Setosphlides A-D, New Isocoumarin Derivatives from the Entomogenous Fungus Setosphaeria rostrate LGWB-10
Wenbin Gao ·Xiaoxia Wang ·Fengli Chen ·Chunqing Li ·Fei Cao ·Duqiang Luo
Abstract Investigation of the entomogenous fungus Setosphaeria rostrate LGWB-10 from Harmonia axyridis led to the isolation of four new isocoumarin derivatives, setosphlides A—D (1— 4), and four known analogues (5— 8).Their planar structures and the relative confi gurations were elucidated by comprehensive spectroscopic methods.The absolute confi gurations of isocoumarin nucleus for 1— 4 were elucidated by their ECD spectra.The C-10 relative confi gurations for the pair of C-10 epimers (1 and 2) were established by comparing the magnitude of the computed 13C NMR chemical shifts (Δ δcalcd. ) with the experimental 13 C NMR values (Δ δexp. ) for the epimers.All of the isolated compounds (1— 8) were evaluated for their cytotoxicities against four human tumor cell lines MCF-7, MGC-803, HeLa, and Huh-7.
Keywords Entomogenous fungus·Setosphaeria rostrate·Isocoumarin·Absolute confi guration
1 Introduction
Symbiosic microorganisms from insects, which are wellknown as a rich source of bioactive natural products, have attracted widespread attention [1— 3].Especially, due to the special environmental conditions, the bioactive natural products from symbiotic microorganisms as a rich source of various compounds with complex structures and excellent activities, may reshape the experts’ views on the drug ability of natural products [4].Among them, isocoumarin derivatives have been isolated as antifungal, insecticidal, and phytotoxic secondary metabolites from several fungal sources [5].However, it made such a task extremely challenging to assign their absolute confi gurations, when a side chain attached to isocoumarin derivative nuclear, as the high free rotation of the stereogenic centers in chains [6].During our ongoing search for bioactive compounds from fungi,Setosphaeria rostrateLGWB-10 was selected for chemical exploration based on HPLC—DAD and HPLC—MS analyses of its EtOAc extract.Subsequently, eight isocoumarin derivatives, including four new setosphlides A—D (1— 4) and four known analogues, (3R,4R)-4,8-dihydroxy-3-((R)-2-hydroxypentyl)-6,7-dimethoxyisochroman-1-one (5) [5], (3R,4R)-4,8-dihydroxy-3-((R)-2-hydroxypentyl)-6,7-dimethoxyisochroman-1-one (6) [5], (12R)-12-hydroxymonocerin (7) [7], and (12S)-12-hydroxymonocerin (8) [6] (Fig.1) were isolated fromSetosphaeria rostrateLGWB-10.Herein, we report the details of the isolation, structure elucidation, absolute confi guration determination, and bioactivities of them.
2 Results and Discussion
Setosphlide A (1) was isolated as a colorless oil.The molecular formula of 1 was determined as C15H20O7by positive HRESIMS data atm/z335.1093 [M + Na] + (calcd.for 335.1101), with six indices of hydrogen defi ciency.Analysis of the 1D NMR and HSQC data of 1 (Table 1) revealed the presence of two methyl groups (δH3.85 and 0.96), three methylene groups (δH1.50 and 1.42, 2.13 and 1.65, and 1.50), three oxygen-bearing methine groups (δH4.70, 4.42, and 3.91), and one olefi nic proton (δH6.49).Analysis of 13 C NMR and HMBC spectra of 1 (Table 2) indicated the presence of 15 carbons, including six quaternary carbons (δC171.2, 158.5, 157.4, 139.1, 136.3, and 101.5), four methines (δC108.5, 80.6, 67.8, and 67.7), three methylenes (δC41.5, 39.5, and 19.9), and two methyls (δC60.9 and 14.4).Six aromatic carbon signals in the region ofδC101.5—158.5 indicated the existence of a polysubstituted phenyl moiety.All of the proton resonances were assigned to the relevant carbon atoms by the HSQC spectrum.Careful comparison of the 1 H and13C NMR spectra as well as the MS data of 1 with those of (3R,4R)-4,8-dihydroxy-3-((R)-2-hydroxypentyl)-6,7-dimethoxyisochroman-1-one (5) [5], revealed that 1 shared the same scaff old as 5.The detailed comparison of 1D NMR data between 1 and 5 suggested the 6-OCH3in 5 was absent in 1, which was confi rmed by the key HMBC correlations from H-5 and 7-OCH 3 to C-4, C-7 and C-15 (Fig.2).Thus, the planar structure of 1 was assigned.

Fig.1 The chemical structures of compounds 1— 8

Table 1 1 H NMR Data (δ) of 1— 4 (600 MHz,CD 3 OD, J in Hz)
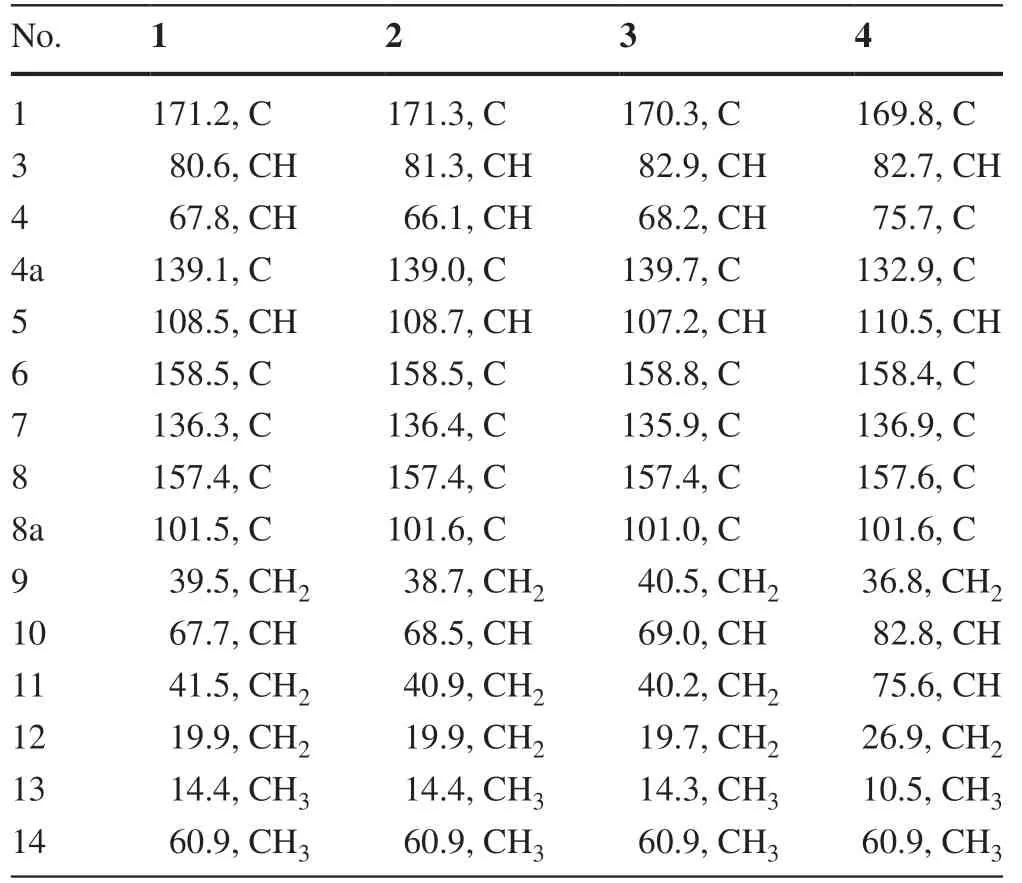
Table 2 13C NMR Data (δ) of 1— 4 (150 MHz,CD 3 OD)
Setosphlides B and C (2 and 3) were obtained with the same molecular formula of 1.Furthermore, the 1 H and 13 C NMR data of 1-3 (Tables 1 and 2) showed striking similarity, suggesting the same structural nucleus of them.In fact, only differences of the NMR signals for CH-3, CH-4, and CH-10 were observed [δH4.70 (1H, td,J= 10.2, 1.8 Hz, H-3), 4.42 (1H, d,J= 1.8 Hz, H-4), 3.91 (1H, m, H-10),δC80.6 (C-3), 67.8 (C-4), and 67.7 (C-10) in 1 vs.δH4.66 (1H, dt,J= 6.6, 1.8 Hz, H-3), 4.52 (1H, d,J= 1.8 Hz, H-4), 3.84 (1H, m, H-10),δC81.3 (C-3), 66.1 (C-4), and 68.5 (C-10) in 2 vs.δH4.53 (1H, m), 4.56 (1H, m), 3.81 (1H, m, H-10),δC82.9 (C-3), 68.2 (C-4), and 69.0 (C-10) in 3], indicating that 1-3 were epimeric isocoumarin derivatives with structural difference at C-3, C-4, and C-10.The above deduction was confi rmed by a detailed analysis of the HSQC,1 H- 1 H COSY, and HMBC spectra (Fig.2).
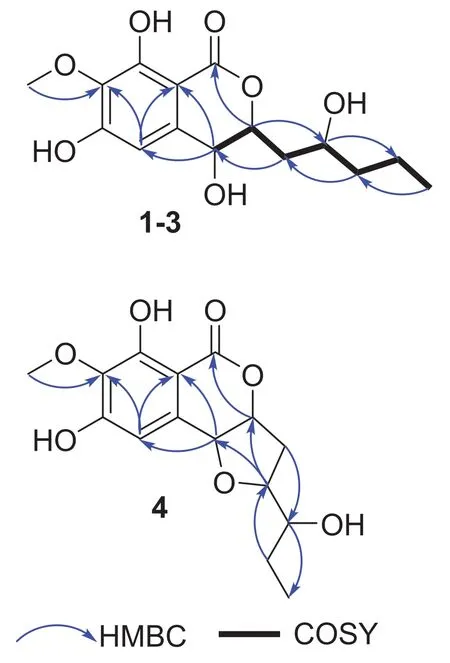
Fig.2 1H— 1 H COSY and Key HMBC correlations of 1-4

Fig.3 Experimental CD spectra of 1-4
The relative confi gurations of C-3 and C-4 in 1— 3 were determined by their NOESY correlations.For compounds 1 and 2, NOE correlations from H-3 to H-4 were observed.While, the NOESY correlations between H-4 and H2-9 were present for 3.In order to assign the absolute confi gurations of C-3 and C-4 in 1— 3, electronic circular dichroism (ECD) was carried out for them.The absolute confi gurations of the C-3 methine carbon in 1— 3 were deduced by the application of the circular dichroism (CD) exciton chirality method.Further more, According to the earlier references [7,8], the negative ECD Cotton eff ect for 1— 3 around 275 nm (Fig.3) indicated the 3R,4R, 3R,4R, and 3S,4Rconfi gurations for 1,2, and 3, respecitively.
However, it was difficult to determine the absolute confi guration of C-10 in 1— 3 due to the high conformational flexibility of the chains in them.Especially, the experimental ECD spectra of 1— 3 were almost identical, indicating that the ECD method had limitations in the assignment of the C-10 absolute confi gurations for them.Recently, computational methods for atomic chemical shift calculations have been developed and used for the relative confi guration identifi cations of complex natural compounds [9— 11].Compounds 1 and 2 are a pair of epimers with more than one stereogenic carbon.The carbons near C-10 in 1 should have different chemical shifts from those of the corresponding carbons in 2.Thus, the confi gurations at C-10 of 1 and 2 could be established by comparing the magnitude of the computed chemical shifts (Δδcalcd.) for two epimers of (10R)-epimer and (10S)-epimer [12].The relative errors (Δδcalcd.) between the computed13C chemical shifts of (10R)-epimer and (10S)-epimer, and the relative errors (Δδexp.) between experimental13C NMR data of 1 and 2 were summarized in Table 3.Based on the relative error magnitudes (Δδcalcd.and Δδexp.), the confi gurations of C-10 for 1 and 2 were suggested to beRandS, respectively.However, the absolute confi guration at C-10 in 4 was undetermined since only one of its C-10 epimer was not obtained.

Table 3 Chemical shift differences of selected carbons in 1 and 2
Setosphlide D (4) was also isolated as a colourless oil with the molecular formula C15H18O7determined by HRESIMS.The NMR spectra (Tables 1 and 2) of 4 showed a high similarity to those of 1— 3.The most signifi cant difference in the 1 H NMR spectra was the presence of an additional methine signal atδH(3.43, m) in 4.Furthermore, the key HMBC from H-9 to C-4 indicated that C-4 and C-9 were connected via an oxygen bridge, forming the third ring, a furan C-ring.The relative confi guration of C-3, C-4, and C-10 of 4 was determined by NOESY experiment, which showed NOE correlations from H-10 to H-3 and H-4.ECD spectrum suggested the 3R,4R,10Rconfi guration of 4 (Fig.3).
All of the isolated compounds (1— 4) were evaluated for their cytotoxicities against four human tumor cell lines MCF-7, MGC-803, HeLa, and Huh-7.However, all of the compounds hardly displayed obvious activity (IC50> 200 μM).
3 Experimental
3.1 General Experimental Procedures
OR and UV data were acquired on Perkin-Elmer 341 and 241 spectrophotometers, respectively.ECD spectra were measured using a JASCO J-715 spectrometer.1D and 2D NMR data were recorded on a Bruker AM-600 spectrometer.HRESIMS spectra were recorded on a Bruker apex-ultra 7.0T spectrometer.HPLC was carried out on a Waters 600—2489 with a YMC column (YMC-Pack ODSA, 250 × 10 mm).Column chromatography (CC) were conducted over silica gel (200—300 mesh) and Sephadex LH-20 gel (25—100 μm).TLC were conducted with silica gel GF 254 plates.
3.2 Isolation of the Fungal Material
The fungal strainSetosphaeria rostrateLGWB-10 was isolated from theHarmonia axyridiscollected in Baoding, Hebei Province, China.The voucher specimen of the fungus was deposited at College of Life Science of Hebei University with Genbank MN 378541.Setosphaeria rostratewas cultured on PDA plate at 28 °C for 7 days, and then inoculated into a 500 mL Erlenmeyer flask containing 200 mL of PDB medium.Flask cultures were incubated at 28 °C on a rotary shaker at 120 rpm/min for 4 days.Solid fermentation was carried out in 100 Erlenmeyer flasks (500 mL), each containing 100 g rice, 80 mL distilled H2O, and 5 mL of culture liquid as seed, and incubated at 28 °C for 40 days.The fermented material was extracted three times with methanol (20 L for each time), and the methanol extract was concentrated in vacuo to yield a yellow oily residue (132.6 g).This residue was subjected to silica gel column and eluted with a gradient elution of petroleum ether (PE)/EtOAc (100:0, 90:10, 80:20, 60:40, 50:50, 40:60, 20:80, 10:90, 0:100 (v/v)) to obtain nine fractions Frs.1—9.Among these fractions, Fr.3, eluted with 60% EtOAc—PE (3:2, v:v), was applied to a Sephadex LH-20 CC (CH2Cl2/MeOH (1:1, v:v)) to remove the pigment to give Fr.3—1 and Fr.3—2.Then, Fr.3—1 was purifi ed by semipreparative HPLC (70% MeOH/H2O, 2.0 mL/min) to give 4 (4.2 mg),7 (6.5 mg), and 8 (6.2 mg).Fr.4 was repeatedly purifi ed by Sephadex LH-20, silica gel CC and semipreparative HPLC to aff ord compounds 1 (4.1 mg) and 2 (4.3 mg),3 (3.5 mg),5 (3.2 mg), and 6 (3.0 mg).
Setosphlide A (1):Colorless oil;+ 10.2 (c1.00, MeOH); UV (MeOH)λmax(logε) 231 (4.67), 274 (0.48), 306 (0.45) nm; ECD (0.50 mM, MeOH) λmax (Δε) 219 (+ 7.76), 273 (- 5.01), 308 (+ 0.95) nm; HRESIMSm/z335.1093 [M + Na] + (calcd for C15H20O7Na, 335.1101).1H and 13 C NMR data, see Tables 1 and 2.
Setosphlide B (2):Colorless oil;+ 5.7 (c1.00, MeOH); UV (MeOH)λmax(logε) 231 (4.65), 274 (0.43), 307 (0.42) nm; ECD (0.50 mM, MeOH) λmax (Δε) 216 (+ 4.44), 274 (- 3.83), 308 (+ 0.64) nm; HRESIMSm/z311.1090 [M + Na] + (calcd for C15H20O7Na, 335.1101).1H and13C NMR data, see Tables 1 and 2.
Setosphlide C (3):Colorless oil;+ 20.6 (c1.00, MeOH); UV (MeOH)λmax(logε) 232 (4.54), 274 (0.43), 307 (0.44) nm; ECD (0.50 mM, MeOH) λmax (Δε) 210 (+ 24.96), 274 (- 6.90), 311 (+ 1.26) nm; HRESIMSm/z311.1094 [M + Na] + (calcd for C15H20O7Na, 335.1101).1H and13C NMR data, see Tables 1 and 2.
Setosphlide D (4):Colorless oil;+ 15.3 (c1.00, MeOH); UV (MeOH)λmax(logε) 233 (4.54), 275 (2.61), 307 (2.84) nm; ECD (0.50 mM, MeOH) λmax (Δε) 215 (+ 9.07), 277 (- 5.10), 341 (- 0.01) nm; HRESIMSm/z311.1125 [M + H] + (calcd for C15H19O7, 311.1120).1H and13C NMR data, see Tables 1 and 2.
3.3 Computational Section
The molecules of (10R)-epimer (1) and (10S)-epimer (2) was constructed and used for conformational searches using the MMFF94S force field by using BARISTA software.A total of 45 stable conformers for 1 and 48 stable conformers for 2 with relative energy within a 10.0 kcal/mol energy window were obtained and optimized at the gas-phase B3LYP/6-311 + G(d) level using the Gaussian 09 package.MPW1PW91 theory at the basis set of B3LYP/6-311 + G(d,p) in the gas phase was applied for 13 C NMR calculation for (10R)-epimer (1) and (10S)-epimer (2).
3.4 Cytotoxity Assay
The cytotoxicities against human breast cancer (MCF-7), human gastric cancer (MGC-803), cervical cancer (HeLa), and human hepatoma (Huh-7) cell lines were evaluated using the MTT method [13].Cisplatin was used as a positive control.
AcknowledgementsThis work was funded by the National Natural Science Foundation of China (31672070) and National Key Research and Development Program of China (2017YFD0201400 and 2017YFD0201401), and the High Performance Computer Center of Hebei University.
Compliance with Ethical Standards
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
 Natural Products and Bioprospecting2021年1期
Natural Products and Bioprospecting2021年1期
- Natural Products and Bioprospecting的其它文章
- Anti-microbial Eff ects In Vitro and In Vivo of Alstonia scholaris
- 3,4-Secocycloartane Triterpenoids from the Cones of Pseudolarix amabilis
- Four New Phloroglucinol-Terpene Adducts from the Leaves of Myrciaria cauliflora
- Asymmetric Total Synthesis of (+)-21- epi-Eburnamonine Via a Photocatalytic Radical Cascade Reaction
- Two New Quinazoline Derivatives from the Moss Endophytic Fungus Aspergillus sp.and Their Anti-inflammatory Activity
- Cytochalasins from Xylaria sp.CFL5, an Endophytic Fungus of Cephalotaxus fortunei
