Two New Quinazoline Derivatives from the Moss Endophytic Fungus Aspergillus sp.and Their Anti-inflammatory Activity
Ning-Ning Wang ·Chun-Yu Liu ·Tian Wang ·Yue-Lan Li ·Ke Xu ·Hong-Xiang Lou
Abstract Two new quinazoline derivatives versicomides E (1) and F (2), and 10 known compounds (3— 12) were isolated from the moss endophytic fungus Aspergillus sp.Their structures were determined on the basis of extensive spectroscopic data analysis and ECD calculations.Among them, the compound 7 (6-hydroxy-3-methoxyviridicatin) was first reported as a natural product.Inhibition on LPS-induced NO production in RAW 264.7 murine macrophages found that compounds 5,7 and 8 showed signifi cant inhibitory eff ects on NO production, with IC 50 values of 49.85, 22.14 and 46.02 μM respectively.
Keywords Endophytic fungus·Quinazoline·Anti-inflammatory activity
1 Introduction
Endophytic fungi are defined as organisms that live in healthy internal tissues of plants without causing any immediate, obvious and negative eff ect [1,2], and have been considered as useful and peerless sources of molecules with potent bioactivity [3], such as cytotoxic [4], antibacterial [5], antifungal [6], antioxidative [7], neuraminidase inhibitory [8] and phosphodiesterase inhibitory activities [9].The moss, as the oldest branch of terrestrial plant evolution, has built extensive contact with fungi [10].The moss endophytic fungus is also a great resource for bioactive compounds, reports of which were not as many as other plant endophytic fungi.Previously, there were some compounds including alkaloids, cyclic peptide, anthraquinone with antifungal activity [11], cytotoxic activity [12,13], immunosuppressive activity [14] and allelopathy activity [15] that were discovered in moss endophytic fungi.In the present research, two new quinazoline derivatives [16] versicomides E (1) and F (2) and 10 known compounds (3— 12) were obtained from the moss endophytic fungusAspergillussp.It is also the first report of the presence of compound 7 (6-hydroxy-3-methoxyviridicatin) as a natural product.Furthermore, anti-inflammatory assay with lipopolysaccharide (LPS)-activated RAW 264.7 murine macrophages found that compounds 5,7 and 8 showed strong inhibitory eff ects on NO production with IC50values of 49.85, 22.14 and 46.02 μM respectively.
2 Results and Discussion
Versicomide E (1) was isolated as faint yellow powder with the molecular formula C19H25N3O4by means of HRESIMS (m/z360.1919 [M + H] + , calcd.for 360.1923).The 1D NMR and HSQC spectra of compound 1 indicated that there was a methoxy (δH3.82,δC55.5,CH3-22) and a 1,3,4,5-tetrasubstituted benzene (δC131.1, C-6;δC154.1, C-7;δH6.83,δC107.7, C-8;δC58.6, C-9;δH6.99,δC97.0, C-10;δC120.9, C-11).Its13C NMR and HMBC spectra confi rmed the existence of a Val moiety and a similar Ile moiety, which was also validated by COSY.Then, compared with signals of quinazoline derivatives [16], compound 1 had similar chemical shifts with versicomide A.The difference between them was that versicomide A had 1,3,4-trisubstituted benzene ring while 1 had 1,3,4,5-tetrasubstituted with and a hydroxyl group (OH-7,δH9.80, s) as revealed by 1 H NMR.Therefore, the planar structure of 1 was elucidated as drawn in Fig.1 which was also supported by the chemical shifts of C-7 (δC154.1) and C-8 (δC107.7).The absolute confi guration was confi rmed by chemical calculation of the ECD spectrum.According to the literature [16], we presumed the absolute confi guration was the same as versicomide A.However, by comparing the experimental ECD data to the calculated result, the experimental ECD was consistent with the calculated ECD of (3R,14R,18R) (Figs.2,3).Then, the absolute confi guration of compound 1 was determined.The optical rotation of= - 88.5 (c0.5, MeOH) also confi rmed that.
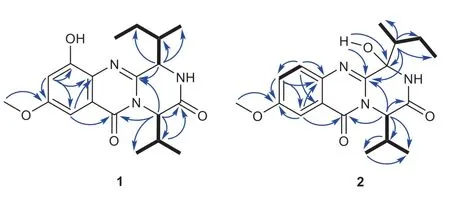
Fig.2 1H— 1 H COSY (H- H), key HMBC correlations (H → C) of compounds 1 and 2
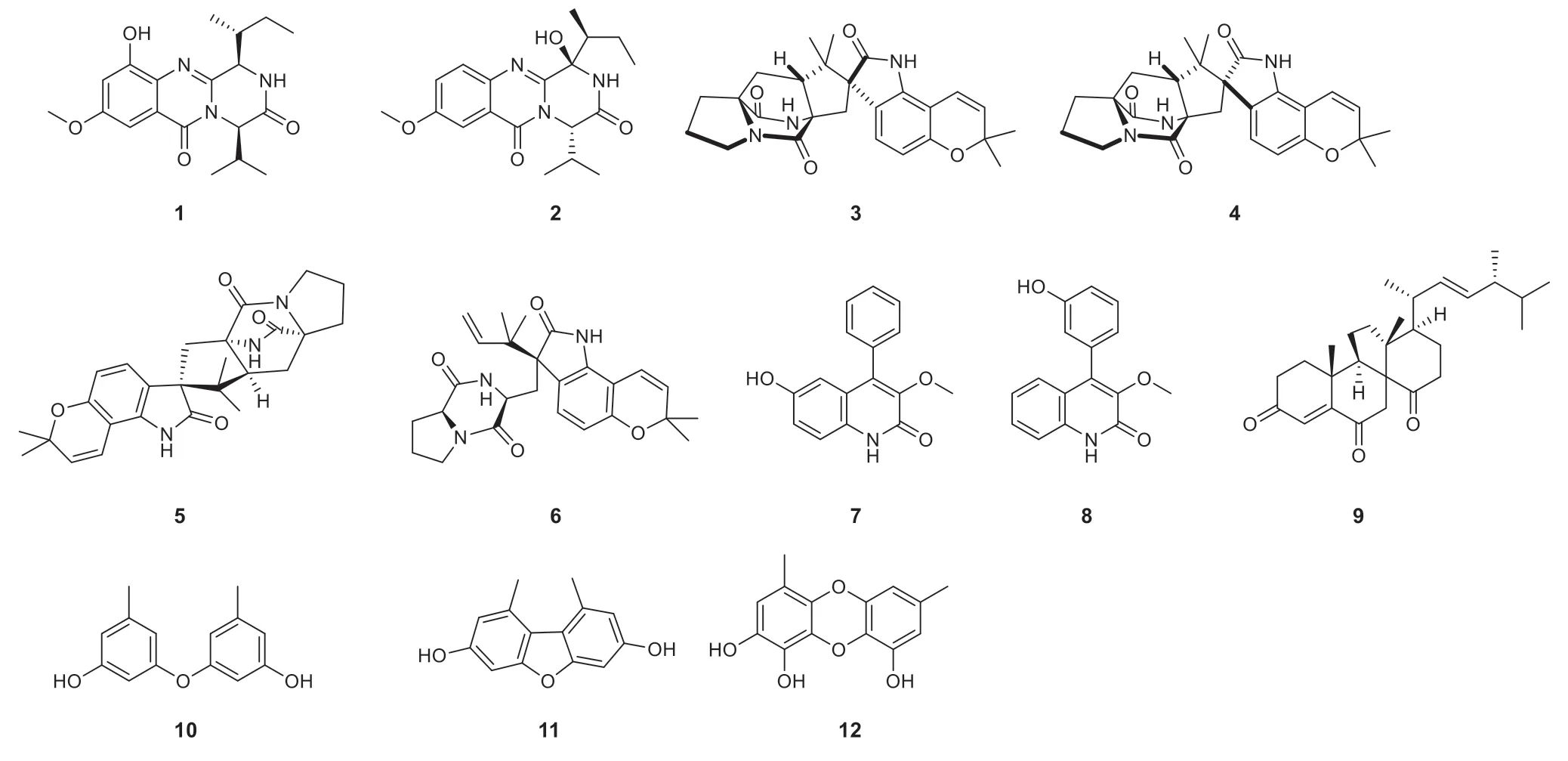
Fig.1 Structures of compounds 1— 12
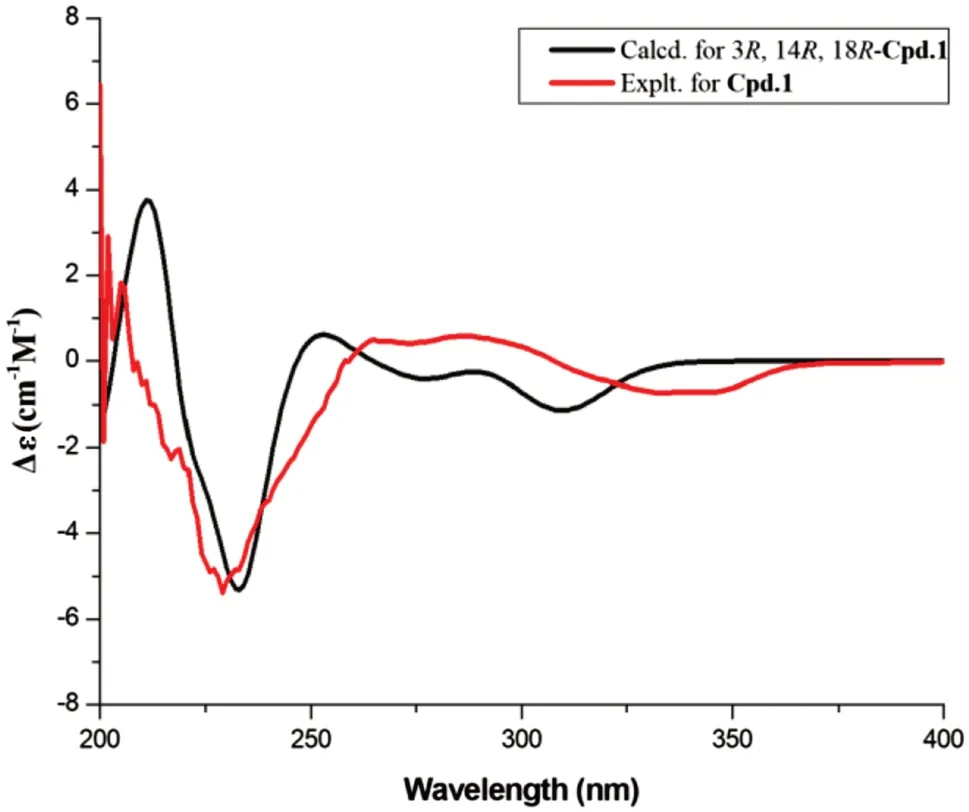
Fig.3 Calculated and experimental ECD spectra for compound 1
Versicomide F (2) was also a faint yellow jelly.The molecular formula was determined to be C19H25N3O4by HRESIMS (m/z360.1919 [M + H] + , calcd.for 360.1923).Its1H NMR showed that there was a different substitution from compound 1, and a different type of hydroxyl group (OH-3,δH6.58, s) from OH-7 (δH9.80, s) in compound 1.Compound 2 had 1,3,4-trisubstituted benzene ring, lacking a hydroxyl substituent was determined for the correlations from H-7 (7.64, d, 8.8 Hz) to C-6, C-8, C-9 and C-11, together with the COSY correlation of H-7 and H-8 in HMBC.HSQC indicated that C-3 (δC84.9) was an oxygenated quaternary carbon.Combination with the HMBC correlations from OH-3 to C-3 and C-4, the planar structure of compound 2 was depicted as in Fig.1.By comparing the ECD of 2 (Fig.S20) with that of the reported compound [16] leads to the final determination of the absolute confi guration of compound 2 as 3R,14S,18S.
Compound 7 was reported as a natural product for the first time in this article.The structure of 7 was determined by comparing spectroscopic data with 3,6-O-dimethylviridicatin in literature [17] and determined as 6-hydroxy-3-methoxyviridicatin.
Other compounds were identifi ed as versicolamide B (3) [18], taichunamide E (4) [19], notoamide B (5) [20], notoamide C (6) [21], 3-O-methylviridicatol (8) [17], dankasterone B (9) [22], diorcinol (10) [23], 3,7-dihydroxy-1,9-dimethyldibenzofuran (11) [24], aspergilol E (12) [25] by direct comparison of their spectral data with the reported.
All compounds were evaluated for the anti-inflammatory activity with the model to inhibit NO production in LPS-stimulated RAW 264.7 murine macrophages.Compounds 5,7 and 8 displayed strong inhibitory eff ects on NO production, with IC 50 values of 49.85, 22.14 and 46.02 μM (Fig.4) respectively.
3 Experimental
3.1 General Experimental Procedures
Optical rotations were acquired using a PerkinElmer 241MC polarimeter (Anton Paar GmbH, Graz, Austria) at 20 °C.UV data were obtained by a UV-2450 spectrophotometer (Shimadzu, Japan).CD spectra were performed on a Chirascan spectropolarimeter (Applied Photophysics Ltd., Leatherhead, UK).NMR spectra were recorded on a Bruker Avance spectrometer operating at 400 (1H) and 100 (13C) MHz or at 600 (1H) and 150 (13C) MHz with tetramethylsilane as an internal standard.HRESIMS data were determined by using a Finnigan LC-QDECA mass spectrometer.IR spectra were recorded on a Nicolet iN 10 Micro FTIR spectrometer.HPLC were performed on an Agilent 1200 G1311A pump equipped with a G1322A degasser, a G1315D DAD detector, and an Eclipse XDBC185 μm column (9.4 × 250 mm).Column chromatography (CC) was carried out using silica gel (200—300 mesh; Qingdao Haiyang Chemical Co.Ltd., Qingdao, China) and Sephadex LH-20 (25—100 mm; Pharmacia Biotek, Denmark).TLC was carried out with precoated silica gel GF-254 glass plates (Qingdao Haiyang Chemical Co.Ltd., Qingdao, China).The compounds were visualized under UV (254 nm) light and by spraying with anisaldehyde—H2SO4followed by heating.
3.2 Fungal Material
The fungusAspergillussp.was isolated from theTrichocoleaceaesp., collected from Wuyanling Nature Reserve Area, Zhejiang Province, China.The strain (No.7-2-1) was identifi ed using nuclear ITS rDNA sequences (Gen-Bank MN310533.1), and was deposited in the Key Lab of Chemical Biology of Ministry of Education, Shandong University, Jinan, China.
The fungus was cultured in eight 300 mL Erlenmeyer flasks, each containing 100 mL of potato dextrose broth (PDB), and put them on a rotary shaker (120 rpm) at 25 °C for 7 days.Large-scale fermentation was conducted in 60 Erlenmeyer flasks (500 mL), containing 80 g of rice and 120 mL of distilled H2O.Then autoclave at 120 °C for 30 min, and after cooling to room temperature, each flask was inoculated with 10 mL seed culture and incubated under static condition at room temperature for 50 days.
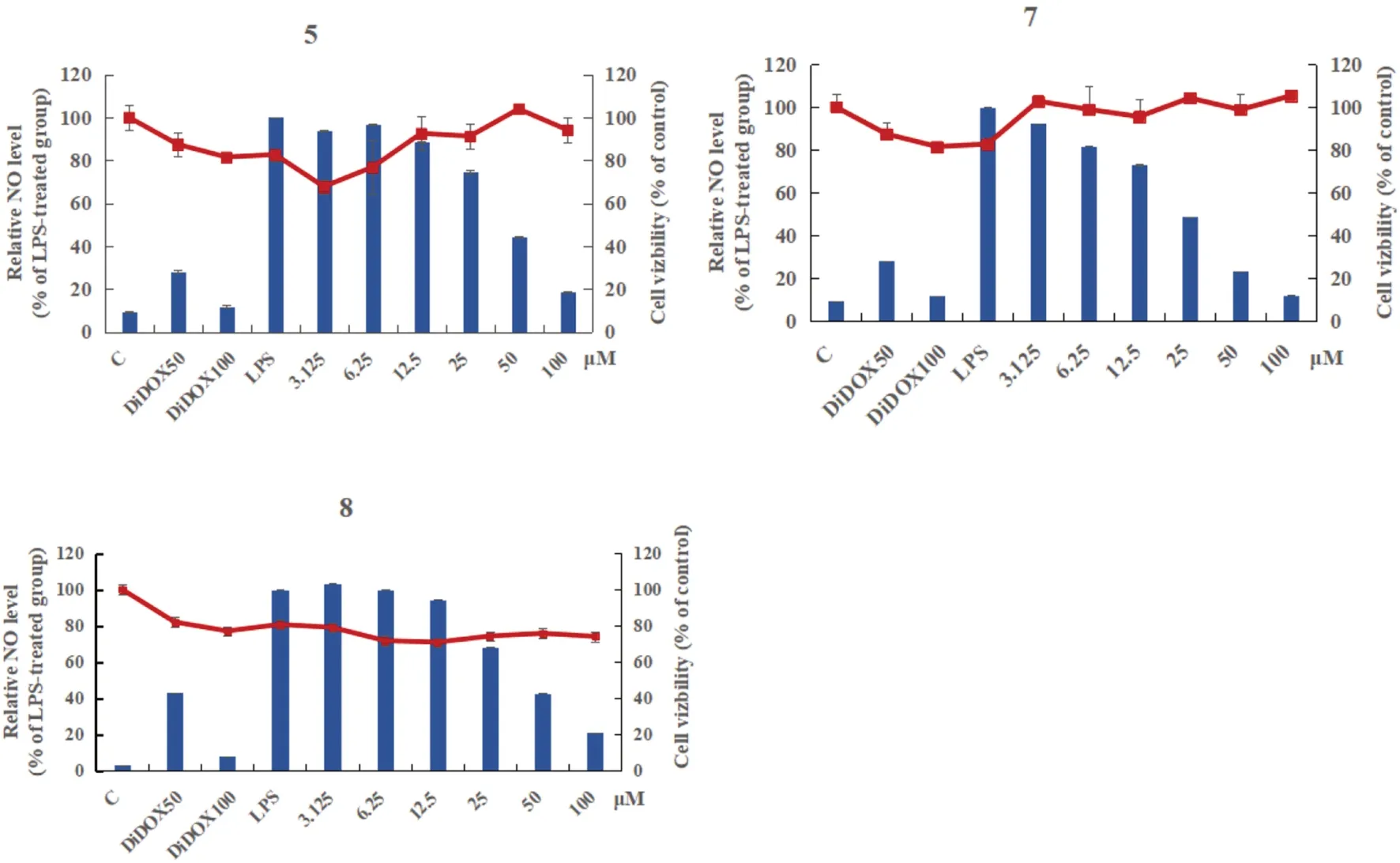
Fig.4 Inhibitory activity of compounds 5,7 and 8 against NO production in RAW 264.7 cells.Cells were treated with various concentrations of compounds along with LPS (1 μg/mL) for 24 h, and the accumulation of nitrite was evaluated by Griess reagent.Values were presented as mean ± SD from three independent experiments.Column relative NO level,Dot cell viability,C control,DiDOX positive control
3.3 Extraction and Isolation
The fermented material including the mycelium was extracted with EtOAc for three times, and the organic solvent was evaporated under vacuum to yield the crude extract (108 g).The crude extract was separated into 15 fractions (A—O) using a silica gel column with a step gradient of CH2Cl2/MeOH from 100:0 to 0:100 (v/v).
Fr.D (4.9 g) was chromatographed by a silica gel column with step gradient of PE/EtOAc from 100:0 to 0:100 (v/v) to yield 15 subfractions (DA—DN).Fr.DM(16.2 mg) was purifi ed by HPLC (80% MeOH/H2O, 1.5 mL) to yield compound 6 (2.4 mg,tR= 14.0 min).Fr.DN(304.7 mg) and Fr.DH(810.0 mg) were both isolated with Sephadex LH-20 CC eluted with CH2Cl2/MeOH (1:2 and 1:1) to aff ord 8 parts (DN1—DN8and DH1—DH8).Fr.DN3(14.4 mg) was prepared with HPLC (72% MeOH/H2O, 1.5 mL) to yield compound 10 (1.0 mg,tR= 13.0 min).Fr.DH2(385 mg) was separated into 17 parts (DH2a—DH2q) using MPLC (ODS, MeOH/H2O from 10:90 to 100:0).Further purifi cation of Fr.DH2f(15.0 mg) and Fr.DH2m(18.3 mg) with HPLC gave compound 1 (40% MeCN/H2O, 1.5 mL, 2.8 mg,tR= 12.2 min),2 (40% MeCN/H2O, 1.5 mL, 6.2 mg,tR= 10.0 min) and 9 (80% MeCN/H2O, 1.5 mL, 6.4 mg,tR= 14.0 min).Compound 11 (2.7 mg,tR= 20.5 min) was obtained by purifying Fr.DH7(60.7 mg) with HPLC (62% MeOH/H2O, 1.5 mL).
Fr.G (8.7 g) was chromatographed by a silica gel column with step gradient of PE/EtOAc from 100:0 to 0:100 (v/v) to yield 23 subfractions (GA—Gw).Fr.GM(3.0 g) was first fractionated by Sephadex LH-20 CC eluted with CH2Cl2/MeOH (1:1) into seven subfractions (GM1—GM7).Fr.GM5(3.0 g) was isolated into twelve parts (GM5a—GM5l) by MPLC (ODS, MeOH/H2O from 10:90 to 100:0), and then, Fr.GM5j(69.9 mg) was subjected to Sephadex LH-20 CC by elution with MeOH to obtain six parts (GM5j1—GM5j6).Preparation of Fr.GM5j5(49.5 mg) with HPLC (77% MeOH/H2O, 1.5 mL) gave compound 12 (2.0 mg,tR= 4.2 min).
Fr.I (553.5 mg) was first fractionated using Sephadex LH-20 CC with the mobile phase of CH2Cl 2 /MeOH (2:1), and 11 subfractions (I1—I11) were obtained.Fr.I2(69.0 mg) was purified by HPLC (45% MeCN/H2O, 1.5 mL) to aff ord compound 3 (15.8 mg,tR= 15.0 min),4 (5.7 mg,tR= 16.0 min), and 5 (3.9 mg,tR= 20.0 min).Fr.I5(53.8 mg) was isolated using Sephadex LH-20 CC eluted with MeOH, and got 7 subfractions (I51—I57).Purifi cation of Fr.I53(42.7 mg) by HPLC (54% MeOH/H2O, 1.5 mL) gave compound 7 (2.4 mg,tR= 17.0 min) and 8 (2.6 mg,tR= 23.8 min).
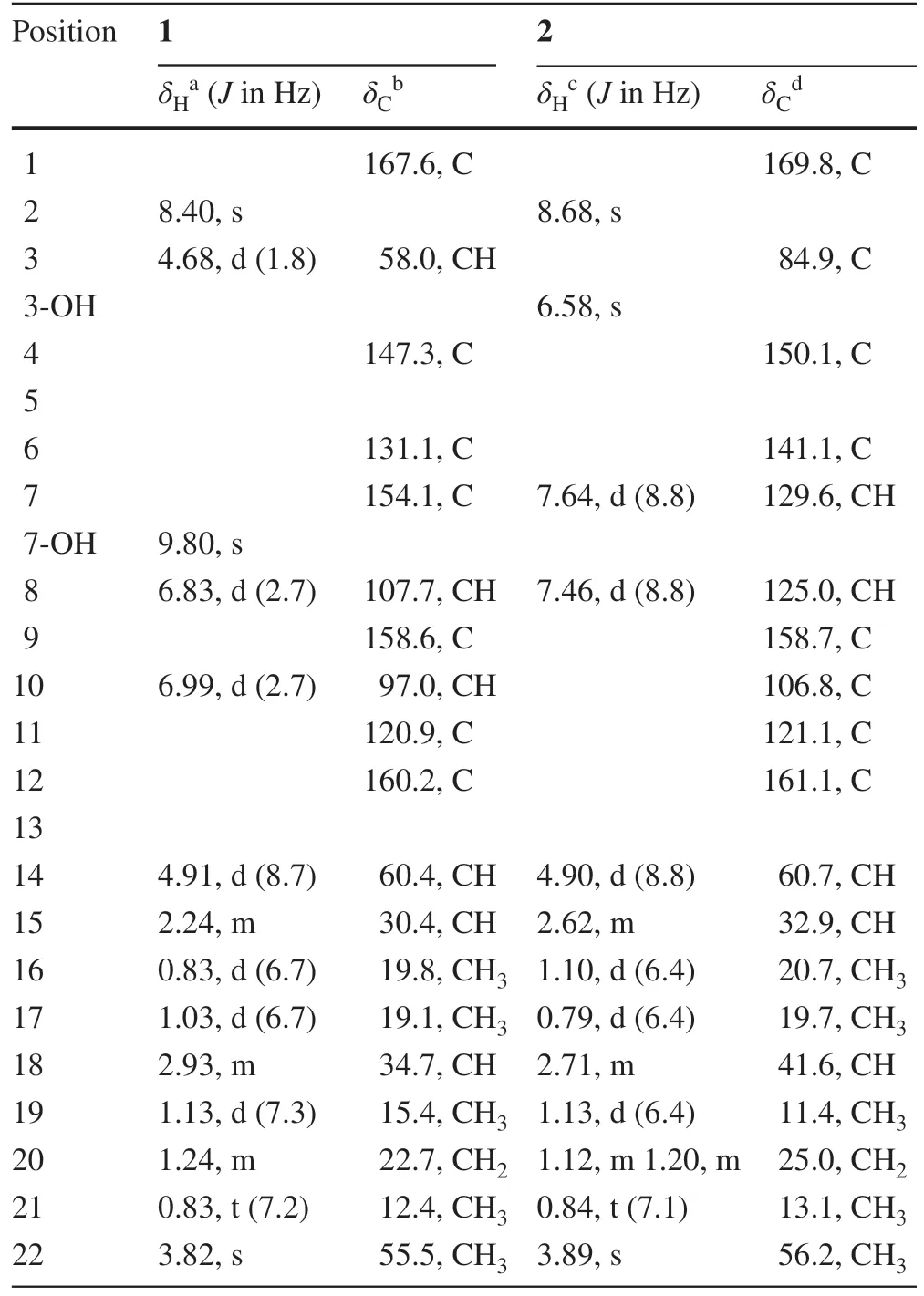
Table 1 1H and 13 C NMR spectral data for compounds 1 and 2 in DMSO- d6
3.4 Spectroscopic Data of Compounds
3.4.1 Versicomide E (1)
3.4.2 Versicomide F (2)
3.5 Anti-inflammatory Activity Test
The inhibition of NO production assay was performed according to the reported procedures [26].DiDOX was set up as a positive control group.Then the percentage of NO production inhibition was calculated to get the IC50values.
AcknowledgementsThe authors gratefully acknowledge the National Natural Science Foundation of China (Nos.81874293 and 81630093) for financial support and Major Basic Research Program of Shandong Province (ZR2019ZD26).
Compliance with Ethical Standards
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
 Natural Products and Bioprospecting2021年1期
Natural Products and Bioprospecting2021年1期
- Natural Products and Bioprospecting的其它文章
- Setosphlides A-D, New Isocoumarin Derivatives from the Entomogenous Fungus Setosphaeria rostrate LGWB-10
- Anti-microbial Eff ects In Vitro and In Vivo of Alstonia scholaris
- 3,4-Secocycloartane Triterpenoids from the Cones of Pseudolarix amabilis
- Four New Phloroglucinol-Terpene Adducts from the Leaves of Myrciaria cauliflora
- Asymmetric Total Synthesis of (+)-21- epi-Eburnamonine Via a Photocatalytic Radical Cascade Reaction
- Cytochalasins from Xylaria sp.CFL5, an Endophytic Fungus of Cephalotaxus fortunei
