玉米田两种阔叶杂草藜及苍耳对草甘膦的敏感性测定
贾芳 崔海兰 李香菊 于惠林
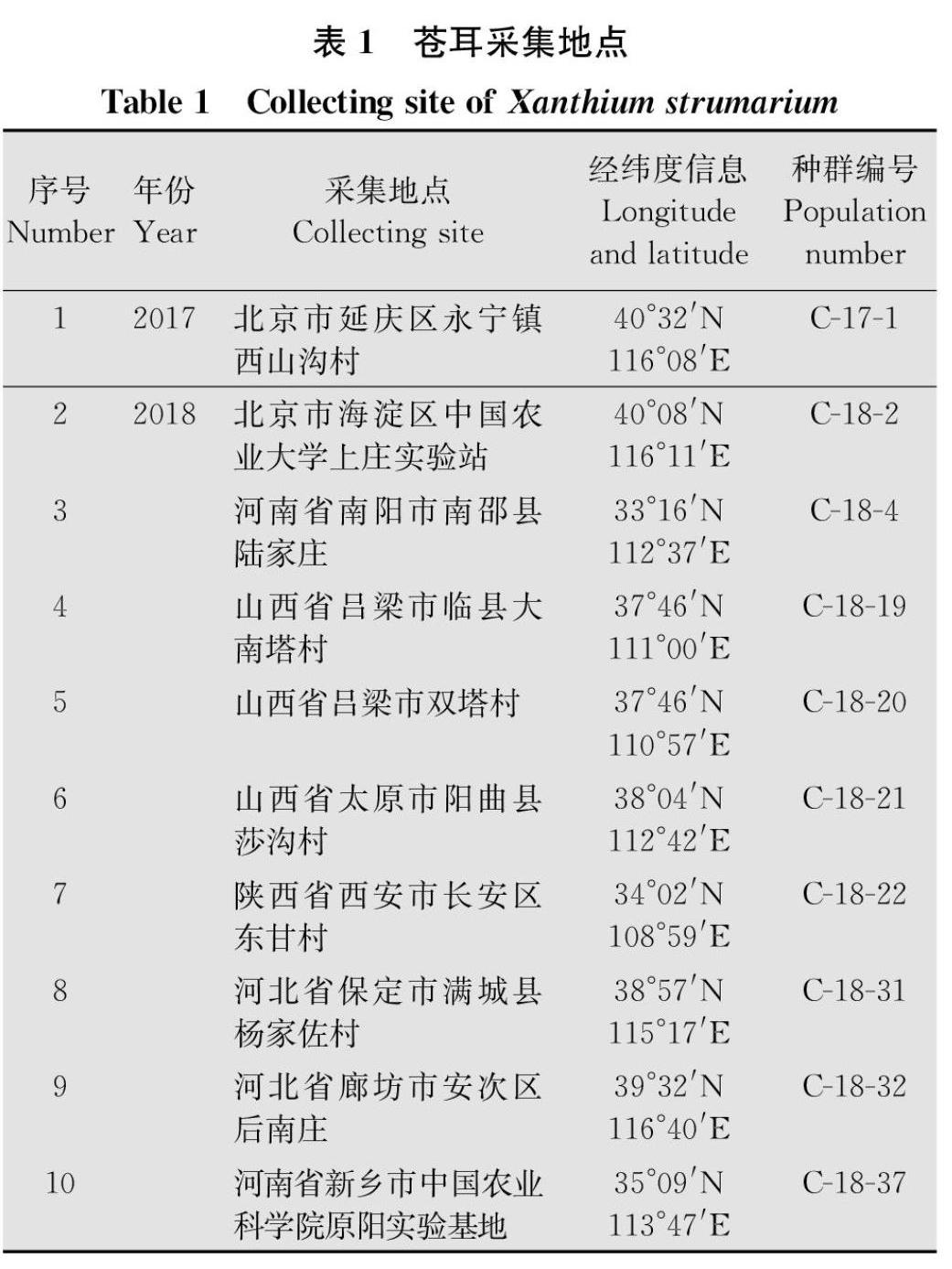
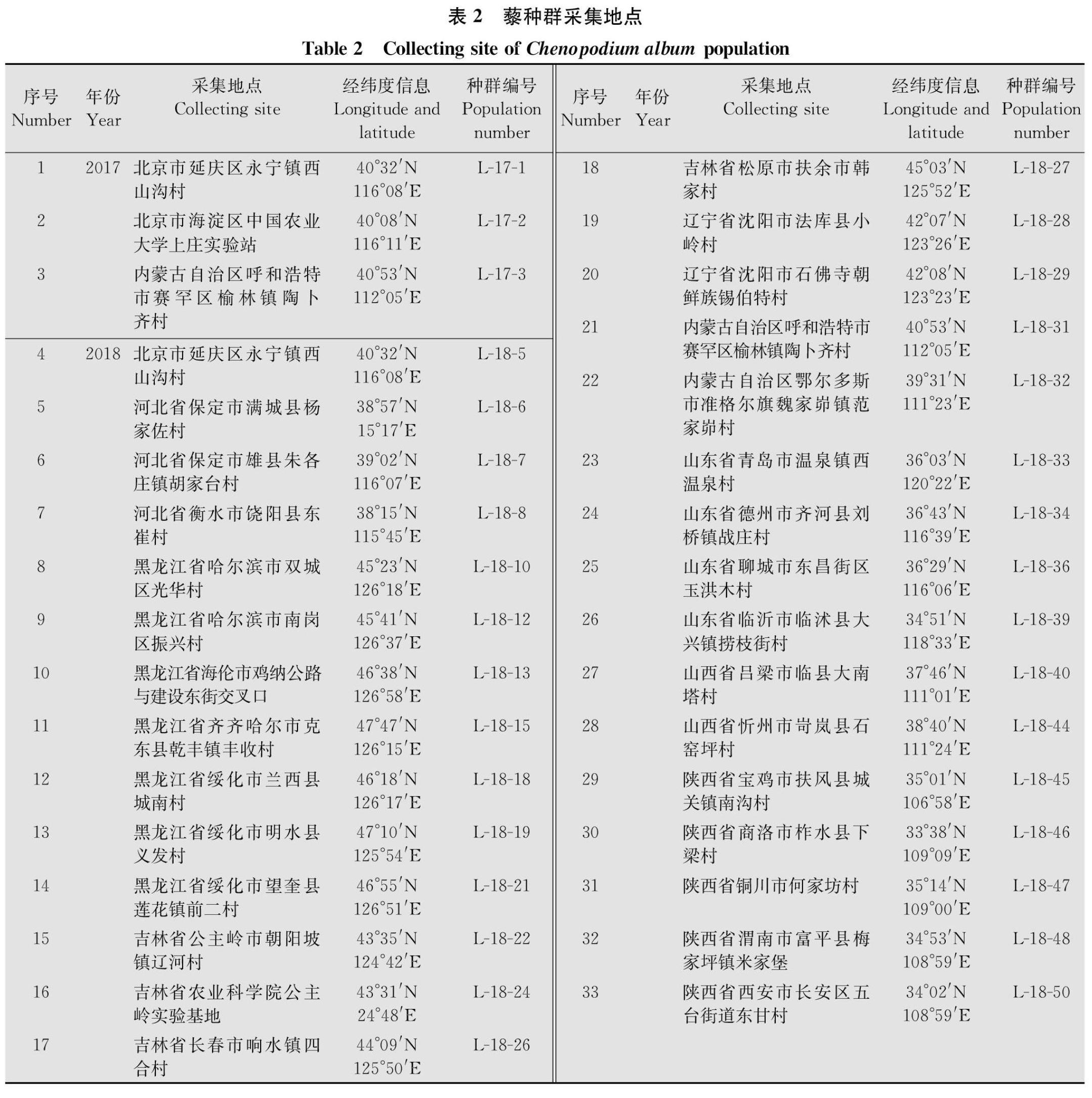
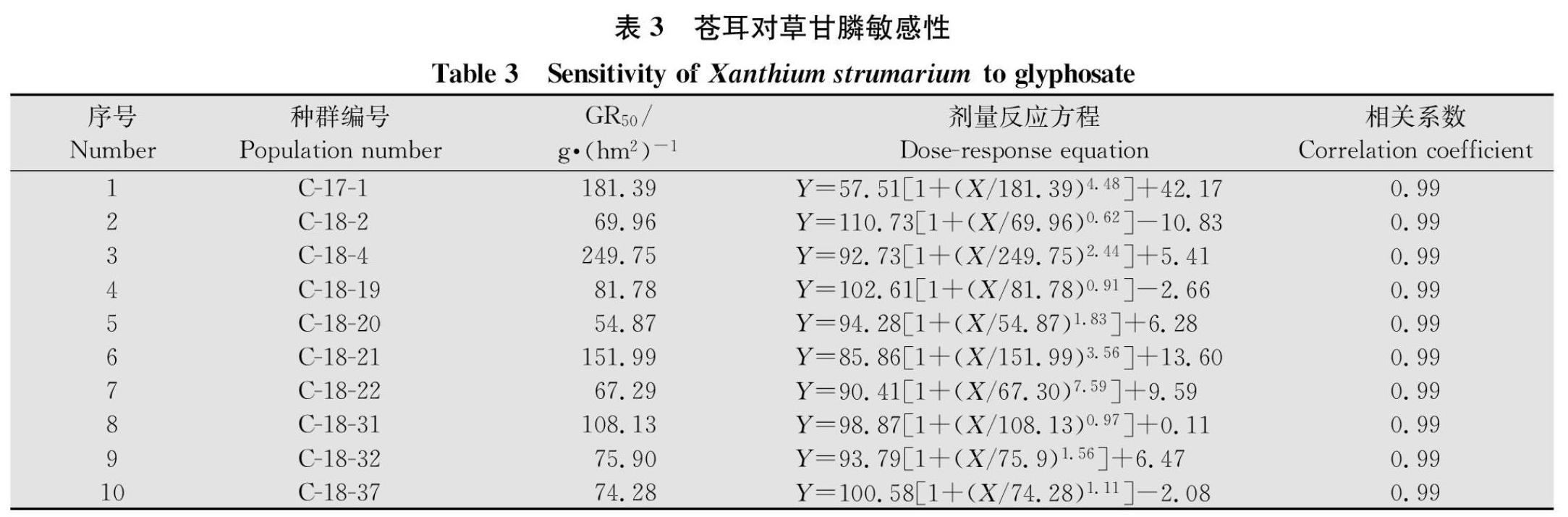
摘要 :蒼耳Xanthium strumarium和藜Chenopodium album是常见的两种阔叶杂草,其在玉米田发生严重,影响了玉米的生长和产量。本文利用整株生物测定法对我国东北及黄淮海玉米产区采集到的苍耳及藜种群对草甘膦的敏感性进行了测定。种子室内培养至5~6叶期,喷施草甘膦后14 d称量鲜重,计算抑制杂草种群50%个体生长的草甘膦剂量(GR50)。草甘膦对10个苍耳种群GR50在54.87~249.75 g/hm2,平均值为(111.53±20.02)g/hm2,均低于450 g/hm2(1/2倍推荐剂量),表明10个苍耳种群均对草甘膦敏感;草甘膦对33个藜种群GR50范围在97.05~920.86 g/hm2,平均值为(313.88±24.70)g/hm2,93%的种群GR50低于450 g/hm2,表明绝大多数藜种群对草甘膦比较敏感,仅发现一个藜种群GR50大于900 g/hm2(田间推荐剂量),表明其对草甘膦具有一定耐受性。
关键词 :杂草; 玉米; 草甘膦; 敏感性
中图分类号: S 451.22
文献标识码: B
DOI: 10.16688/j.zwbh.2019596
Sensitivity of two broadleaf weeds Xanthium strumarium and Chenopodium album to glyphosate in corn fields
JIA Fang, CUI Hailan, LI Xiangju, YU Huilin*
(Institute of Plant Protection,Chinese Academy of Agricultural Sciences, Beijing 100193,China)
Abstract :Xanthium strumarium and Chenopodium album are two common broadleaf weeds, and their occurrence seriously affected corn growth and reduce yield in corn fields. In our study the whole plant bioassay was used to determine the sensitivity to glyphosate of ten populations of X.strumarium and 33 populations of C.album collected from two major corn producing areas, Northern China and Huanghuaihai. The seeds of two weeds were sowed in greenhouse, and the glyphosate was applied at the 56 leaf stage of the weeds. 14 days after glyphosate application, fresh weight of plants was weighted and GR50 values were calculated, respectively. GR50 values of glyphosate to ten populations of X.strumarium ranged from 54.87 to 249.75 g/hm2 with the average value of (111.53±20.02)g/hm2, which were lower than 450 g/hm2 (1/2 recommended dose), showing that all populations of X.strumarium were sensitive to glyphosate. Among C.album populations, GR50 values ranged from 97.05 to 920.86 g/hm2 and its average value was (313.88±24.70)g/hm2. The GR50 values of 93% C.album populations were less than 450 g/hm2, indicated that the vast majority of C.album populations were sensitive to glyphosate. And only one population of C.album showed a GR50 value of above 900 g/hm2 (recommended dose), indicating that it had a certain degree of tolerance to glyphosate.
Key words :weed; corn; glyphosate; sensitivity
玉米是重要的粮食、经济和饲料作物,在我国农业生产和国民经济中占据重要地位。杂草的发生与危害是制约玉米高产的重要因素[1]。玉米田杂草的防除主要依赖于化学防除,然而近年来越来越多的抗除草剂(包括莠去津和乙酰乳酸合成酶抑制剂等)杂草种类被发现[2],不但降低了除草效果还增加了除草成本[3]。自1996年美国孟山都公司首次推出转基因抗草甘膦(glyphosateresistant,GR)大豆以来,使得灭生性除草剂草甘膦应用于作物田,杀死杂草而不伤害作物,除草方式不但变得简单灵活,而且提高了除草效果、降低了除草成本,近年来随着全球转基因技术研究与产业应用快速发展,种植转基因抗除草剂玉米已成为玉米田杂草防除的一个重要手段。至2018年,全球转基因抗除草剂(包括IR/HT复合性状)玉米种植面积为0.53亿hm2[4]。
2.2 藜对草甘膦的敏感性
藜不同种群对草甘膦的耐受水平如表4所示,GR50的范围在97.05~920.86 g/hm2,GR50低于450.00 g/hm2的种群占全部种群的93.94%(图1),GR50平均值为(313.88±24.70)g/hm2;黄淮海地区藜种群的GR50均低于450.00 g/hm2(图1),平均值为(273.46±22.79) g/hm2(图3);北方地区藜种群GR50低于450.00 g/hm2的种群占北方地区全部种群的87.50%(图1),平均值为(356.83±30.02)g/hm2(图3)。内蒙古陶卜齐村连续两年采集到的种群L173和L1831的种群GR50最高,分别为545.33和920.86 g/hm2(表4)。在各个省份藜种群GR50平均值中,内蒙古最高,为(596.42±196.32)g/hm2;山东省最低,为(127.61±30.53)g/hm2(图2)。
3 讨论
本文从黄淮海玉米产区采集到的10个苍耳种群,其GR50均值为(111.53±20.02)g/hm2,所有種群对草甘膦均敏感;从北方及黄淮海玉米产区采集33个藜种群GR50均值为(313.88±24.70)g/hm2,其中大多数种群很敏感,但个别种群如内蒙古陶卜齐村藜种群GR50为920.86 g/hm2,对草甘膦有一定的耐受性,需引起密切的关注。
天然对草甘膦具有耐受性的杂草与经过长期筛选而存活下来的抗性杂草相比,因其天然耐受除草剂的能力可遗传,对转基因抗草甘膦作物的推广和种植危害更大[17]。随着转基因耐草甘膦作物种植面积日益增长,草甘膦的使用量逐渐增加[33],越来越多的对草甘膦有天然耐受性的杂草种类被发现[34],其对草甘膦的耐受机制也被解析。比如杂草有独特的EPSPS蛋白结构(如田旋花)、EPSPS拷贝数及基因表达量增加(如阔叶山麦冬Liriope muscari)、杂草植株有利的形态生理特性(瘤梗番薯Ipomoea lacunosa)和有效的代谢及传导(如苘麻Abutilon theophrasti)等赋予了杂草对草甘膦的天然耐受性,并且一些杂草对草甘膦的耐受性同时存在多种机制[14]。
在美国,1989年Barrentine和McWhorter首次发现在密西西比的大豆田中苍耳对ALS类除草剂氯嘧磺隆和咪唑乙烟酸产生抗性[35],此后越来越多关于苍耳抗ALS类除草剂(灭草喹、咪唑烟酸、咪唑乙烟酸、氯酯磺草胺等)的报道[3640]。玉米田中常见杂草绿穗苋Amaranthus hybridus、长芒苋A.palmeri、反枝苋A. retroflexus、豚草Ambrosia artemisiifolia、地肤Kochia scoparia、牛筋草Eleusine indica等对ALS类除草剂也产生了抗性,使得ALS类除草剂难以在玉米田继续使用[2]。在我国东北地区玉米田莠去津应用已有十几年历史,在这种用药背景下苍耳已经演替为优势杂草并在局部大发生[41]。且莠去津在环境中残留期较长,不但对后茬作物造成影响还污染环境[42]。草甘膦作为低毒、低残留、广谱除草剂,可有效防治苍耳。Clay等曾报道用420 g/hm2的草甘膦喷施处于结实期的苍耳,可以使苍耳种子百粒重减少69%、单株种子量下降70%[43]。草甘膦处理后植物体内莽草酸积累量可作为判断植物是否对草甘膦敏感的一个指标[44],Mueller等检测9种杂草在喷施540 g/hm2草甘膦后的莽草酸积累量,发现苍耳体内莽草酸含量最高,为2 000 mg/L[45],本试验中检测到黄淮海地区苍耳的GR50远低于田间推荐剂量,目前也未有苍耳对草甘膦抗性或耐性的报道。
Loux等发现长期喷施草甘膦的转基因大豆田中,藜对草甘膦的敏感性降低[46],而在长期施用草甘膦8年的转基因抗草甘膦玉米田中,藜已经演变成优势杂草[47]。长期单一喷施草甘膦,导致农田中藜对草甘膦耐受水平显著提高,当与不施用任何除草剂农田中生长的藜同时喷施草甘膦后,长期单一喷施草甘膦的农田中的藜死亡率显著下降[48]。Schuster等发现当藜植株高度为2.5 cm,草甘膦对其生长抑制GR50在430~560 g/hm2;当植株高度为15 cm,GR50在1 010~2 770 g/hm2 [49], 有研究证明,在藜20 cm高时喷施草甘膦,草甘膦的GR50会比10 cm喷施时高1.9~3倍[50]。刘小龙从无草甘膦用药史的地区采集到藜并通过整株生物测定法测定了草甘膦对藜5叶期时的GR50,为215.27 g/hm2 [51]。本文研究发现绝大多数藜种群对草甘膦没有耐受性。东北地区的藜种群较黄淮海地区种群对草甘膦的耐受性强,可能是由于地区间植物形态差异造成的,虽所有种群生长时间相同,但东北地区种群与黄淮海地区种群比较,植株普遍偏高,植株生物量增加,因此单位面积的植物组织所接收到的有效成分减少;另外植株生物量增加,使得植物组织中的钙含量也相应增加,而植物组织中的阳离子钙与除草剂阴离子具有拮抗效应[49,52]。
在GR作物田中少耕或免耕的耕作制度和单一依赖草甘膦控制杂草策略在很大程度上影响了杂草群落组成和密度,为特定适应的杂草种类增长提供了生态机会,并导致杂草种群的重大变化,以至于不可避免地发生种群的演替。这种种群演替带来的结果将增加杂草防除难度和成本,限制GR作物的可持续应用[34]。虽然在我国并未商业化种植转基因抗草甘膦玉米,但应借鉴国外长期种植转基因抗除草剂作物的经验,在转基因抗草甘膦作物田防除杂草时,应避免长期、单一地喷施草甘膦,可以使用不同作用模式的除草剂如2,4滴、莠去津、硝磺草酮等混用;结合苗前除草和生长期除草,苗前除草可使用甲草胺、乙草胺等,生长期可使用烟嘧磺隆、砜磺隆、莠去津[5354]。
参考文献
[1] 谢树章, 杨小艳, 林清, 等. 抗草甘膦转基因玉米研究进展[J]. 中国农业科技导报, 2013, 15(3): 3641.
[2] HEAP I. The international survey of herbicide resistant weeds [EB/OL].[20190916].http:∥www.weedscience.org.
[3] 李香菊, 梁帝允, 袁会珠. 除草剂科学使用指南[M]. 北京: 中国农业科学技术出版社, 2015: 58.
[4] JAMES C. ISAAA brief 54:Global status of commercialized biotech/GM crops in 2018 [DB/OL].The International Service for the Acquistion of Agribiotech Applications,Ithaca,NY: 2018. http:∥www.isaaa.org/resources/publications/briefs/54.
[5] 张翼翾.全球抗草甘膦杂草的概况[J].世界农药, 2018, 40(3): 3845.
[6] GOTTRUP O, OSULLIVAN P A, SCHRAA R J, et al. Uptake, translocation, metabolism and selectivity of glyphosate in Canada thistle and leafy spurge [J]. Weed Research, 1976, 16(3): 197201.
[7] ULLOA S M, OWEN M D K. Response of Asiatic dayflower (Commelina communis) to glyphosate and alternatives in soybean [J]. Weed Science, 2009, 57(1): 7480.
[8] 刘延. 田旋花和打碗花对草甘膦的耐药性研究[D]. 北京: 中国农业科学院, 2008: 5584.
[9] SHERRICK S L, HOLT H A, HESS F D. Effects of adjuvants and environment during plant development on glyphosate absorption and translocation in field bindweed (Convolvulus arvensis) [J]. Weed Science, 1986,34(6): 811816.
[10]SANTOS I C, SILVA A A, FERREIRA F A, et al. Efficiency of glyphosate in the control of Commelina benghalensis and Commelina diffusa [J]. Planta Daninha, 2001, 19(1): 135143.
[11]YUAN C I, CHAING M Y, CHEN Y M. Triple mechanisms of glyphosateresistance in a naturally occurring glyphosateresistant plant Dicliptera chinensis [J]. Plant Science (Shannon), 2002, 163(3): 543554.
[12]SANTOS S A D, TUFFISANTOS L D, SANTANNASANTOS B F, et al. Influence of shading on the leaf morphoanatomy and tolerance to glyphosate in Commelina benghalensis L. and Cyperus rotundus L. [J]. Australian Journal of Crop Science, 2015, 9(2): 135142.
[13]GOMEZ J M. Glyphosatetolerant Asiatic dayflower (Commelina communis L.): ecological, biological and physiological factors contributing to its adaptation to Iowa agronomic systems [D]. USA: Iowa State University, 2012: 6699.
[14]賈芳, 崔海兰, 李香菊, 等. 耐草甘膦杂草的研究现状[J].杂草学报, 2019, 37(1): 19.
[15]中国科学院中国植物志编辑委员会. 中国植物志[M]. 北京: 科学出版社, 1993.
[16]强科斌, 丁伟, 强小蓉. 野生油脂植物——苍耳的观察研究[J].甘肃农业大学学报, 1992(3): 262265.
[17]强胜. 杂草学[M]. 第2版.北京: 中国农业出版社, 2009: 216219.
[18]马承忠. 图说农田杂草识别及防除[M]. 第2版.北京: 中国农业出版社, 2013: 204205.
[19]李扬汉. 中国杂草志[M]. 北京: 中国农业出版社, 1998.
[20]OGG A G, DAWSON J H. Time of emergence of eight weed species [J]. Weed Science, 1984, 32(3): 327335.
[21]THARP B E, KELLS J J. Influence of herbicide application rate, timing, and interrow cultivation on weed control and corn (Zea mays) yield in glufosinateresistant and glyphosateresistant corn [J]. Weed Technology, 1999, 13(4): 807813.
[22]PANDEY H N, MISRA K C, MUKHERJEE K L. Phosphate uptake and its incorporation in some crop plants and their associated weeds [J]. Annals of Botany, 1971, 35(2): 367372.
[23]VENGRIS J, COLBY W G, DRAKE M. Plant nutrient competition between weeds and corn 1 [J]. Agronomy Journal, 1955, 47(5): 213216.
[24]MULUGETA D, STOLTENBERG D E. Influence of cohorts on Chenopodium album demography [J]. Weed Science, 1998, 46(1): 6570.
[25]魏守輝, 张朝贤, 翟国英, 等. 河北省玉米田杂草组成及群落特征[J]. 植物保护学报, 2006, 33(2): 212218.
[26]代伟程, 高兴文, 马成立, 等. 泰安市夏玉米田杂草种类及群落构成研究[J]. 山东农业科学, 2013, 45(9): 9698.
[27]吕跃星, 王权. 吉林省中部地区玉米田杂草种类及其优势种群调查报告[J]. 吉林农业科学, 2002(S1): 4647.
[28]张杰. 周口地区农业耕作模式对田间杂草的影响[D]. 新乡: 河南师范大学, 2014.
[29]黄春艳, 郭玉莲, 王宇, 等. 不同耕作模式对玉米大豆轮作区玉米田土壤潜杂草群落的影响[C]∥植保科技创新与农业精准扶贫——中国植物保护学会2016年学术年会论文集.北京: 中国农业科学技术出版社, 2016:505.
[30]潘思杨.黑龙江省玉米田主要杂草调查及对除草剂敏感性的研究[D]. 哈尔滨:东北农业大学, 2015.
[31]郑丽敏.安阳地区夏玉米田杂草发生规律与防治技术研究[D].郑州: 河南农业大学, 2009.
[32]STREIBIG J C. Herbicide bioassay [J]. Weed Research, 1988, 28(6): 479484.
[33]杨益军. 2018年中国(全球)草甘膦市场分析[J]. 农药市场信息, 2018(5): 2731.
[34]OWEN M D. Weed species shifts in glyphosateresistant crops [J]. Pest Management Science, 2008, 64(4): 377387.
[35]BARRENTINE W L, MCWHORTER C G. Chlorimuron and imazaquin rates for postemergence control of common cocklebur in soybeans [J]. Research Report, Mississippi Agricultural and Forestry Experiment Station, 1989,14(9): 3.
[36]WESLEY R A, SHAW D R, BARRENTINE W L. Incorporation depths of imazaquin, metribuzin, and chlorimuron for common cocklebur (Xanthium strumarium) control in soybeans (Glycine max) [J]. Weed Science, 1989,37(4):596599.
[37]SPRAGUE C L, STOLLER E W, WAX L M. Common cocklebur (Xanthium strumarium) resistance to selected ALSinhibiting herbicides [J]. Weed Technology, 1997,11(2):241247.
[38]OHMES G A, KENDIG J A. Inheritance of an ALScrossresistant common cocklebur (Xanthium strumarium) biotype [J]. Weed Technology, 1999,13(1):100103.
[39]SCHMIDT L A, TALBERT R E, MCCLELLAND M. Management of acetolactate synthase (ALS)resistant common cocklebur (Xanthium strumarium) in soybean [J]. Weed Technology, 2004,18(3):665674.
[40]MARIC D, KONSTANTINOVIC B. Resistance study of Xanthium strumarium L. species population to the herbicide imazethapyr in the south Banat [J]. Herbologia, 2014,14(1):7179.
[41]孙会杰. 辽宁省玉米田杂草群落调查及反枝苋对莠去津抗性研究[D].沈阳: 沈阳农业大学, 2007.
[42]邱罡, 谢凝子.农药莠去津的危害与非生物降解研究进展[J].广东化工, 2008, 35(1): 7377.
[43]CLAY P A, GRIFFIN J L. Weed seed production and seedling emergence responses to lateseason glyphosate applications [J]. Weed Science, 2000, 48(4): 481486.
[44]侯晓玉. 龙葵对草甘膦抗性机理的研究[D].哈尔滨:东北农业大学, 2016.
[45]MUELLER T C, ELLIS A T, BEELER J E, et al. Shikimate accumulation in nine weedy species following glyphosate application [J]. Weed Research, 2008, 48(5): 455460.
[46]LOUX M M, STACHLER J M, MILLER B A, et al. Response of common lambsquarters to glyphosate in the greenhouse and growth chamber [C]∥North Central Weed Science Society Proceedings, 2005, 60: 202.
[47]JESCHKE M R, STOLTENBERG D E. Weed community composition after eight years of continuous glyphosate use in a cornsoybean annual rotation [C]∥Milwaukee: North Central Weed Science Society Proceedings, 2006, 58: 59.
[48]KNISS A R, MILLER S D, WESTRA P H, et al. Glyphosate susceptibility in common lambsquarters (Chenopodium album) is influenced by parental exposure [J]. Weed Science, 2007, 55(6): 572577.
[49]SCHUSTER C L, ALKHATIB S K. Response of common lambsquarters (Chenopodium album) to glyphosate as affected by growth stage [J]. Weed Science, 2007, 55(2): 147151.
[50]SIVESIND E C, GASKA J M, JESCHKE M R, et al. Common lambsquarters response to glyphosate across environments [J].Weed Technology, 2011, 25(1): 4450.
[51]刘小龙. 鐵苋菜(Acalypha australis L.)对草甘膦的耐受性机理研究[D]. 北京: 中国农业科学院, 2016: 2935.
[52]HALL G J, HART C A, JONES C A. Plants as sources of cations antagonistic to glyphosate activity [J]. Pest Management Science, 2000, 56(4): 351358.
[53]WESTHOVEN A M, STACHLER J M, LOUX M M, et al. Management of glyphosatetolerant common lambsquarters (Chenopodium album) in glyphosateresistant soybean [J]. Weed Technology, 2008, 22(4): 628634.
[54]杜丽娟. 玉米田化学除草的药害及方法[J].农业科技通讯, 2015(6): 227230.
(责任编辑:杨明丽)

