Early visual function outcomes of topography-guided FS-LASlK and SMlLE in treatment of myopia and myopic astigmatism
Lin-Juan Yang, Xuan Liu, Sheng-Jian Mi, Le Sun, Meng-Xin Chen
Department of Ophthalmology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an 710061, Shaanxi Province, China
Abstract
INTRODUCTION
Advance in surgical techniques and innovation in examination methods have led to better refractive surgeries outcomes in corneal laser surgery in treatment of myopia and astigmatism. As the popularity of refractive surgery increases, the patient’s expectation towards postoperative quality of vision has been elevated. Previous studies have shown that the incidence of intraoperative and postoperative complications were not minimal after traditional corneal refractive surgery[1]. Despite postoperative uncorrected quality of vision 20/20, some patients may still suffer from visual disturbances such as multiple-imaging, glare, and reduced night-vision, which could affect driving and working at night. The main causes include surgically induced spherical aberration or coma caused by small ablation zone or decentered ablation, as well as relatively large amount of preoperative irregular astigmatism[2-3]. Topography guided femtosecond laser-assisted laser in situ keratomileusis (FS-LASIK) aims not only at correcting refractive error, but also at optimizing the postoperative anterior corneal surface. It has been proven to be able to achieve good postoperative acuity of vision and quality of vision[4-5]. Small incision lenticule extraction (SMILE) is a newly developed surgical technique in refractive surgery. It has gained worldwide popularity as a flapless approach. It is minimally invasive, with less postoperative reduction in corneal sensitivity and lower degree of surgically induced spherical aberration. Several studies have compared the efficacy and safety of SMILE vs FS-LASIK[6-9]. In the current study, we aimed to compare the outcomes of topography-guided FS-LASIK and SMILE for myopia and myopic astigmatism in terms of objective and subjective quality of vision measured by corneal higher-order aberrations (HOAs) and contrast sensitivity (CS).
SUBJECTS AND METHODS
Ethical ApprovalThe study adhered to the Declaration of Helsinki and all patients signed informed consent for the surgery.
PatientsThis retrospective comparative study included 49 patients that underwent corneal laser refractive surgery between April and September 2019. Data from the right eye of each patients were analyzed. Totally 23 eyes were treated with Contoura Vision topography-guided FS-LASIK (FS-LASIKCV), while 26 eyes were treated with SMILE. The inclusion criteria were as follows:1) age ≥18y with a stable refraction of more than 2y, i.e. the increase in myopia of no more than 0.50 D per year; 2) spherical equivalent (SEQ) of between -1.00 and -9.00 D; 3) astigmatism not exceeding -3.00 D; 4) preoperative best corrected visual acuity (BCVA) of 20/20 or better; 5) central corneal thickness of at least 500 μm; 6) reliable corneal topography data in eyes treated with FS-LASIKCV. Exclusion criteria were: 1) topographic indication of keratoconus or other corneal diseases; 2) systemic medication, pregnant or active ocular diseases; 3) undergone any ocular surgeries; 4) failed cyclotorsional tracking during FS-LASIKCV. The patients were told to stop using soft contact lens for at least 1wk, rigid gas permeable contact lens for at least 1mo, and orthokeratology lenses for at least 3mo, before the preoperative examinations.
Pre- and Post-operative Evaluation and MedicationAll patients underwent a comprehensive set of preoperative examination including measurements of uncorrected visual acuity (UCVA), BCVA, near visual acuity, and intraocular pressure. Slit lamp examination of the anterior segment and dilated fundus examination were performed to exclude active ocular disease. The same optician performed cycloplegic refraction and manifest refraction (RT-5100, NIDEK, Aichi, Japan). Sirius (Construzioni Strumenti Oftalmici, Firenze, Italy) corneal topography was performed preoperatively and at 1 and 3mo postoperatively to exclude corneal abnormalities such as keratoconic. Zernike coefficients including root mean square (RMS) of total corneal HOAs, spherical aberration, coma, and trefoil aberration under 6 mm diameter pupil were recorded at each visit. CS measurement was performed under photopic condition, scotopic condition with and without glare using the CSV-1000 standardized CS testing instrument (Vector Vision, Greenville, OH, USA). The patients were tested with BCVA preoperatively, and with UCVA at 1 and 3mo postoperatively. The values were converted to log unit for further analyses. Postoperatively, UCVA and autorefraction (AR-1, NIDEK, Aichi, Japan) were performed at 1d, 1wk, 1, and 3mo.
Prior to the surgery, patients were instructed to use 0.3% levofloxacin (Santan Pharmaceutical Co, Ltd., Osaka, Japan) and 0.3% sodium hyaluronate (Santan Pharmaceutical Co, Ltd., Osaka, Japan) eye drops four times a day for 1 to 3d. Postoperatively, 0.3% levofloxacin was prescribed 4 times a day for 7d, 0.1% fluorometholone (Santan Pharmaceutical Co, Ltd., Osaka, Japan) with gradual tapering from 4 to 1 time a day over the course of 40d, and 0.3% sodium hyaluronate 4 times a day for 3mo.
Preoperative topographies acquired by Topolyzer VAIRO (Alcon Laboratories, Inc., Erlangen, Germany) were used for topography-guided custom ablation design in FS-LASIK-CV group. The topographies needed to satisfy the following criteria to be included: 1) At least 4 images with good repeatability (regional difference between images ≤0.5 D); 2) The edges of the pupil and the cornea were normal and clearly recognizable, and the diameter of the pupil was be between 2.5-4.0 mm; 3) The reflection of the Placido ring shall cover at least 75% of the corneal area; 4) Iris images were clearly visible; 5) Images were acquired within 24h prior to the surgery.
Surgical TechniqueThe surgeries were all programmed and performed by the same senior surgeon. Desired refractive outcome was emmetropia in all eyes. Three to five minutes prior to the surgery, local anesthesia was achieved by instillation of 0.5% oxybuprocaine hydrochloride (Santan Pharmaceutical Co, Ltd., Osaka, Japan) twice. Right after the surgery, one drop of mixture of tobramycin and dexamethasone (Alcon, Puurs, Belgium) was given before the application of the protective shield. All surgeries were uncomplicated.
In the FS-LASIK-CV group, Alcon/Wavelight FS200 femtosecond laser (Alcon Laboratories, Inc., Erlangen, Germany) was used for creation of an oval-shaped flap with meridian length of 8.5 to 9.0 mm. The intended flap thickness was 105 to 110 µm. The hinges were set to be perpendicular to the astigmatism axis. After the flap was lifted, stromal ablation was performed with the Wavelight Ex500 excimer laser (Alcon Laboratories, Inc., Erlangen, Germany) with an optical zone of 6.0 or 6.5 mm in diameter. Iris registration was successful in all cases, and no interruption of the laser occurred during the surgeries. After the excimer laser ablation, the interface between the flap and stromal bed was irrigated with balanced salt solution before repositioning of the flap.

Figure 1 FS-LASIK and SMILE visual outcomes A: UDVA at 1d postoperative; B: UDVA at 1wk postoperative; C: UDVA at 1mo postoperative; D: UDVA at 3mo postoperative; UCVA: Uncorrected visual acuity.
The SMILE procedure was performed using the Visumax 3.0 500 kHz femtosecond laser system (Carl Zeiss Meditec AG, Jena, Germany). The pulse energy was set to be 135-140 nJ. The designed cap thickness and diameter was 115 µm and 7.0-8.0 mm, respectively. The optical zone of the lenticule was determined to be 6.0-6.5 mm. The minimum lenticule thickness was set to 10-15 µm. The side cuts were set to locate at 120° with circumferential width of 2 mm. The treatment center was determined by the surgeon under the surgical microscope with fixation target, taking into consideration of the Kappa angle from preoperative topography. The lenticule was removed after the cut by the femtosecond laser.
Statistical AnalysisData were analyzed using SPSS 23.0 software. Kolmogorov-Smirnov test was applied to examine the normality of the data distribution. Continuous variables showed normal distribution and were expressed as mean±standard deviation (SD). Independent t-test was applied to compare the values between the two groups. Repeated measures ANOVA was used to analyze the changes in logCS at different time points. Two-tailed test was used, and a P value <0.05 was considered statistically significant.
RESULTS
Patients’ CharacteristicsTwenty-three patients were included in the FS-LASIK-CV group (11 males, 47.8%; and 12 females, 52.2%), with a mean age of 23.7±5.18y (range, 19 to 38y). The mean SEQ was -5.99±1.51 D (range, -2.5 to -8.50 D), and mean cylindrical refraction was -0.95±0.51 D (range, -0.75 to -1.75 D). The SMILE group comprised of 26 patients (12 males, 46.2%; and 14 females,53.8%) with a mean age of 23.4±4.09y (range, 18 to 34y). The mean SEQ was -5.38±1.10 D (range, -3.50 to -7.75 D), and mean cylindrical refraction was -0.91±0.56 D (range, 0.00 to -1.75 D). There was no significant difference between the two groups in terms of age, preoperative SEQ, cylindrical refraction, central corneal thickness, corneal HOAs (measured at 6 mm pupil size), or intended size of optical zone (P>0.05 in all comparisons; Table 1). No complications such as postoperative infection, delayed corneal epithelial healing, elevated intraocular pressure, or decentered ablation were registered.
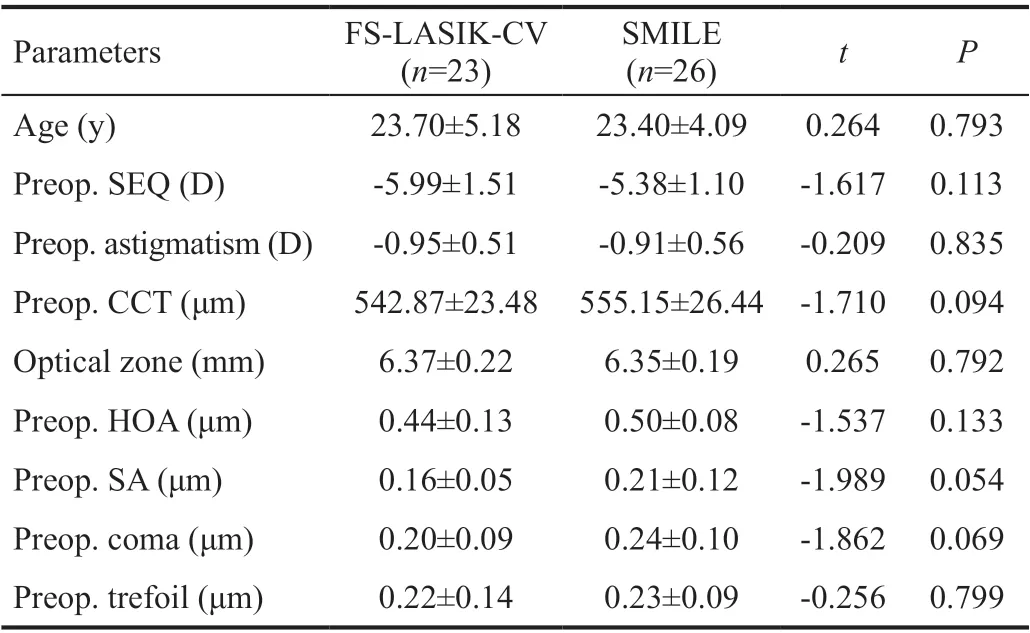
Table 1 Comparison of patients’ characteristics between the two groups mean±SD

Figure 2 The Attempted spherical equivalent refraction versus the achieved spherical equivalent refraction in FS-LASIK-CV group (A) and SMILE group (B).
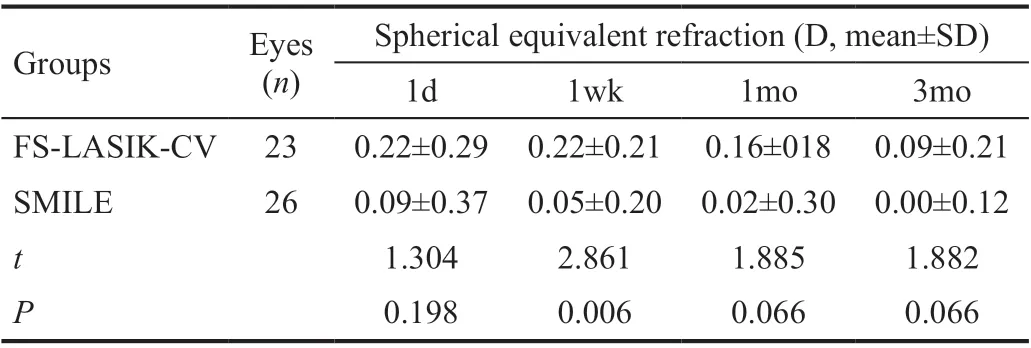
Table 2 Comparison of postoperative spherical equivalent refraction between the two groups
Comparison of Visual Acuity and Refractive Error OutcomesThe visual outcomes was shown in Figure 1. In both groups, 100% of the eyes obtained a UCVA of 20/20 or better at 1wk, 1, and 3mo postoperatively. But UCVA in FSLASIK-CV group was better than in SMILE group at 1d (FSLASIK-CV group 100% obtained a UCVA of 20/20, SMILE group 77% obtained a UCVA of 20/20; Figure 1A) and 3mo Postoperatively (FS-LASIK-CV group 30% obtained a UCVA of 20/13, SMILE group 11% obtained a UCVA of 20/13; Figure 1D). The SEQ was lower in SMILE group than in FS-LASIKCV group at 1wk postoperatively (0.05±0.20 D vs 0.22±0.21 D, t=2.861, P=0.006), but reached target refraction in both groups at 3mo postoperatively (Table 2). At 3mo postoperatively, the mean predictability in FS-LASIK-CV group was 0.09±0.21 D, the regression line value was 0.9837x+0.0005, the mean predictability in SMILE group was 0.00±0.12 D, the regression line value was 1.0003x+0.0002 (Figure 2). Postoperative astigmatism was slightly lower in FS-LASIK-CV group compared to that of SMILE group at all visits, however, the difference was not of statistically significance (P>0.05). In both groups, the residual astigmatism gradually reduced with time (Table 3).
Comparison of Surgically Induced Corneal Higherorder AberrationsAt 3mo postoperatively, the spherical aberration and coma measured at 6 mm pupil size were significantly higher than preoperative levels in both groups (P<0.05). In the FS-LASIK group, the trefoil was lower after surgery (P<0.05), while HOAs was not significantly different between pre- and postoperative measurements. Similarly, the postoperative changes in total HOAs and trefoil were not statistically significant in the SMILE group (Tables 4 and 5). Inter-group comparisons showed less increase in total HOAs in FS-LASIK-CV group than in SMILE group (P<0.05), whereas changes in other higher order aberrations did not show statistically significance (P>0.05; Table 6).

Table 3 Comparison of residual astigmatism between the two groups

Table 4 Comparison of preoperative and 3mo postoperative higher order aberrations under 6 mm pupil size in the FS-LASIKCV group µm, mean±SD

Table 5 Comparison of preoperative and 3mo postoperative higher order aberrations under 6 mm pupil size in the SMILE group µm, mean±SD
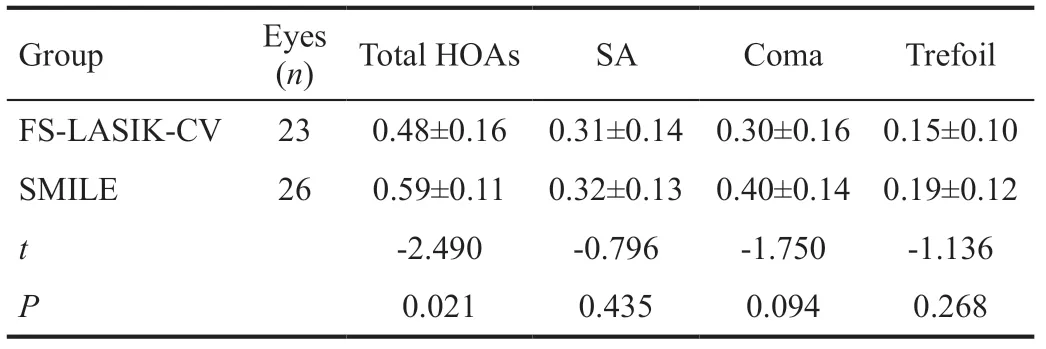
Table 6 Inter-group comparison of higher order aberrations under 6 mm pupil size at 3mo postoperatively µm, mean±SD

Table 7 Inter-group comparison of preoperative logCS under photopic condition and scotopic conditions without glare and scotopic conditions with glare mean±SD
Inter-group Comparison of Contrast Sensitivity FunctionPreoperatively, no significant difference in logCS was detected between the two groups under photopic conditions, scotopic conditions without glare and scotopic conditions with glare (P>0.05; Tables 7 and 8).
Repeated measures ANOVA showed that at 3mo postoperatively, the logCS were better than preoperative levels under scotopic conditions without glare and scotopic conditions with glare in both groups (P<0.05; Tables 9 and 10). Under scotopic conditions without glare and scotopic with glare (12.0 c/d and 18.0 c/d), the inter-group differences and changes with time were all statistically significant (scotopic condition without glare, 12.0 c/d, Fintergroup=22.503, P<0.001; Ftime=10.192, P<0.001; 18.0 c/d; Fintergroup=7.670, P=0.008; Ftime=5.217, P=0.010; scotopic conditions with glare, 12.0 c/d;Fintergroup=10.902, P=0.002; Ftime=6.468, P=0.004; 18.0 c/d: Fintergroup=20.936, P<0.001; Ftime=7.753, P=0.002). Moreover, under scotopic conditions without glare and scotopic conditions with glare (12.0 c/d and 18.0 c/d), there were interactions between logCS and time (F=4.162 and 4.263, P<0.05; F=5.328 and 4.572, P<0.05), indicating that postoperatively, FS-LASIK-CV group had better logCS than SMILE at high spatial frequencies under scotopic conditions without glare and scotopic conditions with glare. LogCS under the other spatial frequencies did not show significant inter-group difference (P>0.05; Tables 9 and 10).
DISCUSSION
The current study showed that both topography-guided FSLASIK and SMILE were efficient, safe, and predictable in treatment of myopia and myopic astigmatism over a 3mo period. There were some differences in the outcomes betweenthe two procedures in terms of postoperative improvement of UCVA and CS.

Table 8 Changes in logCS with time (photopic conditions) mean±SD

Table 9 Changes in logCS with time (scotopic conditions without glare mean±SD

Table 10 Changes in logCS with time (scotopic conditions with glare) mean±SD
Optimal postoperative visual acuity and quality of vision, as well as predictability, stability, and safety are the key factors to success in refractive surgery[9-10]. UCVA directly affects patient’s satisfaction after the surgery, and is thus one of the most important parameters in postoperative assessments. Our outcomes showed that UCVA at 1d postoperatively was better in FS-LASIK-CV group than in SMILE group, whereas no significant difference was detected at 1wk and 1mo postoperatively. This is in line with previous publications comparing FS-LASIK and SMILE[11-12]. the dissociation and extraction of lenticule by a hook during SMILE procedure might increase mechanical injuries of the surrounding stromal tissue, causing slower improvement of visual acuity after SMILE than FS-LASIK[13]. Modern fast repetition rate excimer laser equipped with precise eye tracker system might have contributed to better UCVA in FS-LASIK-CV group at 3mo postoperatively. Moreover, topography-guided customized ablation not only precisely defines the centration of the ablation and compensates for ocular cyclotorsional movement, but also regularizes the irregular astigmatism at the anterior corneal surface, leading to better postoperative UCVA[14-16].
SEQ remained stable after operation in the SMILE group and reached the target refraction at 3mo postoperatively. Figure 2B shows the predictability of the treatment. The equation of y=1.0003x+0.0002 was obtained by performing the linear regression analysis. On the contrary, eyes treated with FSLASIK-CV showed slight overcorrection at early postoperative period, which gradually decreased with time, and reached target postoperative refraction at 3mo postoperatively. The differences in refractive stability between the two surgical approaches might be partly attributed to the differences in postoperative corneal epithelial remodeling behavior[17-18]. In SMILE, Bowman’s layer and anterior corneal stroma are better preserved, which might offer better postoperative biomechanical stability than after FS-LASIK[19]. Furthermore, different nomogram adjustment could have contributed to the differences in postoperative refractive status between the two groups[7].
Centration is one of the challenges during SMILE operation. As a result, despite its efficacy and safety in refractive surgeries outcomes, SMILE has been reported to have induced more HOAs and coma[8,20-21]. The centration of the lenticule in SMILE had significant impact in postoperative HOAs, with less induced postoperative HOAs in corneal vertex normalcentered group than the pupil-centered group[22]. Our outcomes suggested that compensating for the kappa angle using topography as reference have effectively reduced surgical induced HOAs in SMILE. The spherical aberration and coma increased slightly at 3mo postoperatively in both groups (P<0.05), while the trefoil decreased in FS-LASIK-CV group (P<0.05). The changes in corneal morphology could induce the changes of corneal aberrations. The increases in corneal HOAs could be affected by the healing process of the corneal incisions, the stability of the tear film, and the amount of stromal ablation[23]. Therefore, long term follow-up to observe the changes in corneal HOAs is warranted. The increase in total HOAs was lower in FS-LASIK-CV group than SMILE group. The amount of residual astigmatism decreased with time in both groups. Our outcomes also support the superiority of topography-guided FS-LASIK with iris recognition and cyclotorsional compensation in treatment of preoperative anterior corneal HOA and astigmatism.
Compared to visual acuity, CS function test offers more comprehensive evaluation of visual performance and quality under different visual conditions. Previous studies have shown that CS declines after excimer laser refractive surgery, which would gradually improves or even precedes preoperative levels[24-25]. In our study, repeated measures ANOVA showed that the logCS at 3mo postoperatively was better than preoperative level under scotopic conditions without glare and scotopic conditions with glare in both groups (P<0.05). At 1mo postoperatively, the logCS in SMILE group under scotopic conditions without glare and scotopic conditions with glare at spatial frequencies of 12.0 and 18.0 c/d was lower than preoperative level and was lower than the post FS-LASIK-CV level. The CS then gradually increased and surpassed the preoperative level at 3mo postoperatively. This is in consistence with the relatively slow improvement of UCVA in SMILE group during the early postoperative phase, indicating even slower improvement of CSF than UCVA after SMILE. Furthermore, under the scotopic conditions without glare and scotopic conditions with glare at spatial frequencies of 12.0 and 18.0 c/d, there was interaction between logCS and time, indicating that under those conditions, FS-LASIKCV group had better log CS than SMILE group at 1 and 3mo postoperatively. The abovementioned evidences proved that topography-guided FS-LASIK not only improved CS and visual acuity after surgery, but also showed its advantage over SMILE in terms of improvement quality of vision under scotopic conditions.
As a retrospective case study, the current study has some limitations: 1) we lack of data in patient’s subjective evaluation of quality of vision, as well as objective quality of vision assessments such as objective scattering index and modulation transfer function; 2) the follow up time was only 3mo; 3) we designed the non-contralateral eye study, herein we lack of the comparisons of corneal biomechanics and healing results. Studies with long term follow-up and contralateral eye comparisons are warranted. As a conclusion, both surgical approaches demonstrated to have good safety, efficacy, and predictability in treatment of myopia and myopic astigmatism. At early postoperative phase, topography-guided FS-LASIK showed quicker improvement in UCVA and CS under scotopic conditions at high spatial frequencies.
ACKNOWLEDGEMENTS
Conflicts of Interest: Yang LJ,None;Liu X,None;Mi SJ,None;Sun L,None;Chen MX,None.
 International Journal of Ophthalmology2021年3期
International Journal of Ophthalmology2021年3期
- International Journal of Ophthalmology的其它文章
- Protective effects of riboflavin-UVA-mediated posterior sclera collagen cross-linking in a guinea pig model of form-deprived myopia
- Effect of zymosan on the expression and function of the gap-junction protein connexin 43 in human corneal fibroblasts
- LRG1 promotes epithelial-mesenchymal transition of retinal pigment epithelium cells by activating NOX4
- Comparative evaluation of rotational stability of toric lOLs with four-eyelet vs two-eyelet capsular tension rings in eyes with high myopia
- An Ex-Press implant versus trabeculectomy in a fibrotic bleb with late failure after previous trabeculectomy
- Dynamic versus static ultra-widefield fluorescein angiography in eyes with diabetic retinopathy: a pilot prospective cross-sectional study
