicated by insulin resistance, hypertension, dyslipidemia, low-grade inflammation, and oxidative stress[9]. This indicates an association between autonomic imbalance and all the main features of the MetS in the development of cardiac dysfunction in CVD. Moreover, increased oxidative stress in the heart is a common characteristic of CVD in the insulin resistant state and in MetS[10]. Many cardiac pathologies are associated with oxidative stress[11], and increasing evidence shows that reactive oxygen species (ROS) have a crucial role in the development of insulin-resistant cardiomyopathy[12]. These indicate that oxidative stress is involved in the pathogenesis of insulin resistance, cardiovascular structural, and functional alterations in MetS. Therefore, amelioration of oxidative stress, autonomic imbalance, and MetS might reduce the risk of cardiovascular disease.
Rice bran, a major byproduct of the rice milling process, is a natural resource that contains a high level of phytochemicals, including phenolic compounds[13]. Previously, our group has shown the beneficial effects of rice bran hydrolysates (RBH) supplementation in renovascular hypertensive rats, including antioxidant activities, improved endothelial function, amelioration of hypertension through angiotensin-converting enzyme inhibitory, and vasodilatory effects[14]. In addition, RBH alleviates cardiovascular risk factors by strongly reducing insulin resistance, oxidative stress, dyslipidemia, inflammation, vascular remodeling, and arterial stiffness in HCHFdiet-fed rats and in vitro experiment[15-17]. However, the effect of RBH on cardiac dysfunction in diet-induced MetS is not well investigated.
A recent study demonstrated that rice bran water extract possesses cardioprotective properties through exerting its antioxidant effect and preventing lipid over-accumulation and oxidative damage to the heart tissue in rats fed with a high-fat diet[18]. However, little is known about the effect of RBH on the alterations of HRV, cardiac oxidative stress, and cardiac structural changes in diet-induced MetS rats. Recently, a new RBH product was developed by our group from the cold-pressed defatted rice bran (CDRB) by using mild-subcritical alkaline water extraction and hydrolysis with protease enzyme. This archived RBH contains more protein and phenolic contents than RBH previously extracted by our group[19]. Considering the beneficial effect of RBH on metabolic and cardiovascular parameters developed in hypertensive and diet-induced MetS rats, and a higher amount of protein and phenolic compounds in this RBH, it is expected that RBH will be able to alleviate cardiac dysfunction in MetS rats. Therefore, in the present study, we determined the effect of RBH on metabolic disorders, HRV, cardiac oxidative stress, and cardiac structural changes in rats with high fat and high fructose (HFHF) diet-induced MetS.
2. Material and methods
2.1. RBH extraction
CDRB was obtained from Medifoods (Thailand) Co., Ltd. (Chaiyaphum province, Thailand). RBH was prepared by using mild-subcritical alkaline water extraction and hydrolyzed with protease G6 and protease GN process as described previously, with some modifications[19]. In brief, the CDRB was sieved through a 50-mesh screen, suspended in water, and subjected to a mild-subcritical alkaline water condition at pH 9.5, 120 ℃ for 120 min, followed by hydrolysis process using commercial enzymes. The CDRB mixture was adjusted to the optimal hydrolysis condition for protease G6 (EC 3.4.21.62) (DuPontGenencorScience, USA) (pH 8.0, 55 ℃) prior to adding the enzyme. Protease G6 was added at a ratio of enzyme per CDRB of 20 mL/kg and incubated for 4 h to hydrolyze the protein. Then, the protease GN was added at the same concentration to the mixture and incubated for 2 h. The enzymatic reaction was stopped by heating at 90 ℃ for 15 min. The mixture was then cooled down to ambient temperature (28±2) ℃ and centrifuged at 4 023 ×g for 5 min. The supernatant was collected and its pH was adjusted to 7.5, and the liquid was evaporated at 60 ℃ under vacuum condition to obtain the final 20° Brix solution. The concentrated solution was added with maltodextrin (DE 10-13) to obtain the final soluble solid content of 25° Brix. The solution was heated at 130 ℃ for 30 min to destroy microorganisms and then spray dried using inlet and outlet air temperature of 190 ℃ and 90 ℃, respectively to obtain RBH powder. The RBH powder was stored in air tight containers and kept at -20 ℃. The proximate compositions of RBH were; protein content: 27.47% (wet basis), fat: 3.45%, and moisture: 1.44%. The phenolic compounds in RBH were analyzed and reported by the Laboratory Service (Central Laboratory, Bangkok, Thailand) as shown in Table 1.

Table 1. Phenolic compounds in rice bran hydrolysates (RBH).
2.2. Animals and diets
Male Sprague-Dawley rats (220-250 g body weight) were supplied by the Nomura Siam International Co., Ltd, Bangkok, Thailand. During the experimental period, the rats were housed in a temperature-controlled room (25±1) ℃ and maintained on a 12 h light/dark cycle with free access to standard pellet diet and water at the Northeast Laboratory Animal Center (Khon Kaen University, Khon Kaen, Thailand). After acclimatization for 7 d, the rats were randomly divided into two groups, which received either a standard rat chow diet with tap water (control group) or an HFHF diet (lard 40%, fructose 20%) with fructose supplemented (10%) drinking water (HFHF group) ad libitum for 16 weeks. The HFHF diet was prepared in our laboratory, whereas a standard chow diet was purchased from Perfect Companion Group Co. Ltd. (Thailand). Food and water intake were recorded every day, and body weight (BW) was recorded weekly.
At week 10, rats were randomly assigned into five subgroups (n = 8/group): Control+ deionized water (DI), Control+RBH (1 000 mg/kg/day), HFHF+DI, HFHF+RBH (500 mg/kg/day) and HFHF+RBH (1 000 mg/kg/day), which received treatments orally for further 6 weeks. The doses of RBH were chosen on the basis of our preliminary observation and previous study[17] where these doses could reduce fasting blood glucose and blood pressure of MetS rats. Lipid profiles, fasting blood glucose (FBG), oral glucose tolerance test (OGTT), and indirect systolic blood pressure (SBP) were measured before and after 6 weeks of RBH treatment for all experimental groups.
2.3. Determination of metabolic variables and blood pressure
Rats were fasted for 8-12 h and blood samples were taken from the lateral tail vein to determine FBG using a glucometer (Roche Diagnostics, Sydney, Australia). The OGTT was performed following a procedure described previously[16] and an area under the curve (AUC) of blood glucose concentration-time over the period of 120 min was calculated to determine insulin sensitivity in HFHF groups during the experimental period. Fasting serum was used to determine triglyceride (TG) and high-density lipoprotein-cholesterol (HDL-C) using enzymatic and colorimetric methods (Roche diagnostics, Bangkok, Thailand). Non-invasive inflatable tail-cuff connected to a Rat Tail-Cuff Blood Pressure System (IITC/Life Science Instrument model 299 and model 179 amplifier; Woodland Hills, CA, USA) was used to measure SBP in conscious rats as previously described[17].
2.4. HRV measurement
At the end of 16 weeks, HRV was used to determine cardiac autonomic tone balance using PowerLab (ADInstruments, Australia) and the Chart 7.0 program[20]. For HRV analysis, the electrocardiogram (ECG) lead Ⅱ was recorded in each rat using a noninvasive method previously described[21], with some modifications. Briefly, before ECG was recorded, the animals were anesthetized using pentobarbital sodium (30 mg/kg). Anesthesia was assessed by pedal reflex. The ventral area of the front leg and the pelvic region (inguinal) of each animal was carefully shaved and placed on a heat pad to maintain body temperature. Surface ECG electrodes (3M Deutschland GmbH, Health Care Business, Germany) were placed on the ventral area of the front leg and the area of pelvic region (inguinal) in direct contact to the skin. Lead Ⅱ on the ECG was recorded continuously for 10 min. The negative electrode was attached to the skin of the right front leg and the positive electrode to the left side of pelvic region. For each record, the most stable 5 min continuous segment was chosen for HRV analysis. To evaluate the autonomic nervous system activity in each animal, the frequency domain method was used to analyze HRV. Low-frequency (LF: 0.2-0.75 Hz) and high-frequency (HF: 0.75-2.5 Hz) domains were analyzed by integrating the spectrum for each bandwidth[22]. The HF component represents cardiac parasympathetic activity, whereas LF component usually represents cardiac sympathetic and parasympathetic activity. Increased LF/HF ratio or a reduction in HRV (i.e. depressed HRV) was used as a measure of cardiac autonomic imbalance[6,7].
2.5. Hemodynamic measurements
At the end of the study, rats were anesthetized with pentobarbital sodium (60 mg/kg body weight, i.p.) and a tracheotomy was performed for spontaneous breathing. Thereafter, SBP, mean arterial blood pressure (MAP), heart rate (HR), and pulse pressure (PP) were measured via femoral artery, using the AcqKnowledge data acquisition and analysis software (Biopac Systems Inc., Santa Barbara, CA, USA). After hemodynamic measurements, rats were sacrificed by pentobarbital sodium overdose. Blood samples and arterial tissue were collected for assaying plasma oxidative stress markers and measuring the vascular superoxide production (O
). The heart, left ventricle (LV), liver, and abdominal fat (retroperitoneal, epididymal, and omental fat) were separated and weighed. Organ weights were normalized relative to the body weight (mg/g body weight). Samples of the LV were divided into three parts for determining cardiac oxidative stress and antioxidant markers, measuring LV hypertrophy and myocardial fibrosis, and performing Western blotting analysis of protein expression of endothelial nitric oxide synthase (eNOS).
2.6. Oxidative stress and antioxidant biomarkers
2.6.1. Vascular superoxide production (O)

2.6.2. Determination of cardiac and plasma MDA level
The level of MDA, a marker of lipid peroxidation, was determined in plasma and heart tissue by measuring TBA reactive substances, following a previously described method[23]. For measuring cardiac MDA level, heart tissue was homogenized in phosphate buffer (pH 7.4) and incubated on ice for 30 min. The tissue was then centrifuged at 10 000 g for 10 min at 4 ℃, after which the supernatant was collected to assess cardiac MDA level using the same procedure as previously used for plasma samples[23]. Total protein levels in cardiac tissues were measured by Bradford dye-binding method. MDA concentration in the cardiac tissues was normalized against the cardiac tissue protein concentration and expressed as μmol/mg of protein.
2.6.3. Determination of cardiac and plasma protein carbonyl level
Protein carbonyl, a marker of oxidizing protein damage in plasma and cardiac tissue, was assessed by measuring the formation of carbonyl groups based on the reaction with dinitrophenylhydrazine, as previously described[23]. In brief, plasma samples were incubated with and without 15 mM 2,4-dinitrophenylhydrazine in 3.6 M HCl for 1 h in the dark. Subsequently, protein was precipitated, washed, and dissolved in 6 M guanidine. The carbonyl content was determined from the absorbance at 360 nm after subtraction of HCltreated blanks, using a molar absorption coefficient of 22 000/M/cm. The plasma protein content was analyzed by Bradford dyebinding method. For measuring cardiac protein carbonyl, heart tissue was homogenized and the supernatant was collected with the same procedure used in cardiac MDA level determination. Then, the supernatant was used to assess protein carbonyl level using the same procedure with the plasma samples. Protein carbonyl concentration in the cardiac tissue was normalized against tissue protein concentration and the result was expressed as nmol/mg of protein.
2.6.4. Measurement of catalase (CAT) activity in cardiac tissue
CAT activity in the heart was assessed according to the previously described method with some modifications[24]. The enzymecatalyzed decomposition of HOwas determined. Briefly, 10 μL of sample was added to 50 μL of cold 6 mM HOfor initiating the enzymatic reactions. The reaction was stopped after 3 min by adding 5 mol/L HSOand mixing thoroughly, after which 150 μL of 0.005 mol/L KMnOreagent was added and mixed thoroughly. The reading was taken at 490 nm within 30-60 s and the data were expressed as units/mg protein.
2.7. Western blotting analysis
Expression of eNOS protein was measured in heart tissue homogenates following a previously described method with some modifications[14,23]. In brief, heart tissue samples (n = 4) were homogenized and the protein concentration was estimated by Bradford dye-binding method. The homogenates were run on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDSPAGE) system. The proteins were transferred electrophoretically onto a polyvinylidene difluoride (PVDF) membrane and blocked with 5% skimmed milk in Tris buffer saline with 0.1% Tween-20 for 2 h at room temperature. Following this, they were incubated overnight at 4 ℃ with primary antibody of mouse monoclonal antieNOS (1:2 000 dilution; Cat: 610296; BD Biosciences, San Jose, CA, USA). Membranes were then repeatedly washed with Tris buffer saline and incubated for 2 h at room temperature with the secondary antibody, horseradish peroxidase goat anti-mouse immunoglobulin G (IgG) (1:2 500 dilution; sc-2031; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The blots were developed in the ECL substrate solution (Thermo Fisher Scientific, Rockford, IL, USA). Densitometric analysis of specific eNOS and β-actin bands was performed using an ImageQuant400 imager (GE Healthcare Life Science, Piscataway, NJ, USA). Band intensity of eNOS protein was normalized to β-actin expression from the same sample and data were presented as percentages of the values from the heart of the control group.
2.8. Histology
The LV from rats (n = 6-8) were dissected, then fixed with 4% paraformaldehyde, and embedded in paraffin blocks using a standard histological process. Sections of 5 μm thickness were cut using a microtome and stained with hematoxylin and eosin (Bio-Optica Milano SpA., Milano, Italy) to assess LV hypertrophy. Heart imaging was performed using a stereoscope (Nikon SMZ745T with NIS elements D 3.2). Heart morphometric evaluation was conducted using Image J morphometric software (National Institutes of Health, Bethesda, MD, USA). Myocardial interstitial fibrosis was determined using picrosirius red-stained LV sections. The stained sections were examined and captured with a digital microscope camera (Nikon DS-Ri 1 Camera) and analyzed by Image-pro plus software (Media Cybernetics, MD, USA). LV fibrosis was presented as a percentage of the positively stained area to media area.
2.9. Statistical analysis
Results were presented as mean ± standard deviation (SD). Oneway analysis of variance (ANOVA) and the Student-Newman-Keuls post hoc test were used to evaluate the difference between groups. A value of P < 0.05 was considered statistically significant.
2.10. Ethical statement
All procedures and protocols performed in this study were approved by the Institutional Animal Care and Use Committee of Khon Kaen University (ACUC-KKU-53/60).
3. Results
3.1. Daily intake, body weight, and organ weights
After 16 weeks of HFHF diet consumption, the HFHF group gained more body weight than the control group [Control: (199±6)% vs. HFHF: (251±15)%], and liver weight and abdominal fat pad weight were also significantly higher in the HFHF group (P<0.001). RBH-administered rats (500 and 1 000 mg/kg) showed significant decreases in the liver and abdominal fat pad weight compared with the HFHF group (P<0.05; Table 2). The heart weight and the LV weight relative to the body weight were significantly lower in the HFHF group as compared with the control group (P<0.05; Table 2). No effect of RBH treatment on the final body weight, heart, or LV weight was observed in the HFHF groups.
3.2. Effects of RBH on metabolic parameters and SBP
The level of FBG, AUC, and serum TG were significantly increased, whereas serum HDL-C was significantly decreased in the HFHF group compared with the control group (P<0.05), suggesting impaired glucose tolerance, insulin resistance, and dyslipidemia. After the treatment of RBH (500 and 1 000 mg/kg) for 6 weeks, these HFHF diet-induced changes were improved. The metabolic parameters in the HFHF groups treated with RBH of both doses were not significantly different.
SBP was significantly elevated in the HFHF group compared with the control group (P<0.001). RBH treatment significantly prevented the increased blood pressure in a dose-dependent manner (P<0.001; Table 2). No significant differences in the metabolic parameters and indirect SBP were observed between the vehicle control groups in the absence and presence of RBH treatment (Table 2).
3.3. Effects of RBH on HRV in HFHF-diet-fed rats
At the end of 16 weeks of experimental periods, the LF/HF ratio of HRV was measured as an index of cardiac autonomic balance. As presented in Figure 1, the LF/HF ratio was significantly increased in the HFHF group compared with the control group (P<0.001), indicating depressed HRV. After treatment with a high dose of RBH (1 000 mg/kg) for 6 weeks, the LF/HF ratio was markedly decreased and returned to normal levels compared with HFHF untreated group (P<0.001).
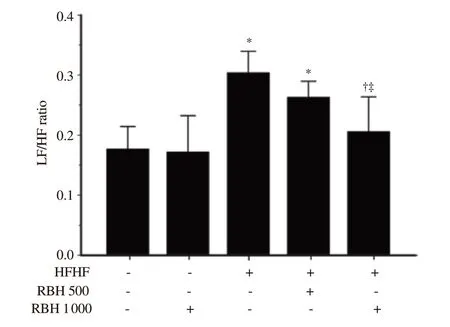
Figure 1. Effect of RBH on heart rate variability in all experimental groups. Results are expressed as mean ± SD (n = 6-8). *P < 0.05 vs. the control group, †P < 0.05 vs. the HFHF group, ‡P < 0.05 vs. HFHF + RBH 500 mg/kg. LF/HF: low frequency/high frequency.
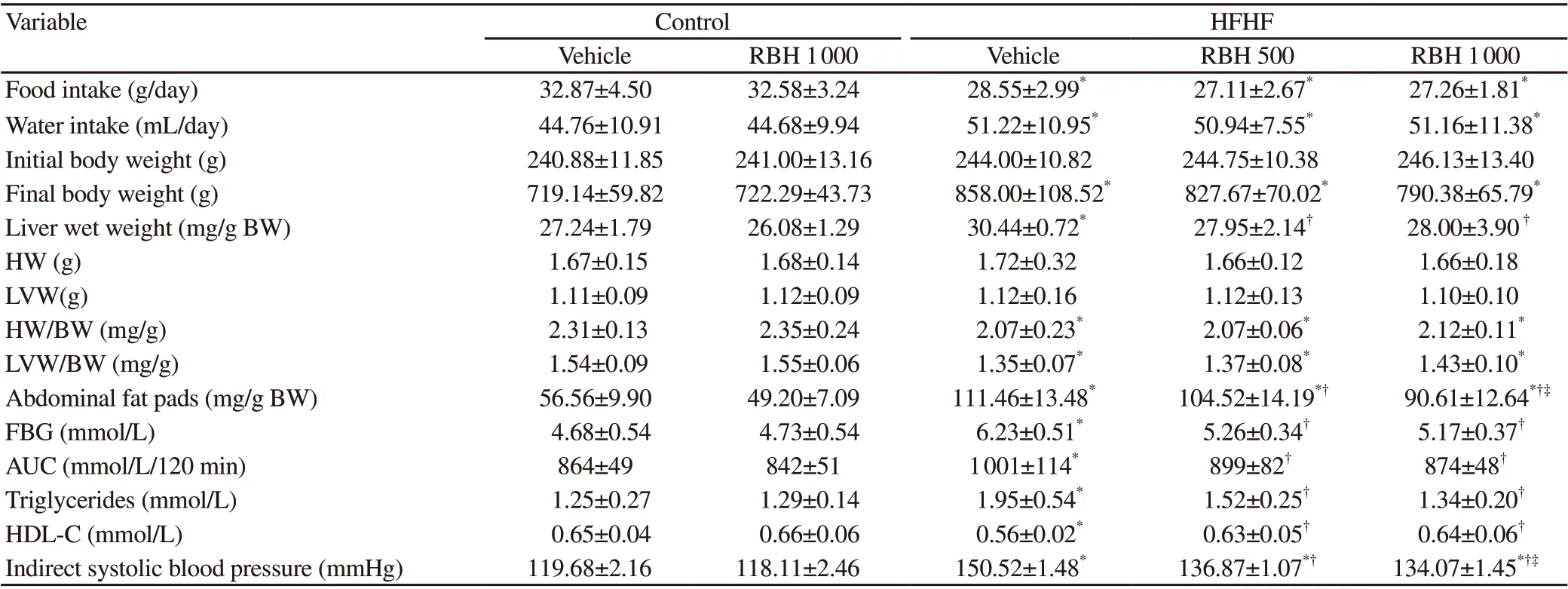
Table 2. Physiological parameters, organ weights, metabolic parameters, and indirect systolic blood pressure in all experimental groups.
3.4. Effects of RBH on hemodynamic parameters
As shown in Figure 2, all hemodynamic parameters (SBP, MAP, PP, and HR) were significantly increased in the HFHF group compared with those of the control group (P<0.05). RBH (500 and 1 000 mg/kg) prominently alleviated hemodynamic alterations in SBP, MAP, and HR in HFHF rats (P<0.001; Figure 2A, B, and D). PP was significantly improved in HFHF rats treated with 1 000 mg/kg of RBH (P = 0.045; Figure 2C).
3.5. Effects of RBH on oxidative stress and antioxidant markers
Compared with the control group, the level of MDA was significantly higher in plasma and cardiac tissue in the HFHF group (P<0.05; Figure 3A and C). In addition, markedly increased protein carbonyl level in cardiac tissue was also observed in the HFHF group (Figure 3D), confirming the oxidative damage due to continuous HFHF diet feeding. RBH administration, especially at a high dose, significantly decreased the level of MDA in plasma and reduced both MDA and protein carbonyl levels in cardiac tissue (P<0.05; Figure 3A, C, and D). Furthermore, the activity of the enzymatic antioxidant CAT in cardiac tissue was significantly increased in the HFHF group treated with a high dose of RBH (P=0.003; Figure 3F). RBH also prevented HFHF diet-induced vascular Oproduction (P<0.001; Figure 3E).

Figure 2. Effect of RBH on hemodynamic parameters; systolic blood pressure (A), mean arterial blood pressure (B), pulse pressure (C), and heart rate (D) in all experimental groups. Values are expressed as mean ± SD (n = 6-8). *P < 0.05 vs. the control group, †P < 0.05 vs. the HFHF group, ‡P < 0.05 vs. HFHF + RBH 500 mg/kg.

Figure 3. Effect of RBH on oxidative stress and antioxidant markers; plasma MDA levels (A), plasma protein carbonyl (B), cardiac MDA levels (C), cardiac protein carbonyl (D), vascular O2·- production (E) and CAT activity in heart (F) in all experimental groups. Results are expressed as mean ± SD (n = 6-8). *P < 0.05 vs. the control group, †P < 0.05 vs. the HFHF group, ‡P < 0.05 vs. HFHF + RBH 500 mg/kg. MDA: malondialdehyde; CAT: catalase.
3.6. Effects of RBH on cardiac protein expression of eNOS
Western blotting assay showed downregulated cardiac eNOS protein expression in the HFHF group as compared with the control group (P=0.027; Figure 4). A high dose of RBH (1 000 mg/kg) prominently upregulated cardiac eNOS protein expression in the HFHF group after 6 weeks of treatment (P=0.032; Figure 4). No effect of RBH treatment on cardiac eNOS protein expression was observed in the control group.
3.7. Effects of RBH on cardiac hypertrophy
Figure 5 shows representative sections of LV tissue stained with hematoxylin and eosin. HFHF diet feeding for 16 weeks induced morphological changes in ventricles, including a significant increase in the LV wall thickness (P<0.001) and a decrease in the ventricular luminal area (P = 0.002) with no significant change in the ventricular cross-sectional area (P = 0.804), compared with the control group (Figure 5). Interestingly, RBH administration (500 and 1 000 mg/kg) attenuated these structural changes as evidenced by significantly reduced LV wall thickness and increased ventricular luminal area in the HFHF group. Myocardial interstitial fibrosis was evaluated from the left ventricular sections stained with picrosirius red. Continuous HFHF diet feeding induced a significant increase in myocardial fibrosis when compared with the control group (Figure 6). However, the changes induced by HFHF diet were alleviated in the RBHtreated groups at both doses tested, compared with HFHF untreated group (P<0.001; Figure 6).
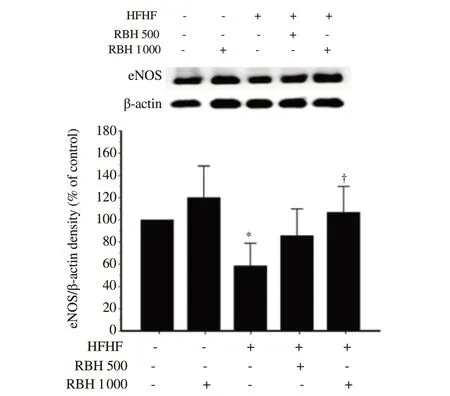
Figure 4. Effect of RBH on eNOS protein expression in cardiac tissue of all experimental groups. Values are presented as a percentage of normal control values and expressed as mean ± SD, n = 4/group. *P < 0.05 vs. the control group, †P < 0.05 vs. the HFHF group. eNOS: endothelial nitric oxide synthase.
4. Discussion
The major finding of this study was that all of these pathologic features of MetS in rats fed with an HFHF diet (obesity, hypertension, dyslipidemia, hyperglycemia, impaired glucose tolerance, cardiac remodeling, and oxidative stress) were alleviated by RBH supplementation. These beneficial effects of RBH are consistent with previous studies in HFHF-diet-fed rats[25], high carbohydrate and high fat diet-fed rats[16,17], obese Zucker rats[26], and high-fat diet-fed rats[18].
A previous study demonstrated that long-term feeding of HFHF diet in rats could cause insulin resistance[25]. A possible mechanism of insulin resistance in HFHF feeding is enhancement of de novo lipogenesis in the liver, where fructose is predominantly metabolized[27]. This may result in lipid overload and promote intra-hepatic fat accumulation leading to impairment of insulin signaling[16,25,27]. In this study, we found that chronic feeding with an HFHF diet induced insulin resistance, as indicated by increased FBG and impaired glucose tolerance. After treatment with RBH at 500 and 1 000 mg/kg/day, these metabolic parameters were significantly improved. These beneficial effects of RBH are in agreement with previous studies in which RBH ameliorated insulin resistance and dyslipidemia in HCHF-diet-fed rats[16,17]. The underlying mechanisms of RBH to mitigate insulin resistance and improve insulin sensitivity may be associated with the suppression of lipogenic genes, decreased levels of proinflammatory cytokines, and upregulation of PPAR-γ[16]. Moreover, it has been shown that insulin resistance can cause oxidative stress by increasing ROS production, leading to CVD[12,28]. As insulin resistance is strongly correlated with oxidative stress, reducing oxidative stress has been found to improve insulin sensitivity[29]. Previous studies showed that RBH has antioxidant properties that scavenge ROS and decrease plasma MDA in rats with hypertension and MetS[14,17]. Consistent with previous studies, we found that RBH, especially in a high concentration, reduced oxidative stress as indicated by decreased vascular Oproduction, reduced plasma and cardiac MDA levels, and decreased protein carbonyl level in cardiac tissue in HFHF-diet-fed rats. Although insulin signaling was not examined, we demonstrated that RBH significantly reduced oxidative stress, and this coincided with amelioration of insulin resistance in our MetS rats.

Figure 5. Effect of RBH on cardiac structural changes in all experimental groups. (A) The top panel shows representative photographs of heart sections that were stained with hematoxylin and eosin and imaged using a stereoscope with a 1×objective lens to measure left ventricular wall thickness (B), ventricular luminal area (C), and cross-sectional area (CSA) (D) of the left ventricular. Scale bar = 5 mm. Results are expressed as mean ± SD (n = 6-8). *P < 0.05 vs. the control group, †P < 0.05 vs. the HFHF group.
Autonomic imbalance in the form of sympathetic overactivity and vagal withdrawal is the central pathophysiological mechanism involved in the genesis of obesity and its associated co-morbidities, such as dyslipidemia, insulin resistance, diabetes, hypertension, and cardiovascular dysfunction[9]. LF/HF ratio, one of several HRV parameters, has been used to measure cardiac autonomic tone balance[6]. Increased LF/HF ratio (i.e. depressed HRV) indicates cardiac autonomic tone imbalance[6,7]. Previous studies demonstrated that MetS is associated with cardiovascular autonomic dysfunction[30,31]. Similarly, we found a significant increase in LF/HF ratio in our rat model of MetS induced by an HFHF diet, indicating that long-term consumption of HFHF diet caused autonomic imbalance and treatment with RBH restored HRV. Insulin resistance causes autonomic imbalance[32] and stimulates sympathetic outflow, leading to HRV depression[33]. Therefore, amelioration of insulin resistance by RBH treatment is a plausible means to restore HRV. In addition, we found that HR in rats fed an HFHF diet was significantly higher than that in rats fed a normal diet, and that HR could be decreased by RBH supplementation, supporting the notion that RBH can improve HRV. Apart from insulin resistance, other cardiovascular risk factors including dyslipidemia, hypertension, and oxidative stress have been reported to cause autonomic imbalance[31,34,35]. In the present study, RBH supplementation ameliorated all of these cardiovascular risk factors in HFHF-diet-fed rats. These beneficial effects of RBH therefore may account for the improvement we observed in HRV.
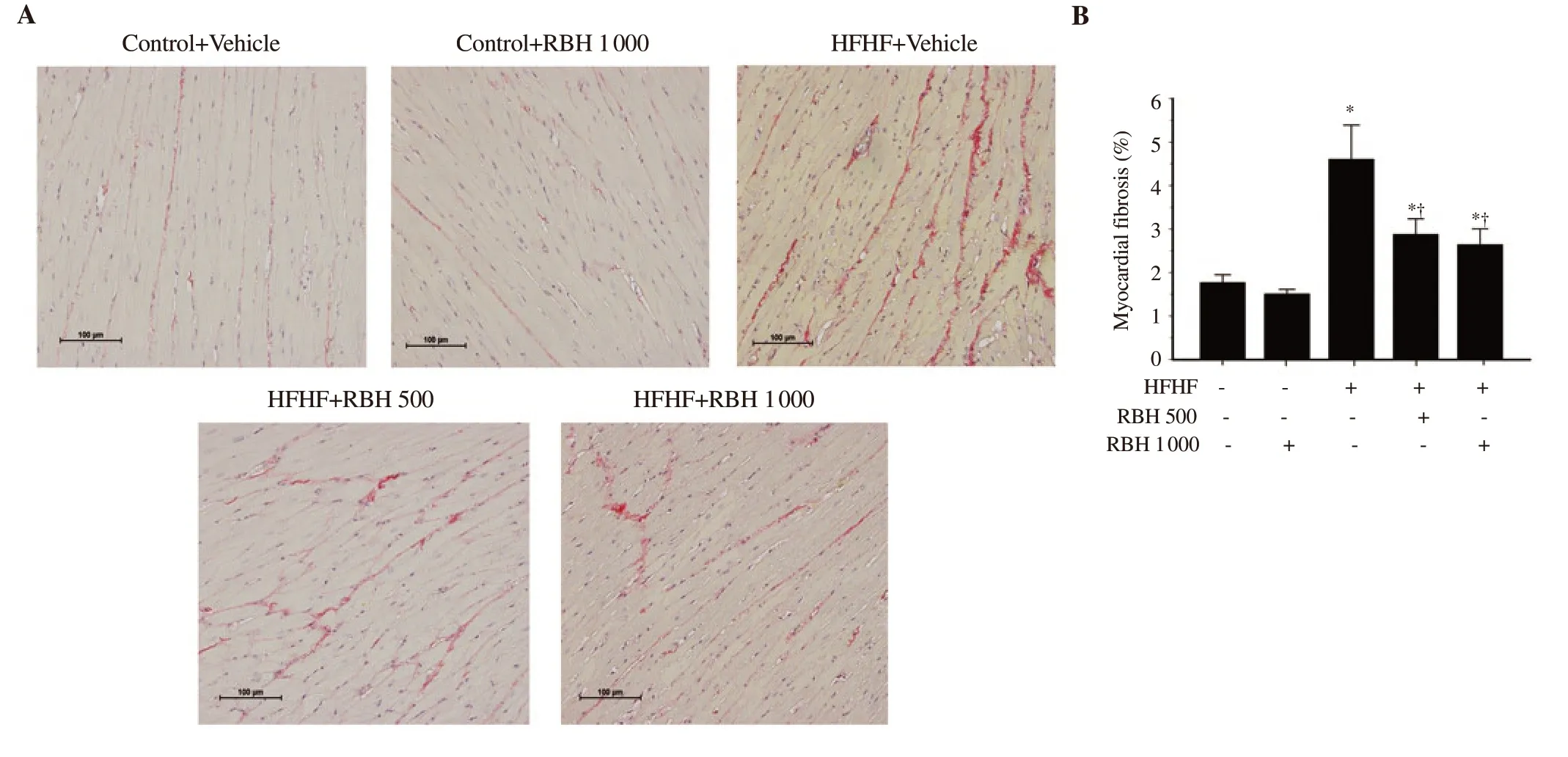
Figure 6. Effect of RBH on myocardial fibrosis in all experimental groups. (A) Representative images of myocardial fibrosis are shown under the light microscope using a 20×objective lens (scale bar = 100 μm). (B) Percentage area of myocardial fibrosis. Results are expressed as mean ± SD (n = 6-8). *P < 0.05 vs. the control group, †P < 0.05 vs. the HFHF group.
Nitric oxide (NO) influences the regulation of cardiovascular function and provides cardioprotection. In the heart tissue, coronary and endocardial endothelial cells and cardiac myocytes are major sources of NO[36]. The major physiological functions of NO derived from eNOS and neuronal nitric oxide synthase are reduction of contractile frequency of cardiomyocytes, attenuation of cardiac contractility, acceleration of relaxation, increasing distensibility of cardiomyocytes, and improvement in the efficiency of myocardial oxygen consumption[36]. Cardiac diastolic dysfunction has been reported to be associated with NOS uncoupling and cardiac oxidative stress[37]. Moreover, dysfunction of NOS may contribute to contractile dysfunction, decreased LV function, adverse remodeling, and myocardial hypertrophy[38]. Apart from NO, increasing evidence supports the idea that enhanced ROS and oxidative stress promote the pathogenesis of cardiac dysfunction, myocardial hypertrophy, and the process of myocardial remodeling[39-41]. The increased oxidative stress impairs NO availability and its signaling in the heart, facilities activation of various pro-inflammatory mediators, and ultimately causes cardiac dysfunction and remodeling[36]. Previous studies have found a marked decrease in eNOS dimer ratio and NO production and an increase in MDA levels in the LV myocardium in HFHF-diet-fed rats[42]. Cardiac expression of eNOS and phospho-Akt were reduced in diabetic OLETF rats[43] and heart MDA levels were increased in high-fat diet-fed rats[18]. Moreover, studies on long-term high fructose diet also showed that elevated myocardial superoxide production preceded diastolic dysfunction and cardiac hypertrophy[44,45]. In agreement with previous reports, we found that HFHF-diet-fed rats showed a significant decrease in eNOS protein expression and an increase in MDA and protein carbonyl levels in the LV myocardium. In addition, we observed cardiac remodeling in HFHF-diet-fed rats, as indicated by a significant increase in LV wall thickness, a decrease in LV lumen area, and a significant increase in myocardial fibrosis in LV. RBH treatment, especially in a high concentration, decreased cardiac MDA and protein carbonyl levels, enhanced eNOS protein expression, and alleviated cardiac structural alterations in HFHF-diet-fed rats. Therefore, our findings suggest the beneficial effect of RBH on the attenuation of cardiac remodeling in HFHF-diet-fed rats. The mechanism of this effect may involve a decrease in cardiac oxidative stress and an increase in NO production through upregulation of eNOS protein expression. However, oxidative stress and impaired NO availability were also associated with cardiac dysfunction, an investigation of RBH supplement on cardiac function is required for elucidating its potential effects in HFHF-diet-fed rats.
Recent studies by our group in hypertensive[14] and MetS rat[17] found that RBH possesses antioxidant activity, increases NO production via upregulation of eNOS, and decreases vascular Oproduction through downregulation of p47phox NADPH oxidase subunit, contributing to the prevention of endothelial dysfunction and vascular remodeling. Furthermore, a study of rats fed on a highfat diet showed that rice bran treatment prevents oxidative damage in the cardiac tissue by increasing cardiac free radical-scavenging activity[18]. In this study, although cardiac free radical-scavenging activity of RBH was not examined, we found that RBH reduced cardiac MDA and protein carbonyl levels, decreased vascular Oproduction and elevated CAT activity which is an antioxidant enzyme in cardiac tissue of HFHF-diet-fed rats. These findings support the beneficial effects of RBH on the alleviation of cardiac oxidative stress in HFHF-diet-fed rats.
There are limitations in this study that should be taken into consideration. Firstly, we implied that HFHF-diet-fed rats developed insulin resistance state as indicated by hyperglycemia and an impaired glucose tolerance test, some parameters including, fasting insulin and homeostatic model assessment - insulin resistance were not measured in this study. Secondly, we recorded ECG while the animal was under anesthesia and this could have some interference on cardiac autonomic control and the HRV index obtained. However, cardiovascular and autonomic effects of anesthetic agents used in this study have already been described and the applied dose of this agent also meets the guideline to induce anesthesia in rats (30-60 mg/kg b.w.)[22]. Moreover, because all animals were under the same condition during ECG recording, the findings on the effect of HFHF diet feeding as well as the effect of RBH on the HRV could still be compared.
In conclusion, chronic consumption of HFHF diet for 16 weeks leads to MetS, autonomic imbalance, cardiac remodeling, and oxidative stress. Treatment with RBH alleviated metabolic abnormalities, restored autonomic balance, improved hemodynamic parameters, reduced LV remodeling, and relieved cardiac oxidative stress. These findings provide evidence of the cardioprotective effects of RBH against cardiovascular complications in MetS. However, additional studies are needed to identify the underlying mechanisms of these effects and their exact role.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgments
We would like to acknowledge Dr. Justin Thomas Reese for editing the manuscript via Publication Clinic KKU, Thailand.
Funding
This work was financially supported by the Young Researcher Development Project of Khon Kaen University, 2018.
Authors
’contributions
KS conceived and designed the research, performed experiments, analyzed the data, and wrote the manuscript. ST and AJ performed the RBH extraction. PS, GT and WS performed experiments. UK conceived, contributed analysis tools, and supervised the work. All authors read and approved the final manuscript.
 Asian Pacific Journal of Tropical Biomedicine2021年5期
Asian Pacific Journal of Tropical Biomedicine2021年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Origanum vulgare L. leaf extract alleviates finasteride-induced oxidative stress in mouse liver and kidney
- Aloin attenuates chronic constriction injury-induced neuropathic pain in rats by inhibiting inflammatory cytokines and oxidative stress
- Argemone mexicana extract alleviates gastrointestinal disorders by stimulating muscarinic receptors and blocking voltage-gated L-type calcium channels
- Honokiol attenuates oxidative stress-induced cytotoxicity in human keratinocytes via activating AMPK signaling
