Protective effects of riboflavin-UVA-mediated posterior sclera collagen cross-linking in a guinea pig model of form-deprived myopia
Ding Han, Mei-Nan He, Ying Zhu, Yan Zhang, Rui-Hua Wei
Tianjin Key Laboratory of Retinal Functions and Diseases, Eye Institute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China
Abstract
INTRODUCTION
Myopia is the most commonly seen refractive error that causes vision damage and blindness worldwide[1-2]. The length of the vitreous chamber of myopic population is increased[3]. During the past decade, the prevalence of myopia has been on the rise, and the age of myopic population becomes younger[4]. In China, more than 70.0% of the children are myopic[5-6]. The percentage of high myopia also exhibits a rising tendency, reaching 40% in the Asian countries[7]. Complications caused by high myopia, including glaucoma and retinal degeneration, have also occurred at a higher frequency[8-11].
The main characteristic of myopia is continuing elongation of axial length, which is accompanied by sclera degeneration and dilation. Although many approaches have been used to manage myopia, including spectacle glasses, contact lens, and refractive surgery, these approaches can only correct refractive errors, none of them stops axial elongation.
However, recent studies have shown that collagen crosslinking induced by riboflavin-ultraviolet A (UVA) can enhance the anti-traction function of sclera and anti-hydrolysis function of its protein components[12-15]. In 2003, Wollensak et al[12]first reported the safety and effectiveness of riboflavin-UVA-induced cross-linking for treatment of progressing keratoconus[12]. The riboflavin-UVA-induced cross-linking’s effect on axial elongation was first demonstrated in 2004[15]. Subsequently, a series of studies were set to test its safety and efficacy culture system and on sclera in rabbits[13-14,16-17].
These studies mainly observed the impact of the cross-linking on normal subjects, very few study has explored the effect of the sclera crosslink on animal models of induced myopia[18-19]. Moreover, most of the previous studies applied riboflavin to the sclera anterior to or at equatorial region. This is surprising given the fact that myopia is predominantly caused by elongation of sclera at the posterior pole[20]. Therefore, in the current study, the riboflavin-UVA-induced cross-linking was performed at the posterior sclera of the eyeball, and the effects of the cross-link was evaluated in a guinea pig model of formdeprived myopia.
MATERIALS AND METHODS
Ethical ApprovalAll the experimental procedures conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision research, and were approved by the Laboratory Animal Care and Use Committee of Tianjin Medical University (No.SYXK2009-0001).
Animals and Designing ExperimentTwenty-five pigmented guinea pigs at three weeks of age were purchased from the experimental animal farm, Lutown, Danyang City (Jiangsu Province, China). The animals were subjected to ocular and optometric examinations upon arrival, and those with eye diseases, myopia, and anisometropia more than 1.5 diopters were excluded. The rest of the guinea pigs were raised at 25ºC±1ºC with a relative humidity of 40%-70% and a 12h light: 12h dark illumination cycle in the animal facility of Tianjin Medical University Eye Institute. The animals were fed ad lib with food, water and vegetables.
The included guinea pigs were randomly divided into four groups: the normal control group (NOR, n=7) that did not receive any treatment; the form-deprived myopia group (FDM, n=7) that revived from-deprivation by wearing a mask over the right eye; the normal control with cross-linking group (NOR+CL, n=5) in which normal controls received riboflavin-UVA-induced cross-link; and the form-deprived myopia and cross-linking group (FDM+CL, n=6) that received riboflavin-UVA-induced cross-link first and then underwent form deprivation. For all the animals, the right eyes were subjected to experimental manipulations and the left eyes served as selfcontrols. Retinoscopy, A-scan, and keratometry were used to measure refractive error, axial length, and corneal curvature, respectively, at baseline and on a weekly basis after the form deprivation started. Having been form deprived for four weeks, the animals were sacrificed for the morphological and molecular analyses.
Figure 1 The form-deprived myopia model of guinea pig A: The uncovered eye; B: The covered eye.
Riboflavin-UVA-Mediated Cross-Linking of Posterior ScleraThe guinea pigs in the riboflavin-UVA-mediated cross-linking groups underwent systemic anesthesia by an intramuscular injection of ketamine hydrochloride. Oxybuprocaine hydrochloride eye drops were topically applied as local anesthesia. The upper nasal quadrant conjunctiva was incised, the retractor muscle and the superior oblique muscle were dissected at the scleral insertion point. Two scleral sutures (Dacron 10-0) were placed at 2 mm posterior to the limbus to rotate the eye ball and expose the posterior and equatorial sclera of the upper nasal quadrant. Photosensitizer solution containing 0.1% riboflavin (dissolved in 20% dextrin, Solarbio, China) was dripped onto the posterior and equatorial sclera, which was immediately irradiated through a UVA diode (CL01, Weishijian Suzhou, China) at a wavelength of 370±5 nm for 30min. The UVA irradiation was applied at 6 mm from the sclera with surface irradiance of 3 mW⁄cm2and treatment area of 6.6 mm2. The riboflavin-containing solution was applied every 5min during the irradiation to avoid dryness of the sclera. After irradiation, the scleral sutures were removed, tobramycin ointment was topically applied, and the conjunctiva was closed with a 10-0 suture. The animals were then treated with or without FDM induction.
Form-Deprived Myopia ModelFacemask was made from white latex balloon of size 6 (Xiong-Xian Latex Factory, China) with 5%-7% light transmission. The facemasks only covered the right eyes of guinea pigs, leaving the left eyes, nose, mouth, and ears uncovered (Figure 1).
Ocular RefractionEye drops of 0.5% tropicamide (Xingqi, Shen Yang, China) was administered to completely dilate pupil. Retinoscopy was performed in a dark room using a streak retinoscopy (YZ24, Su Zhou, China) and trial lenses by a single experienced examiner. Each eye was measured three times, and the mean value of the equivalent spherical was recorded as the refractive error.
Axial LengthA-scan (KN1800, Wuxi, China) was used to measure the axial length. The ultrasonic frequency wasset to 10 MHz. The 0.5% oxybuprocaine hydrochloride (Santen, Japan) was administered as topical anesthesia. The ultrasonic probe directly touched the cornea during the axial measurement and each eye was measured 5 times, the average was recorded as the axial length.
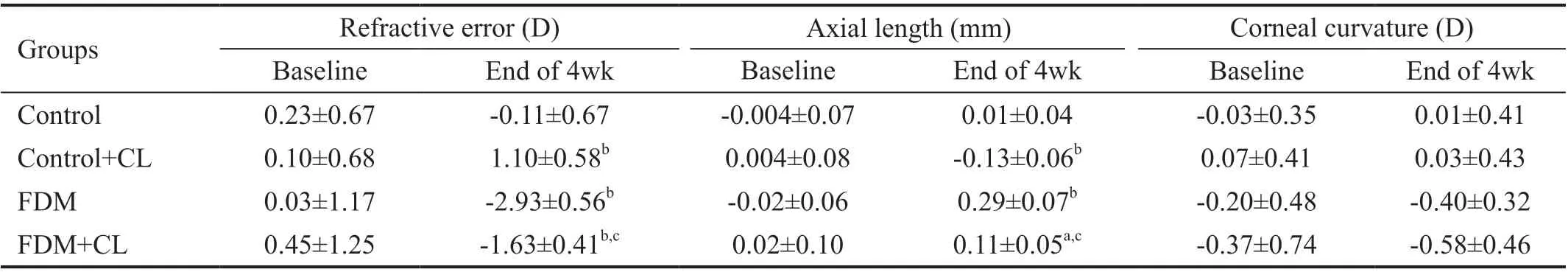
Table 1 Interocular difference in refractive error, axial length, and corneal curvature
KeratometryCorneal curvature was measured with a keratometer (BL-8001, China). A +8.0 D lens was attached to the front of the keratometer to magnify the cornea to be measured. Three readings were obtained for each cornea, and the real corneal curvature of guinea pigs was calculated by multiplying the mean of these three readings with a coefficient of 0.451[21].
Biomechanical TestAt the end of the experiment, the anterior eye segment of the eye ball was removed by a circumferential incision around limbus. A small rectangular scleral strip (3.8 mm2), containing the cross-linking area, was acquired from the posterior eye cups. The scleral strip with the similar shape was obtained as a control from the contralateral eye at the comparable position. The thickness of the scleral strips was determined with a mechanical micrometer caliper. Stress-strain measurements were performed on the sclera strips through clamping it horizontally between the jaws of a microcomputercontrolled biomaterial tester (MTF-100; ZSL-1; Tianjin University of Technology). The initial distance between the two jaws was 3 mm, and the strain was increased at 1 mm per minute, the stress was measured till tissue rupture. The ultimate stress r (MPa) and ultimate strain ε (%) of the sclera strips were recorded. The slope of the stress-strain plot was considered as the elastic modulus E (MPa).
Histological ExaminationAt the end of the experiment, two eyes from each group were selected randomly for histology examinations. The posterior eyecups were fixed in 4% neutralbuffered formalin for at least 24h and embedded in paraffin. A 7-µm paraffin sections were stained with haematoxylin and eosin. The stained sections were observed and the pictures were captured under a light microscope (OLYMPUS DP73, Japan) at magnifications of 400 times.
Statistical AnalysisStatistical analyses were performed with SPSS software Version 19.0. A linear regression with mixed effect model was used to analyze the interocular difference (right eye minus the left eye) of refractive errors, corneal curvature, and axial length. Post hoc comparison between individual groups was conducted with Tukey-test. The interocular difference in biomechanical properties was analyzed with one-way ANOVA, and comparison between individual groups was done by with Bonferroni test. P<0.05 was deemed as statistically significant.
RESULTS
Refractive ErrorThe interocular difference in refractive errors (IDRE=RE of right eye-RE of left eye) was examined immediately before and at weeks 1, 2, 3, and 4 following FDM induction. At baseline, there was no significant difference in IDRE among the four groups (all P>0.05). As time proceeds, the IDREs in the two FDM groups, including FMD group and FDM+CL group, became progressively greater, while the two groups without FDM treatment (normal control and the control+CL) showed little change in IDRE. Mixed-effect linear model revealed a significant effect of FDM (P<0.01). It also revealed that riboflavin-UVA-mediated cross-linking had a significant effect on reducing IRDE in the FDM groups as compared to the groups that had not received FDM induction (P<0.001). However, there was no interaction between FDM and CL (P=0.191) treatment factors (Table 1 and Figure 2).
Axial LengthThe interocular difference in axial length (IDAL=axial length of right eye-axial length of left eye) was monitored on a weekly basis after FDM induction. At baseline, there was no significant difference in IDAL among the four groups. However, as time proceeds, the IDALs in the two groups received FDM treatment (the FMD only and the FDM+CL) became progressively longer, while the two groups without FDM treatment (the control and the control+CL) showed little change in IDAL. Mixed-effect linear model revealed significant effect of FDM (P<0.001). It also revealed that the cross-link had a significant effect on reducing the IRAL in the groups that had received CL versus the groups that had not undergone CL (P<0.01). However, there was no interaction between FDM and CL (P=0.342; Table 1 and Figure 3).

Figure 2 Line plot showing the changes in interocular difference in refractive errors (IDRE) in the normal control (open square), the FDM (open circle), the control+CL (filled square), and the FDM + CL (filled circle) bCompared to control group, P<0.01; cCompared to FDM group, P<0.01.

Figure 3 Line plot showing the changes in IDAL in the normal control (open square), the FDM (open circle), the Control+CL (filled square), and the FDM+CL (filled circle) aCompared to control group, P<0.05; bCompared to control group, P<0.01; cCompared to FDM group, P<0.01.

Figure 4 Line plot showing the changes in IDCC in the normal control (open square), the FDM (open circle), the control+CL (filled square), and the FDM+CL (filled circle) aCompared to control group, P<0.05; cCompared to FDM group, P<0.01.

Figure 5 Line plot showing the changes in interocular difference of biomechanical properties in the normal control (open square), the FDM (open circle), the control+CL (filled square), and the FDM+CL (filled circle).
KeratometryThe interocular difference in corneal curvature (IDCC=corneal curvature of right eye-corneal curvature of left eye) was also evaluated during the experimental period. At baseline, there was no significant difference in IDCC among the 4 groups. The IDCCs in the two groups received FDM (the FMD only and the FDM+CL) did not exhibit significant difference from that in the groups without FDM (the control and the control+CL). Mixed-effect linear model revealed no significant effect of FDM (P=0.062). It also revealed that cross-link did not generate a significant effect on IDCC among the experimental groups (P=0.3615). There was no interaction between FDM and CL either (P=0.252; Table 1 and Figure 4).
Biomechanical MeasurementsThe interocular difference in biomechanical measurements (biomechanical measurement of right eye-biomechanical measurements of left eye) was determined at the end of 4wk. The two groups that had received riboflavin-UVA-mediated cross-linking showed significantly higher ultimate stress than the corresponding control groups (Table 2). The elastic modulus values were also exhibited the similar trends among the experimental groups. However, there was no significant difference in the ultimate strain among the four groups (Table 2 and Figure 5).
Histological EvaluationIn the cross-linked eyes, the cornea, lens, optic nerve and retina were intact and without abnormalities compared to the self-control eyes. There was no evidence of pathological findings, such as inflammation, degeneration, atrophy, gliosis, or necrosis in the sclera. There was no histological difference in the retina among the four groups (Figures 6 and 7).

Figure 6 The histological picture of the retina A: NOR group; B: FDM group; C: NOR+CL group; D: FDM+CL group.

Figure 7 The histological picture of sclera A: NOR group; B: FDM group; C: NOR+CL group; D: FDM+CL group.

Table 2 Interocular difference in biomechanical properties
DISCUSSION
In this study, the effects of riboflavin-UVA-mediated crosslinking on sclera on ameliorating myopic changes in a guneia pig model of FDM. In the discussion, we will address the issues of effectiveness, safety, and extra cares taken by this study, and the potential mechanisms underlying the findings.
There are two key steps in our study. The first one was to induce myopia. As shown in the results, all the form-deprived eyes, in both FDM and FDM+CL groups, developed myopia, with the diopter ranging from 0.03±1.17 D and 0.45±1.25 D at the baseline to -2.93±0.56 D and -1.63±0.41 D, respectively at 4wk post FDM as compared to the contralateral self-control eyes. Meanwhile, the axial length elongated from -0.02±0.06 mm and 0.02±0.10 mm at baseline to 0.29±0.07 mm and 0.11±0.05 mm, respectively at 4wk relative to the contralateral self-control eyes. These results were consistent with those of the study conducted by Lu et al[21].
The second one was to slow the progression of myopia with riboflavin-UVA-mediated cross-linking. This was confirmed by the significantly reduced myopia in the FMD+CL group when compared to the FMD only group. We also noticed that, even in the control+CL group, the cross-linked eyes developed a mild hyperopia compared with the contralateral eyes, whose diopter ranging from 0.10±0.68 D at baseline to 1.10±0.58 D at 4wk post FDM induction. It was statistically significant when compared with the NOR group, which ranging from 0.23±0.67 D at baseline to -0.11±0.67 D at 4wk (P<0.01). While cross-linking can reduce the refractive error in the control group, there was almost no elongation of the axial length. This result is similar to the study by Dotan et al[22].
One interesting observation in our study was that the development of myopia did not proceed with an even speed. At the beginning of the FDM, the corneal curvature became flattened, but with time, the elongation of the axial length played an important role in myopia progression. And we found that the myopia of FDM group changed from 0.03±1.17 D to -2.16±0.69 D at 2wk and to -2.93±0.56 D at 4wk. The progression of FDM during the first 2wk took up 74% of the changes occurred during the 4-week period. This may indicate that myopia development intervention could be more effective at the beginning of the body growth.
A therapeutic strategy should also be safe if it has any value in the future application to the clinic. In the study, the changes should happen localized in the sclera without damage to the retina, which is critical for visual function. The main characteristic of myopia is that the continuing elongation of the axial length, which inducing sclera thinning and posterior scleral staphyloma. Previous studies on myopic sclera have reported a derangement of collagen fibrils, a larger variation of fibril diameters, and an increase of collagen fibrils with unusual cross-sectional shapes[23]. The ultramicroscopic alterations seen in our study agreed with those previous findings. In the FDM group, the fiber buddle in sclera was found to be considerably thinner than normal, and there appeared some decrease in the number of fibroblasts. This suggested a derangement of the growth and organization of the collagen fibrils—either due to abnormal fibril formation or due to the presence of accentuated breakdown or catabolism of the sclera[24-25].
In the cross-linked eyes, the sclera collagen fiber thickness was incrassation and the fiber buddle arrangement was more regular. Moreover, after the 4wks’ experiment, there were no histological signs of retina or retinal pigment epithelium damage in the cross-linked guinea pig eyes compared to the contralateral eyes. Whether there are functional changes in the retina remains unclear. As a next step, we plan to investigate the changes of electroretinogram (ERG) and visual evoked potentials (VEP) in the treatment of riboflavin-UVA in FDM of guinea pigs to test any potential functional alterations.
As reported in previous literatures, the development of myopia is affected by many factors. To successfully demonstrate the effectiveness of certain therapeutic strategy, great cares have to be taken to isolate the desired effect from the noisy factor. In this study, we took extra care in the following several aspects. First, previous studies usually chose the equatorial sclera as the target area, but in this study, the posterior sclera was chosen as the target for the reason of that the posterior sclera makes an initial change in myopia progression. It can be thought that this area may take a credible role in the progression of the high myopia[26-27].
Second, in contrast to chemical cross-linking methods such as glyceraldehyde injection[28-29], the extent of the treatment area was easily controllable with UVA irradiation because of the visible UVA-light-induced fluorescence of riboflavin. Therefore, this physical cross-linking method is especially advantageous for localized pathology at posterior sclera such as staphyloma. The limitations of this cross-linking include exposure of the sclera and lower efficiency compared with the chemical cross-linking. Therefore, the UVA irradiation is safer than the glutaraldehyde with fewer toxins and less extent of treatment.
Third, many factors may influence the experimental results, such as the scleral thickness which changes with dryness, the degree of sclera saturation by riboflavin[30], the duration of crosslinking[31], and the volume of choroidal blood filling. In this study, we took great care to soak the sclera sufficiently with riboflavin before and during the irradiation to maximize the UVA absorption and to avoid the drying of the sclera. To avoid side effects on the retinal and RPE cells by scattered UVA light, the UVA diode was strictly positioned perpendicular to the scleral surface.
Studies have shown that in the process of myopia development, the scleral remodeling take an important role, and Riboflavin-UVA collagen cross-linking could induce the proliferation of the scleral fiber to strengthen the sclera and enhance the antienzyme ability to inhibit myopia progression[12,15,32]. Riboflavin-UVA-induced collagen cross-linking has been applied to treat progressive keratoconus[12,33-36]. Since the changes of collagen in the posterior sclera of high myopia are similar with the changes occurred in keratoconus, the same treatment principle could be applied to both diseases[37-38].
In mammals, the scleral tissue contains approximately onethird collagen by weight, consisting predominantly of type I collagen[39]. The study from Hayes et al[32,40]has shown that the riboflavin-UVA collagen cross-linking mainly occurred in type I collagen. The free radicals and reactive oxygen species (ROS) generated by the riboflavin-sensitized UVA photoreaction could induce cross-linking of the collagen type I molecule chains and lead to significantly increased molecular weight[41].
An evaluation of the biomechanical parameters demonstrated an increase in the biomechanical stiffness of cross-linked sclera as compared to the uncross-linked sclera. We think that the biomechanical parameters of sclera are improved by treatment with riboflavin-UVA collagen cross-linking. Cross-linking enhances sclera’s anti-traction and anti-hydrolysis capabilities so that it would have sufficient resistance to intraocular pressure to maintain the size and shape of the ocular globe, and finally to slow down the myopia progression.
In conclusion, the posterior sclera collagen cross-linking induced by riboflavin -UVA can slow down the progress of myopia and increase the sclera biomechanical strength.
ACKNOWLEDGEMENTS
Foundations:Supported by the Tianjin Clinical Key Discipline Project (No.TJLCZDXKQ013); the Research Project of Health Committee in Binhai District, Tianjin (No.2019BWKQ033).
Conflicts of Interest: Han D,None;He MN,None;Zhu Y,None;Zhang Y,None;Wei RH,None.
 International Journal of Ophthalmology2021年3期
International Journal of Ophthalmology2021年3期
- International Journal of Ophthalmology的其它文章
- Corneal stromal mesenchymal stem cells: reconstructing a bioactive cornea and repairing the corneal limbus and stromal microenvironment
- Real-world outcomes of two-year Conbercept therapy for diabetic macular edema
- Role of home monitoring with iCare ONE rebound tonometer in glaucoma patients management
- Comparing posture induced intraocular pressure variations in normal subjects and glaucoma patients
- High interpretable machine learning classifier for early glaucoma diagnosis
- Micropulse laser trabeculoplasty under maximal tolerable glaucoma eyedrops: treatment effectiveness and impact of surgical expertise
