Ursolic Acid Exhibits Protective Property against DSS-induced Ulcerative Colitis in Mice through STAT3 Signaling Pathway
ZHUANG Qunchuan,SHEN Aling, LIU Liya, SANKARARAMAN Senthilkumar,SFERRA Thomas Joseph,CHEN Qi,CHEN Youqin,*
1 Biomedical Research Center of South China, Fujian Normal University, Fuzhou, Fujian 350117, China;
2 Academy of Integrative Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian 350122, China;
3 Fujian Key Laboratory of Integrative Medicine in Geriatrics, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian 350122,China;
4 Fujian Key Laboratory of Innate Immune Biology, Fujian Normal University, Fuzhou, Fujian 350117, China;
5 Department of Pediatrics, Case Western Reserve University School of Medicine, Rainbow Babies and Children's Hospital, Cleveland 44106, OH, USA
*Correspondence:CHEN Qi,E-mail:nfsw@fjnu.edu.cn;CHEN Youqin,E-mail:yxc571@case.edu
ABSTRACT Objective:Anti-inflammatory effects of ursolic acid (UA) on a dextran sulfate sodium (DSS)-induced experimental murine colitis models was investigated to elucidate its possible molecular mechanisms on intestinal epithelial cells (IEC). Methods: For in vivo study, a total of 15 male BALB/c mice weighing (20-22) g were randomly divided into three groups: normal control group, DSS model group and DSS+UA treatment group; the mouse colitis model was induced by 3% dextran sodium sulfate (DSS) for 8 days; one of the DSS-induced groups was pretreated with UA. The body weight and colon length of mice in each group were measured by physical balance and vernier caliper, respectively. The mice in each group were scored according to the clinical disease activity index (DAI) score method. HE staining was used to observe the histopathological changes of colon in each group. The changes of serum amyloid protease A (SAA) and IL-6 expression in colon tissue were measured by ELISA. Using IL-6-stimulated differentiated Caco-2 cells as an in vitro inflammatory model of human intestinal epithelium, the effects of UA on the activation of IL-6/signal transducer and activator of transcription 3 (STAT3) signal pathway in IEC were examined by Western blot for STAT3 phosphorylation. Results: 1)Compared with the normal control group, the DAI score was increased, the length of the colon was shortened,and the histological damage was obvious in the DSS model group (P<0.05); administration of UA significantly reduced the severity of DSS-induced murine colitis as assessed by DAI score,colon length,and histology damage of colon (P<0.05). 2) Compared with the normal control group, the SAA level and the IL-6 level of colon tissue in the DSS model group increased significantly.The DSS-induced increases of SAA and colonic IL-6 levels were reversed by UA treatment (P<0.05). 3) Compared with normal IEC, IL-6 stimulation significantly increased the phosphorylation level of STAT3; STAT3 phosphorylation in IEC-treated with IL-6 and UA was significantly inhibited compared with only IL-6 stimulation (P<0.05). Conclusion: Our findings implicate that UA ameliorates DSS-induced colonic inflammation by blocking IL-6/STAT3 signaling pathway, and therefore indicate that UA may have clinical potential as a novel targeted therapy for ulcerative colitis.
KEY WORDS ulcerative colitis;ursolic acid;inflammation;STAT3 signaling pathway;dextran sulfate sodium
1 Introduction
Inflammatory bowel disease(IBD)consists primarily of ulcerative colitis(UC)and Crohn's disease(CD).These disorders are chronic inflammatory conditions of the intestines and are characterized by the presence in the gut of extensive areas of ulceration,pronounced inflammatory cell infiltrates, and epithelial cell necrosis[1-3]. Though there has been rapid introduction of new therapies for IBD not all patients respond completely and the therapies are associated with side effects including immunosuppression. Thus, the development of new therapies with less toxicity is needed in the care of patients with IBD.
STAT3 is one of the signal transducer and activator of transcription (STAT) protein family members. The activation of STAT3 plays a major role in the pathophysiology of IBD[4-7]. STAT3 can be activated by a diverse group of growth factors and cytokines, including interleukin-6 (IL-6)[8]. STAT3 activation is mediated by phosphorylation at tyrosine 705.The phosphorylation of cytosolic STAT3 induces its homodimerization, nuclear translocation, and DNA binding resulting in the expression of genes involved in inflammatory diseases such as IBD[9-12].Previous studies have showed that IL-6 is elevated in serum of IBD patients and predicts clinical relapse in CD[13-15]and anti-IL-6 therapy might have a therapeutic role[16-19]. Therefore,IL-6/STAT3 pathway is an appealing target for therapy in IBD.
Natural compounds have been used extensively for treating various inflammatory disorders. These compounds are especially sought out by and are useful for those who are unresponsive to or develop significant side effects with conventional treatments. Recently studies have suggested that a large group natural compound have the potential ability to limit the development and severity of certain cancers and inflammatory diseases[20-22].A natural pentacyclic triterpenoid,ursolic acid(UA)has been reported to possess anti-inflammatory and antitumor properties[23-25]. Studies have shown that UA can block nuclear factor-kappaB(NF-κB)-mediated inflammatory signaling and suppress the phosphorylation of STAT3[26-28].However,it is unknown whether UA might have a therapeutic role in IBD.Thus,in the present study we explored the effect of UA in a well-established murine model of ulcerative colitis and elucidated its possible mechanism of action in anin vitromodel of inflamed human intestinal epithelium.
2 Materials and methods
2.1 Reagents, kits and antibodies
Cell culture media and reagents were obtained from Invitrogen Thermofisher (Grand Island, NY, USA).Dextran sulfate sodium(DSS,molecular weight 40 000 Da)was purchased from MP Biochemical(Solon,OH,USA).Ursolic acid was purchased from Sigma Chemicals(St.Louis,MO,USA).Antibodies for Western blot were from Cell Signaling Technology (Danvers, MA,USA). The immunoblot detection system (ECL Plus)was fromPierce(Rockford,IL,USA).Bicinchoninic acid(BCA) protein assay reagent was from Pierce(Rockford, IL, USA). Mouse-specific IL-6 ELISA kit was from Biolegend Inc. (San Diego, CA, USA). Mousespecific serum amyloid A(SAA)ELISA kit was from ImmunologyConsultants laboratory,Inc(Newberg,OR,USA). All the other chemicals, unless otherwise stated,were obtained from Sigma Chemicals (St. Louis,MO,USA).
2.2 Preparation of UA stock solution
UA was dissolved in DMSO(10 mM)and stored at-20 ℃until used in thein vitroexperiments. UA was freshly dissolved in saline for the animal experiments.
2.3 Cell culture
Human colon cancer Caco-2 cells were purchased from the American Type Culture Collection (Cat no.HTB37Rockville, MA, USA). The cells were maintained in Dulbecco's modified Eagle medium(DMEM)(Grand Island, NY, USA) containing 1,000 mg/L of glucose,and supplemented with 20%(v/v)fetal bovine serum (FBS) (Thermofisher, Grand Island, NY, USA)and antibiotics (50 U/mL penicillin and 50 μg/mL streptomycin), cultured at 37 ℃in a humidified air containing 5%CO2.Caco-2 cells usually reached confluence 3 days after seeding and differentiated into enterocyte-like cells 18-20 days post-confluence. The fully differentiated cells were used in the experiments.The media was changed every 2-3 days. The media was removed and supplemented with 0.5%FBS medium before the day of experiment.
2.4 Induction of colitis and treatment with UA
Male BALB/c mice (with an initial body weight of 20-22 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The mice were housed under pathogen-free conditions with a 12 h light/dark cycle and food and water were provided ad libitum throughout the experiments. The mice were acclimatized for 1 week prior to the experiment. The Institutional Animal Care and Use Committee of Fujian University of Traditional Chinese Medicine (Fuzhou, China) approved the animal experiments conducted in this study. Colitis was induced by administering 3% DSS in the drinking water ad libitum from experimental day 1 through day 8, as previously described[29].Starting at the first day of induction of colitis,the mice were randomly divided into three groups(n=5): normal control group in which mice received neither DSS stimulation nor UA treatment; DSS model, or DSS+UA groups in which mice received DSS stimulation and intra-gastric administration of UA(0.075 mg/g)or the saline alone daily for 12 days.
2.5 Evaluation of colitis
The progression of DSS-induced colitis was monitored daily in a blinded manner. This included measurement of body weight, evaluation of stool consistency, and presence of blood in the stools by a guaiac paper test. Stool consistency was assessed using the following 3-point scale:0,normal;2,loose stools;and 4, watery diarrhea. The intensity of the guaiac paper test was scored by the following scale:0,no bleeding;2,slight bleeding(+occult test);and 4,gross bleeding(blood visible in stool or blood stains visible).Disease activity index (DAI) was represented as the sum of scores for weight loss, stool consistency and rectal bleeding,as previously described[21].
2.6 Sample collection
At the end of the experiment, the mice were anesthetized with Avertin. Blood was collected via right heart ventricle puncture with lightly heparinized syringes and kept on ice. Sera were separated by 5 min centrifugation at 5,000×gand stored at-80 ℃prior to the analysis. After sacrifice, the colons were excised and length and weight were measured. One portion of each distal colon was dissected and fixed in 10% formalin for histological examination. The remainder of each distal colon was snap-frozen in liquid nitrogen and stored at -80 ℃for further analysis of the tissue IL-6 level.
2.7 Assessment of histology
The formalin fixed section of the distal colons were processed and stained with hematoxylin and eosin(H&E).The severity of colitis was assessed in a blinded fashion by an experienced pathologist using a validated histological grading score of the colon as described previously[22].
2.8 Enzyme-linked immunosorbent (ELISA) assay
2.8.1IL-6 level Proteins were extracted from the frozen colon using T-PER Tissue Protein Extraction Reagent Kit according to the manufacturer's instructions.Protein concentrations were determined by BCA protein assaykit(Rockford,IL,USA)and equal amounts of protein were utilized in triplicate for each condition.The concentrations of IL-6 in the colons were measured using a mouse IL-6 ELISA kit (Biolegend) according to the manufacturers'instructions.All samples were assayed in triplicate. Absorbance was read at 450 nm using a microplate reader (model ELX800;BioTek Instruments,Inc.,Winooski,VT,USA).Results are presented as mean (expressed in pg/mg total protein) and standard deviations(SD).
2.8.2Serum amyloid A(SAA)level The concentrations of SAA in the sera were measured using a mouse SAA ELISA kit according to the manufacturer's instructions. All samples were assayed in triplicate. Absorbance was read at 450 nm using a microplate reader(model ELX800; BioTek Instruments, Inc., Winooski,VT, USA). Results are presented as mean (expressed in pg/mL)and standard deviations(SD).
2.9 Western blot analysis
Differentiated Caco-2 cells (20 days post-confluence) in 24-well bicameral inserts were pre-incubated with or without UA(20 μM)for 1 hour prior to stimulation with IL-6(10 μg/mL)by addition to the basolateral pole for 15 minutes.At the end of the experiment,cells were washed with ice cold phosphate-buffered saline (PBS) and lysed with mammalian cell lysis buffer (Thermofisher, Grand Island, NY, USA) containing protease and phosphatase inhibitor cocktails(Millipore,Bedford, MA, USA). The lysis buffer containing the disrupted cells was centrifuged at 11,000×gat 4 ℃for 10 min and the protein concentrations of the supernatant quantified with the BCA assay kit. Equivalent amounts of protein were resolved on 4% to 12%Novex Bis-Tris gels(NuPAGE;Grand Island,NY,USA). Proteins were then transferred into nitrocellulose membranes in the iBlot Western blot system (Invitrogen, Grand Island, NY, USA). The membranes were blocked for 1 h at room temperature with super Blocking Buffers(Pierce Biotechnology,Rockford,IL,USA)and incubated at 4 ℃overnight with primary antibodies against pSTAT3(rabbit,polyclonal, 1:1 000, CST,9145),STAT3(rabbit,polyclonal,1:1 000,CST,12640)and β-actin(rabbit,polyclonal,1:2 000,CST,4967)in blocking buffer. Following the primary antibody incubation period the membranes were washed and,subsequently, incubated with appropriate anti-rabbit IgG,HRP-linked secondary antibody(1:5 000,CST,7074)for 1 h at room temperature.Membranes were analyzed using enhanced chemiluminescence Plus reagents and scanned with the Storm Scanner (Amersham Pharmacia Biotech Inc,NJ,USA).
2.10 Statistical analysis
Data are presented as the means±standard deviations(SD) for the indicated number of independently performed experiments and analyzed using Student'st-test or one-wayANOVA, followed byLSD's test orDunnett's test. Data were considered significant whenP<0.05. All the analyses were performed using the SPSS package for Windows(version 22.0;SPSS,Inc.,Chicago,IL,USA).
3 Results
3.1 UA prevents clinical manifestations of DSSinduced ulcerative colitis in mice
To evaluate whether UA can protect mice against DSS-induced ulcerative colitis,we first determined the clinical manifestations in experimental mice. Compared with the normal control group, DSS administration induced weight loss,higher disease activity index(DAI),and shortening of the colon(Fig.1).However,in the DSS+UA group there was a significant amelioration of the weight loss and DAI as compared to DSS-induced group (Fig. 1A and Fig. 1B). Importantly, there was no significant difference in the body weight changes and clinical manifestations between normal control group and those mice receiving saline or UA only(data not shown).
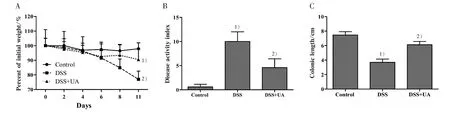
Figure 1 Effect of UA on the clinical manifestations in mice with DSS-induced colitis
3.2 UA prevents the histological damage of colon tissue in mice with DSS-induced ulcerative colitis
To determine whether the reduction in clinical manifestations induced by UA is associated with the prevention of colonic inflammation, the anatomy of and histopathological changes within the colon were assessed. The colon length shortening observed in the DSS treated mice was prevented in the group with DSS + UA (Fig. 1C). Histologic evaluation (Fig. 2),revealed the distal colons from normal control mice has normal colonic histology with an intact epithelium,well-defined gland lengths with no observable inflammation in the mucosa, whereas colon tissues of DSS only showed severe colonic histological damages,including destruction of crypt structure with loss of goblet cells,disruption of the epithelial layer and submucosal infiltration of inflammatory cells.Noticeably,UA treatment showed a significantly reduction in the colonic inflammation. All mice receiving saline only or UA only had normal histology of the colon (data not shown).
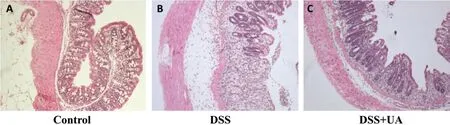
Figure 2 Effect of UA on histopathological damage of colon in mice with DSS-induced colitis (×100)
3.3 UA decreases the SAA level in mice with DSSinduced ulcerative colitis
To further evaluate the inflammatory process in these mice, we measured the serum SAA. Consistent with the result from H&E staining assay,DSS-induced elevation of SAA level was reduced by UA treatment(Fig.3). The SAA level in mice of normal control,DSS model, and DSS+UA group was (292.1±61.7),(1 427.3±452.8), and (444.5±172.8) μg/mL, respectively(P<0.05).
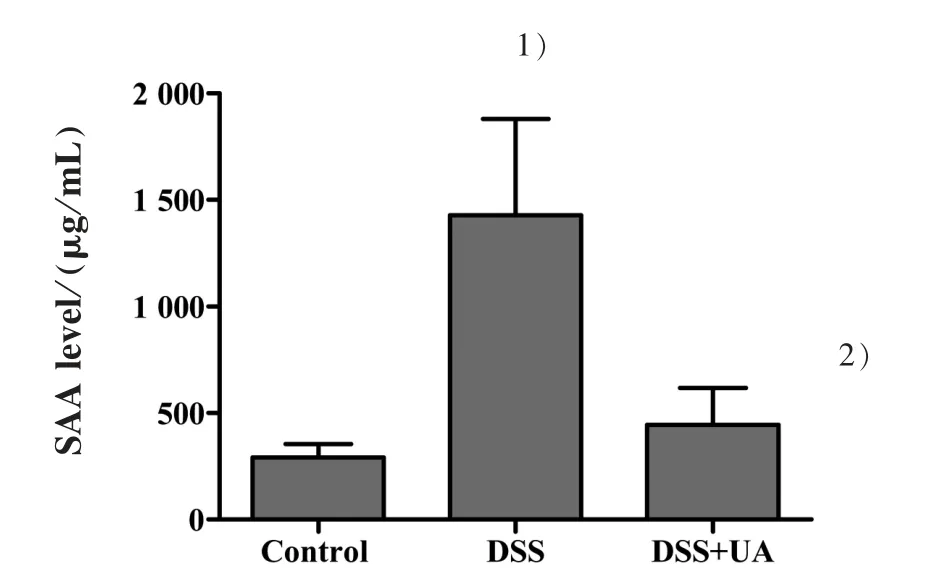
Figure 3 Effect of UA on the level of serum amyloid A in mice with DSS-induced colitis
3.4 UA Reduces IL-6 elevation within the colons of mice with DSS-induced ulcerative colitis
To determine whether UA could reduce IL-6 levels within the colons of mice with induced ulcerative colitis, IL-6 ELISAs were performed on colon tissue protein extracts.UA treatment significantly decreased IL-6 levels in the colons of the DSS-induced mice(Fig.4).The IL-6 levels in the normal control,DSS model,and DSS+UA group were(320.509±78.651),(1 638.707±296.850) or (629.720±188.210) pg/mg total protein,respectively(P<0.05).
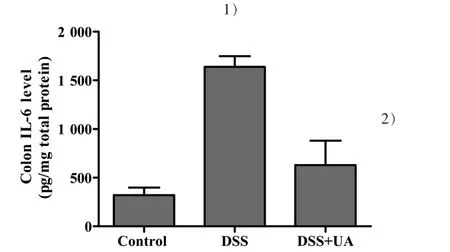
Figure 4 Effect of UA on colonic IL-6 levels in mice with DSS-induced colitis
3.5 UA inhibits IL-6-induced STAT3 phosphorylation in Caco-2 cells
According to the resultsin vivo,related mechanisms were subsequently exploredin vitro. Since the role of IL-6/STAT3 pathway is the best-characterized signal pathway in inflammatory response, we further examined whether the possible mechanism of the effect of UA on DSS-induced colitis by inhibiting IL-6-induced STAT3 phosphorylation.Using IL-6-stimulated differentiated Caco-2 cells as anin vitroinflammatory model of human intestinal epithelium,we examined the effects of UA on IL-6/STAT3 pathway.As shown in Fig.5A and B, IL-6-induced an increase in the expression of phosphorylated STAT3 in Caco-2 cells. However,UA treatment significantly inhibited this effect. The expression of non-phosphorylated STAT3 remained unchanged after the treatment with IL-6 and/or UA.
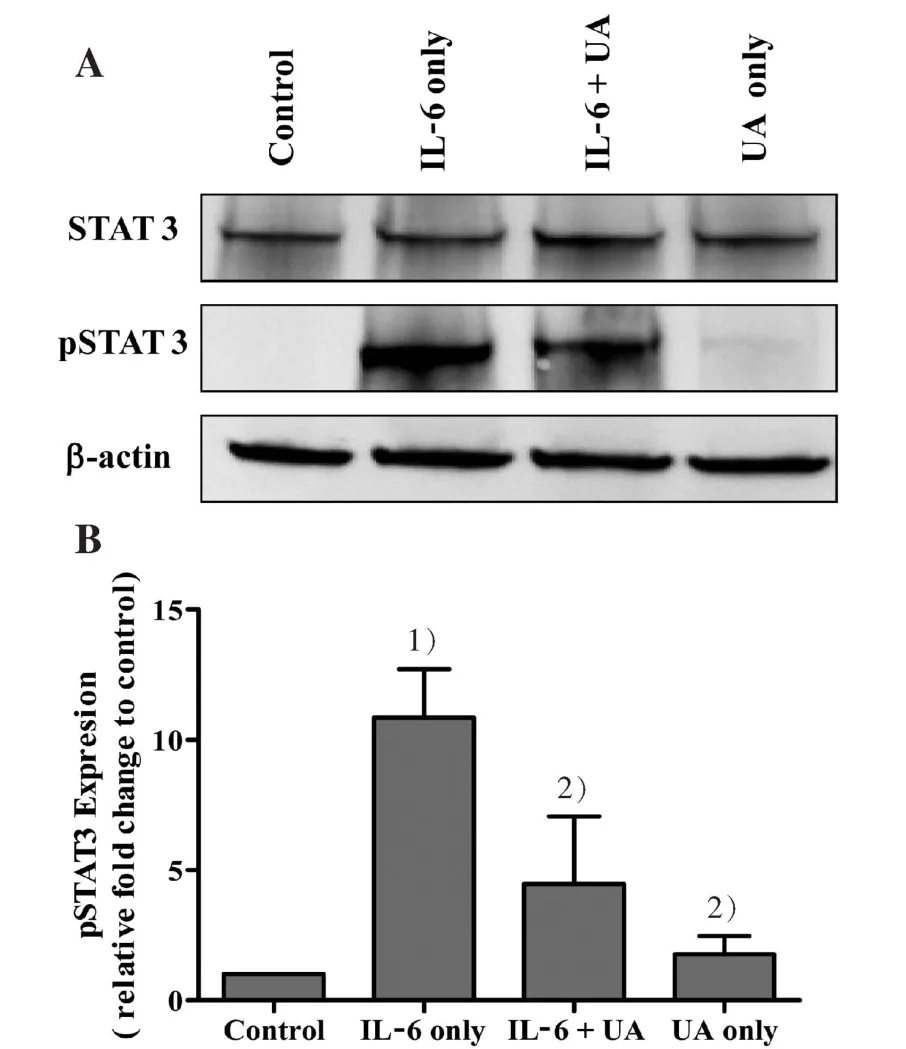
Figure 5 Effect of UA on IL-6 induced STAT3 phosphorylation in Caco-2 cells
4 Discussion
Despite recent advance in the drug for treatment of IBD,important question about limited the use of these drugs is their toxicity. Recently research has focused on natural products as alternative agents that may be equally or more effective, but toxicity-free. UA appears to have pharmacological effects combined with low toxicity[30-31].Previous studiesin vitroandin vivohave demonstrated that UA has biological effects,such as anti-oxidative, anti-inflammation, and an-ticancer activities[23,25-28]. However, there is limited data available evaluated whether UA has an effect on colonic inflammation. In the present study, we first examined the effects of UA on an experimental model of DSSinduced colitis that is a commonly used model for the inflammation component of IBD. Colitis was characterized by weight loss, increased disease activity index, shortened colon length, microscopic inflammation,and increases in serum SAA.In our study,for the first time, we demonstrated that UA has therapeutic efficacy in this model of colitis ameliorating each of these effects.
IL-6 is a cytokine secreted by lamina propria cells in patients with IBD. In patients with IBD serum IL-6 levels positively correlate with severity of intestinal histopathology[14-16]. Previous studies in mice have demonstrated that the colonic expression of IL-6 is increased in acute colitis[32-33]. In the present study, we observed the increased level of IL-6 in the distal colon of mice with DSS-induce colitis, providing supporting evidence for the crucial role of IL-6 in colonic inflammation. Moreover, UA significantly inhibited the production of IL-6 in this model.
In general, IL-6 binds to soluble or membranebound IL-6 receptors (e.g., sIL-6Ra or IL-6Ra) resulting in the activation of Janus kinase 2 (JAK2) and the downstream effectors signal transducer and activator of STAT3 and phosphatidyl inositol 39 kinase[34-35].Recent studies have demonstrated a beneficial effect of anti-IL-6 therapy in Crohn's disease as well as in animal models of colitis[18-19].These data strongly suggested that activation of the IL-6/STAT3 pathway has a key role in the development of IBD and the prevention of activation of this pathway maybe useful in the treatment of IBD. Previous reports have demonstrated that UA down-regulates activation of various pro-inflammatory pathways including NF-κB,STAT3,AKT,and IKKα/β phosphorylation[26-28]. Therefore, UA's anti-inflammatory effect on colitis may through the inhibition of STAT3 phosphorylation. To confirm this hypothesis, we investigated STAT3 activation in IL-6 stimulated Caco-2 cells. Our results indicate that UA strongly suppresses STAT3 phosphorylation and this might be the possible mechanism for its anti-inflammatory action in UC.
In conclusion, our present study demonstrates that UA attenuates intestinal inflammation in the experimental murine colitis model and may mediate this through the inhibition of activation of the IL-6/STAT3 pathway.These findings provide,for the first time,the novel protective effect of UA against inflammation in the colon.The present study suggests that further studies should focus on the clinical development of UA for the treatment of IBD.

