Four New Phloroglucinol-Terpene Adducts from the Leaves of Myrciaria cauliflora
Ming Chen ·Jia-Qing Cao ·Wen-Jing Wang ·Ni-Ping Li ·Yan Wu ·Lei Wang ·Wen-Cai Ye
Abstract Myrcauones A—D (1— 4), four new phloroglucinol—terpene adducts were isolated from the leaves of Myrciaria cauliflora.Their structures with absolute confi gurations were elucidated by combination of spectroscopic analysis, single crystal X-ray diffraction, and electronic circular dichroism (ECD) calculations.Compound 1 was a rearranged isobutylphloroglucinol—pinene adduct featuring an unusual 2,3,4,4a,10,11-hexahydro-1 H-3,11a-methanodibenzo[b, f]oxepin backbone.Compound 4 showed moderate antibacterial activity against Gram-positive bacteria including multiresistant strains.
Keywords Myrciaria cauliflora·Phloroglucinol—terpene adducts·Antibacterial activity
1 Introduction
The plantMyrciaria cauliflorais an evergreen shrub and widely distributed in southern and central Brazil [1].This plant has been traditionally used as a folk medicine to treat asthma, diarrhea, and gastrointestinal diseases [2,3].Previous phytochemical investigations on this plant only reported essential oils and flavonoids [4— 6].As a part of our eff orts to search for structural unique and bioactive constituents from Myrtaceae plants [7— 10], four new phloroglucinol—terpene adducts, myrcauones A—D (1— 4), were isolated from the leaves ofM.cauliflora.Their structures and absolute confi gurations were determined by means of 1D and 2D NMR spectroscopy, X-ray diffraction analysis, and electronic circular dichroism (ECD) calculations.Compound 1 is a rearranged isobutylphloroglucinol—pinene adduct featuring an unusual 2,3,4,4a,10,11-hexahydro-1H-3,11amethanodibenzo[b,f]oxepin backbone.All isolates were evaluated for their antibacterial activities.Herein, we describe the isolation, structural elucidation, and antibacterial activities of these myrcauones A—D (1— 4).
2 Results and Discussion
2.1 Structural Elucidation
Compound 1 was obtained as yellow gum.The molecular formula of 1 was established as C22H32O3by its HRESIMS data (m/z345.2423 [M+H] + , calcd for C22H33O3: 345.2424).The UV spectrum displayed absorption maximum at 206 nm.The IR spectrum showed characteristic absorptions for hydroxyl group (3475 cm -1 ) and aromatic ring (1611 and 1488 cm-1).The1H NMR spectrum of 1 suggested the presence of an olefi nic proton [δH6.23 (1H, s, H-5′)], a hydroxyl group [δH4.75 (1H, s, 2′-OH)], a methoxy group [δH3.76 (3H, s,H 3 -11′)], an isopropyl moiety [δH1.97 (1H, m, H-8′), 0.52 (3H, d,J= 6.8 Hz,H3-9′), and 1.03 (3H, d,J= 6.8 Hz,H3-10′)], and three tertiary methyls [δH2.06 (3H, s,H3-12′), 0.90 (3H, s,H3-9), and 0.79 (3H, s,H3-8)].The13C NMR and DEPT spectra of 1 exhibited 22 carbon signals including 7 quaternary carbons (5 olefi nic ones), 5 methines (an oxygenated and an olefi nic ones), 4 methylenes, and 6 methyls (an oxygenated one).The aforementioned data implied that 1 could be an isobutylphloroglucinol—monoterpene adduct (Fig.1) [11].
A comparison of the NMR data of 1 with those of melaleucadine A [11] indicated the presence of an uncommon rearrangedβ-pinene unit (part 1a), which was further confi rmed by the two spin systems (H-2 to H-6 and H-10 to H-9′/H-10′) in its 1 H— 1 H COSY spectrum (Fig.2) and the HMBC correlations between H3-8/H3-9 and C-1/C-4/C-7 and between H2-10 and C-1/C-2/C-6/C-7.In addition, the HMBC correlations between H-5′ and C-1′/C-3′/C-4′/C-6′, between H3-12′ and C-2′/C-3′/C-4′, between H3-11′ and C-4′, and between H-7′ and C-1′/C-2′/C-6′, allowed the establishment of an isobutylphloroglucinol moiety (part 1b).Furthermore, the HMBC correlations between H-7′ and C-1, and between H2-10 and C-1′ defi ned the connection of 1a and 1b via C-7′—C-10 bond.Finally, the leftover oxygen atom was anticipated to connect C-2 (δC88.6) with C-6′ (δC159.4) to form an uncommon 2,3,4,5-tetrahydrooxepine ring on the basis of the HMBC correlation between H-2 and C-6′ as well as the molecular formula information.
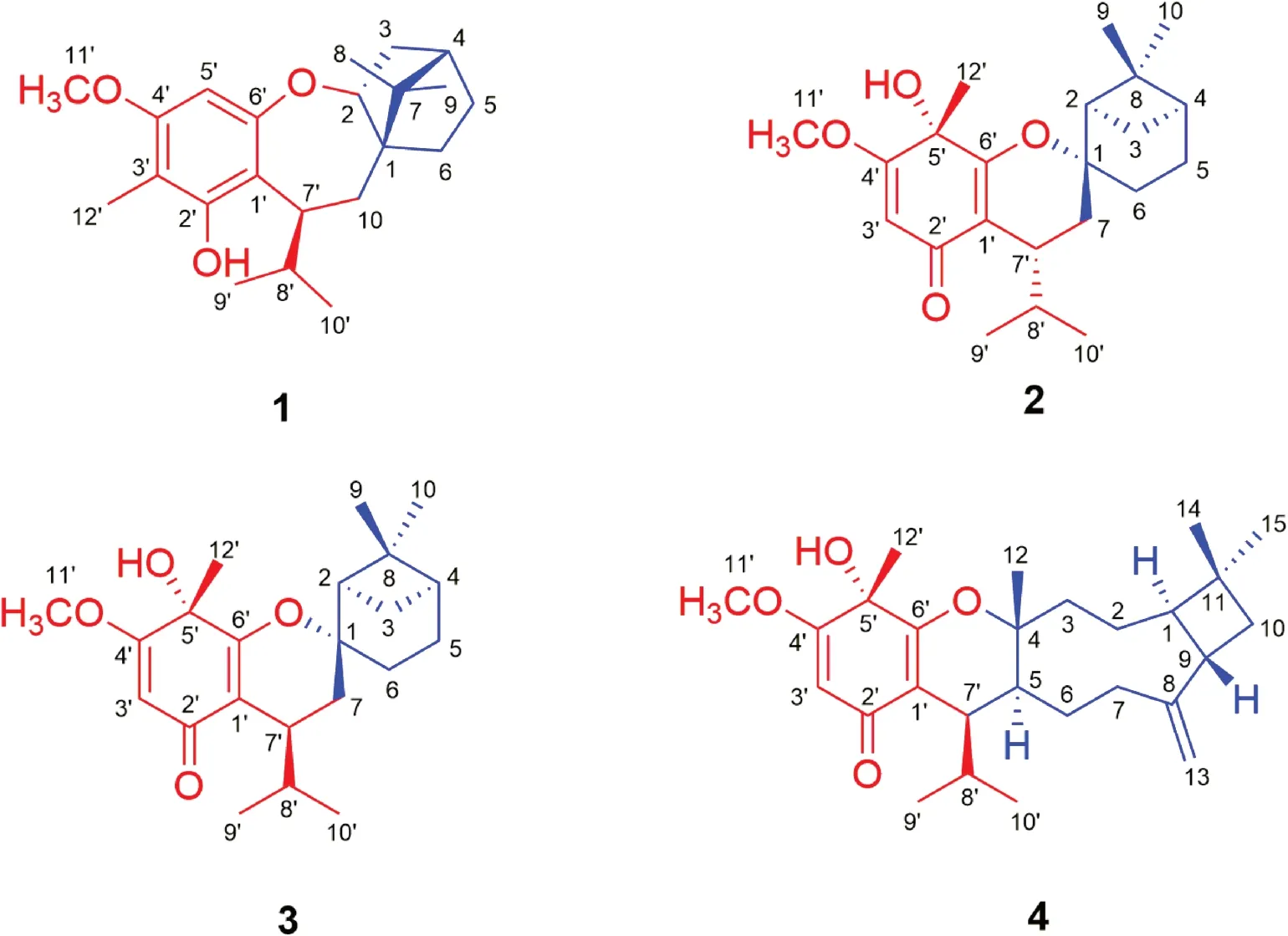
Fig.1 Chemical structures of 1— 4
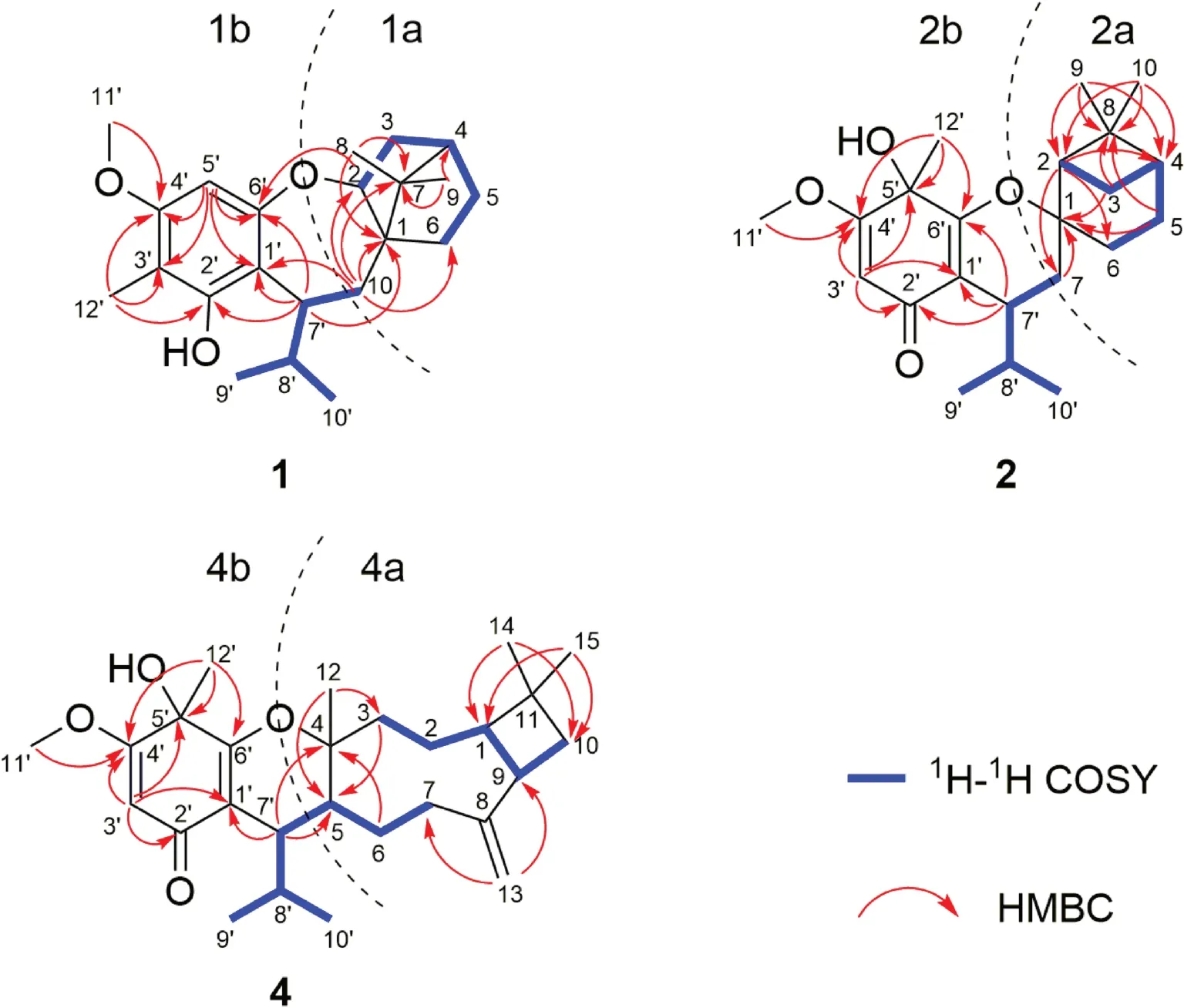
Fig.2 Key 1 H— 1 H COSY and HMBC correlations of 1,2,4
The relative configuration of 1 was established by a NOESY experiment (Fig.3).The NOE correlations between H-10βand H-2/Me-8/H-8′ indicated that H-2, Me-8, and the isopropyl group (C-9′/8′/10′) wereβ-oriented.Meanwhile, the correlations between H-10αand H-6a/H-7′ as well as between H-6a and Me-9 suggested that Me-9 and H-7′ wereα-oriented.To determine the absolute confi gurations of 1, a comparison of its experimental and calculated ECD data was performed.The experimental ECD spectrum of 1 exhibited negative Cotton eff ects at 211 (Δε+ 6.2) and 277 (Δε+ 0.7) nm, and a negative one at 238 (Δε- 0.5) nm, which were similar with those in the calculated CD spectrum for 1R,2R,4S,7′S-isomer (Fig.4).Thus, the absolute confi guration of 1 was determined as 1R, 2R, 4S, and 7′S.
The molecular formula of compound 2 was determined as C22H32O4by its HRESIMS data (m/z361.2386 [M+H] + , calcd for C22H33O4: 361.2373).The IR spectrum showed absorptions of hydroxyl (3357 cm-1), carbonyl group (1656 cm-1), and double bonds (1605 and 1462 cm-1).The1H NMR data (Table 1) for an olefi nic proton [δH5.33 (1H, s, H-3′)], a methoxy group [δH3.75 (3H, s,H3-11′)], an isopropyl moiety [δH2.86 (1H, m, H-8′), 0.61 (3H, d,J= 6.8 Hz,H3-9′), and 0.93 (3H, d,J= 6.8 Hz,H3-10′)], and three tertiary methyls [δH1.51 (3H, s,H3-12′),δH1.23 (3H, s,H3-9), and 0.96 (3H, s,H3-10)] indicated that 2 could be an isobutylphloroglucinol—monoterpene adduct [12].
The1H—1H COSY spectrum of 2 revealed the presence of two spin systems (H-2 to H-6 and H-7 to H-9′/H-10′) (Fig.2).Accordingly, aβ-pinene unit (part 2a) could be established by the HMBC correlations between H-2 and C-4/C-6/C-7, between H 2 -3/H 2 -5 and C-1/C-8, and between H 3 -9/H 3 -10 and C-2/C-4/C-8.Furthermore, comparison of its NMR data with those of the known compound baeckfrutone H indicated the existence of an isobutyrylphloroglucinol moiety (part 2b), which was further confi rmed by the HMBC correlations between H-3′ and C-1′/C-2′/C-4′/C-5′, between H3-12′ and C-4′/C-5′/C-6′, between H3-11′ and C-4′, and between H-7′ and C-1′/C-2′/C-6′ [12].The closure mode of dihydropyran ring which connected the two fragments (2a and 2b) could be deduced on the basis of the molecular formula information and the downfi eld chemical shift at C-1 (δC85.4).

Fig.3 Key NOESY correlations of 1— 4
In the NOESY spectrum, the correlations between H-3b and H-2/H-4/Me-9, between H-2 and H-7′, between H-7βand Me-10/H-7′, as well as between H-3a and Me-12′ indicated that these protons were allβ-oriented (Fig.3).The absolute confi guration of 2 was determined by ECD calculation.The experimental ECD spectrum of 2 displayed positive cotton eff ects at 244 (+ 11.7) and 296 (+ 8.1) nm, and negative ones at 203 (- 19.8) and 337 (- 1.8) nm, which were similar to those in the calculated spectrum for 1R,2R,4S,5′S,7′R- 2 (Fig.4).Thus, the absolute confi guration of 2 was identifi ed as 1R, 2R, 4S, 5′S, 7′R.
Compound 3 possessed the same molecular formula as 2 on the basis of its HRESIMS data (m/z361.2391 [M+H] + , calcd for C22H33O4, 361.2373).Analyses of the NMR data of 3 and comparison with those of 2 indicated that these two compounds had the same planar structure but differed in their relative confi gurations.The downfi eld chemical shifts of C-2 (fromδC45.2 to 53.0) and C-7 (fromδC32.7 to 33.7), as well as the upfi eld chemical shifts of C-6 (fromδC30.8 to 27.4), C-7′ (fromδC33.6 to 32.2) revealed that 3 was a C-7′ epimer of 2.This deduction was confi rmed by the NOE correlations between H-3b and H-2/H-4/Me-9, between H-2 and H-7β, between H-7βand H-8′/Me-9′/Me-10, between H-3a and Me-12′, and between H-7αand H-7′ (Fig.3).Finally, the agreement of the ECD curve of 3 with those of the calculated 1R,2R,4S,5′S,7′S- 3 (Fig.4) allowed the assignment its absolute confi guration.
Compound 4 was obtained as colorless blocks.Its molecular formula was determined to be C27H40O4by its HRESIMS data atm/z429.2996 [M+H] + (calcd for C27H41O4: 429.2999).Comparison of the NMR data of 4 with those of 3 suggested that they had the same isobutyrylphloroglucinol moiety (part 4b) (Fig.2).The remaining NMR signals for 15 carbons implied the presence of a sesquiterpene moiety.The spin systems (from H-3 to H-10 and from H-7 to H-9′/H-10′) established by the 1 H— 1 H COSY spectrum as well as the HMBC correlations between H3-14/ H3-15 and C-1/C-10, between H2-13 and C-7/C-9, and between H3-12 and C-3/C-5 indicated the presence of a caryophyllene unit (part 4a) (Fig.2), which was further confi rmed by comparison of the NMR data of 4 with those of myrtucommulone K [10].Furthermore, the HMBC correlations between H-7′ and C-4/C-5/C-1′ indicated the connection of parts 4a and 4b via a C-5 and C-7′ bond.Finally, the closure mode of dihydropyran ring which connected the two fragments (4a and 4b) could be deduced on the basis of the molecular formula information and the downfi eld chemical shift at C-4 (δC 85.8).
In the NOESY spectrum, the correlations between H-5 and H-1/H-7′, between H-1 and Me-15, between H-9 and Me-14, as well as between Me-12 and H-8′/Me-12′ suggested that the relative confi gurations of C-1, C-4, C-5, C-9, and C-7′ were identical to those of myrtucommulone K (Fig.3).Additionally, the structure and absolute confi guration of 4 was unambiguously determined by X-ray crystallographic analysis using Cu Kαradiation with the Flack parameter [0.08 (13)] (Fig.5).Hence, the absolute confi guration of 4 was defi ned as 1R, 4R, 5S, 9S, 5′Sand 7′R.
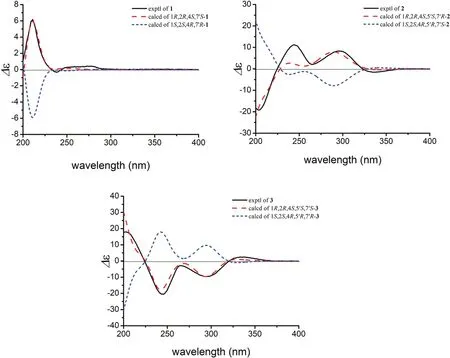
Fig.4 Calculated and experimental ECD spectra of 1— 3
2.2 Bioactivity Evaluation
The antibacterial activities of compounds 1— 4 against Gram-positive strainsStaphylococcus aureusATCC 43300 (MRSA),S.aureusATCC 700699 (VISA),S.aureusATCC 25923 andEnterococcus faecalisATCC 29212 and Gramnegative strainsPseudomonas aeruginosaATCC 27853,Escherichia coliATCC 25922 andKlebsiella pneumoniaeATCC 700603 were measured by broth microdilution method.As a result, compound 4 exhibited moderate antibacterial activity against all Gram-positive strains with MIC value of 32 μg/mL (Table 2).
3 Experimental
3.1 General Methods
Melting points were obtained on a Buchi melting point B-545 apparatus (Buchi Instrument, Switzerland) and are uncorrected.Optical rotations were measured on a JASCO P-2000 digital polarimeter (Jasco Co., Ltd., Tokyo, Japan) at room temperature.IR spectra were determined on a JASCO FT/IR-4600 plus Fourier transform infrared spectrometer (Jasco Co., Ltd., Tokyo, Japan) using KBr pellets.UV spectra were recorded on a JASCO V-550 UV/Vis spectrophotometer (Jasco Co., Ltd., Tokyo, Japan).CD spectra were obtained on a ChirascanqCD (Applied Photophysics Ltd., Surrey, UK).HRESIMS spectra were acquired on an Agilent 6210 LC/MSD TOF mass spectrometer (Agilent Technologies, CA, USA).NMR spectra were measured on Bruker AV-500 or AV-400 spectrometers (Bruker, Switzerland) with TMS as internal standard, and chemical shifts were denoted inδvalues (ppm).X-ray crystallographic analyses were carried out on an Agilent Gemini S Ultra CCD diffractometer with Cu Kαradiation (λ= 1.54178 Å).Silica gel (200—300mesh; Qingdao Marine Chemical, Inc., Qingdao, People’s Republic of China), Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden), and reversed-phase C18silica gel (YMC, Kyoto, Japan) were used for column chromatography (CC).Preparative HPLC was carried out on an Agilent 1260 Chromatograph equipped with a G1311C pump and a G1315D photodiode array detector (Agilent Technologies, CA, USA) with a semi-preparative C18reversed-phase column (Cosmosil, 10 mm × 250 mm, 5 μm).All solvents used in CC and HPLC were of analytical grade (Shanghai Chemical Plant, Shanghai, People’s Republic of China) and chromatographic grade (Fisher Scientifi c, New Jersey, USA), respectively.
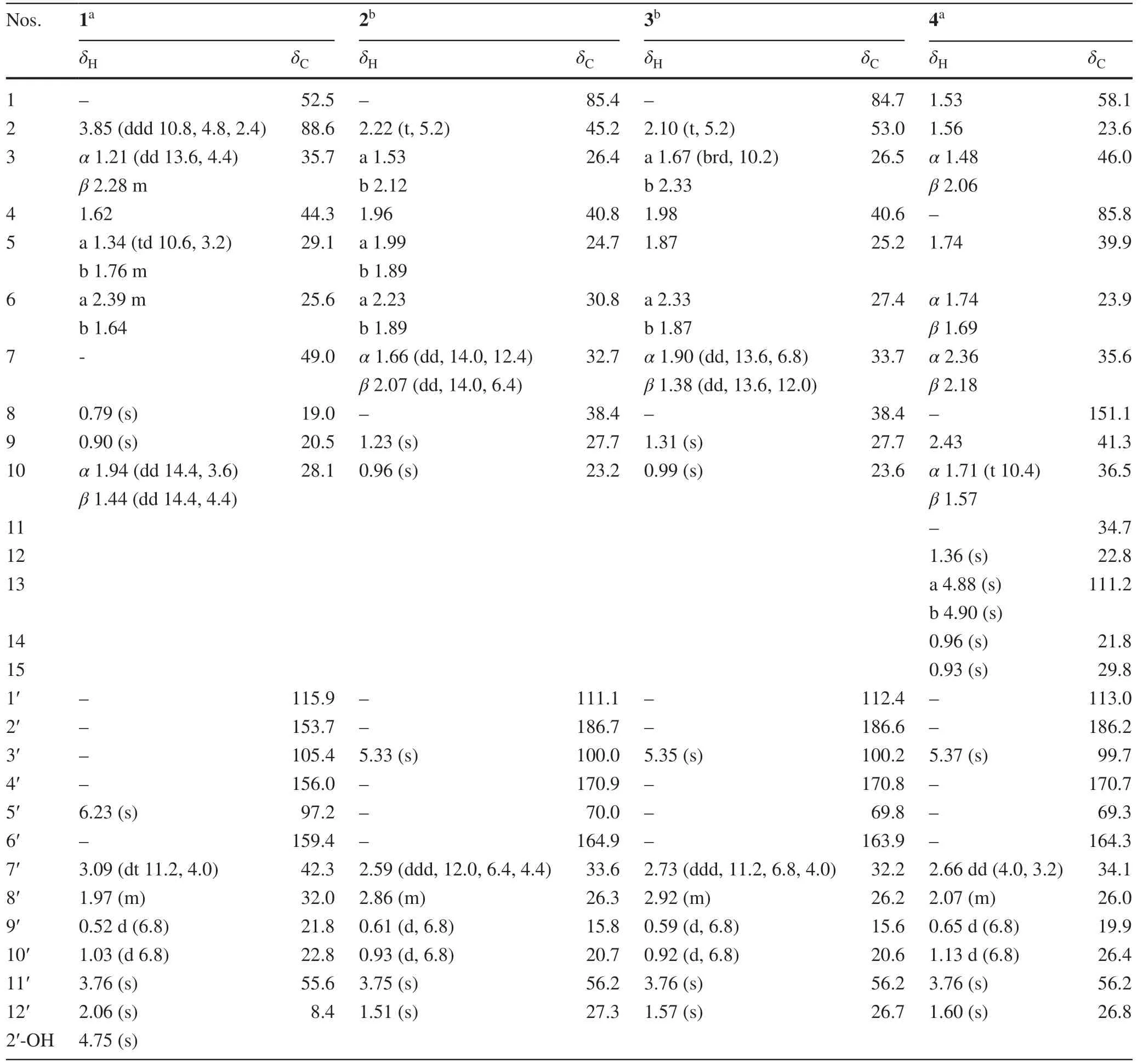
Table 1 1H and 13C NMR data of 1-4 in CDCl 3 (δin ppm,Jin Hz)
3.2 Plant Material
The leaves ofM.cauliflorawere collected from Nanning city, Guangxi Province of People’s Republic of China, in July of 2018.A voucher specimen (No.2018070607) identifi ed by Professor Guang-Xiong Zhou (Jinan University) was deposited in the Institute of Traditional Chinese Medicine and Natural Products, Jinan University, Guangzhou, People’s Republic of China.

Fig.5 X-ray ORTEP drawing of 4
3.3 Extraction and isolation
The air-dried leaves ofM.cauliflora(15 kg) were powdered and extracted with 95% EtOH (v/v, 50 L) at room temperature.The extract (2.2 kg) was suspended in H2O and extracted with petroleum ether (PE, b.p.60—90 °C).The PE extract (673.2 g) was subjected to a silica gel column chromatography using cyclohexane—EtOAc (100:0 → 0:100, v/v) as eluent to aff ord 10 fractions (Frs.A—J).Fr.G (48.3 g) was further separated by silica gel column using a gradient cyclohexane—EtOAc (100:0 → 0:100, v/v) to give 8 subfractions (Frs.G1—G8).Subfraction G5 (10.7 g) was chromatographed on Sephadex LH-20 (CH2Cl2/MeOH, 1:1, v/v) to obtain three subfractions (Frs.G5A—G5C).Subfraction G5B (7.3 g) was subjected to ODS column using MeOH/H2O (50:50 → 100:0, v/v) and further purifi ed by semipreparative reversed-phase HPLC (MeOH/H2O, 70:30, v/v, 3 mL/min) to aff ord 1 (12.5 mg,tR41.8 min),3 (11.3 mg,tR33.7 min) and 4 (15.7 mg,tR49.3 min).Subfraction G6 (5.3 g) was separated on Sephadex LH-20 (CH2Cl2/MeOH, 1:1, v/v) to obtain 2 (7.3 mg).
Compound 1yellow gum (CH3OH);= + 119 (c= 0.50, MeOH); UV (MeOH)λmax(logε) 206 (3.73) nm; IR (KBr)νmax3475, 2977, 2954, 2876, 1611, 1584, 1488, 1445, 1415, 1385, 1307, 1236, 1199, 1132, 1084, 1035, 1014, 982, 904, 831 cm-1;1H NMR (CDCl3, 500 MHz) and13C NMR (CDCl3, 125 MHz), see Table 1; HRESIMSm/z345.2423 [M+H] + (calcd for C22H33O3: 345.2424); ECD (MeCN, Δε) 211 (+ 6.2), 238 (- 0.5), 277 (+ 0.7) nm.
Compound 2yellow oil (CH3OH); [α]D25 = + 59 (c= 0.50, MeOH); UV (MeOH)λmax(logε) 202 (3.85), 245 (3.92), 296 (3.50) nm; IR (KBr)νmax3357, 2957, 2933, 2871, 1656, 1605, 1462, 1385, 1353, 1236, 1137, 1092, 998, 983, 893, 843 cm-1;1H NMR (CDCl3, 400 MHz) and13C NMR (CDCl3, 100 MHz), see Table 1; HRESIMSm/z361.2386 [M+H]+(calcd for C22H33O4: 361.2373); ECD (MeCN, Δε) 203 (- 19.8), 244 (+ 11.7), 296 (+ 8.1), 337 (- 1.8) nm.
Compound 3yellow oil (CH3OH);= - 63 (c= 0.50, MeOH); UV (MeOH)λmax(logε) 202 (3.71), 245 (3.77), 297 (3.32) nm; IR (KBr)νmax3359, 2958, 2924, 2871, 1656, 1603, 1463, 1388, 1372, 1259, 1232, 1136, 1089, 989, 899, 842 cm-1;1H NMR (CDCl3, 400 MHz) and13C NMR (CDCl3, 100 MHz), see Table 1; HRESIMSm/z361.2391 [M+H]+(calcd for C22H33O4: 361.2373); ECD (MeCN, Δε) 203 (+ 18.0), 245 (- 20.5), 293 (- 9.6), 333 (+ 2.5) nm.
Compound 4colorless blocks (CH3OH); m.p.162—163 ℃;= - 42 (c= 0.50, MeOH); UV (MeOH)λmax(logε) 205 (3.86), 247 (3.87), 310 (3.44) nm; IR (KBr)νmax3336, 2957, 2870, 1663, 1615, 1462, 1386, 1364, 1284, 1259, 1233, 1176, 1140, 999, 941, 886, 844 cm-1;1H NMR (CDCl3, 500 MHz) and 13 C NMR (CDCl3, 125 MHz), see Table 1; HRESIMSm/z429.2996 [M + H]+(calcd for C27H41O4: 429.2999).

Table 2 Antibacterial activities of compounds 1— 4 (MIC, μg/mL)
3.4 X-Ray Analysis
Crystal data for 4C27H40O4, monoclinic, space groupP21,a= 10.2148 (3) Å,b= 24.2552 (4) Å,c= 20.4139 (5) Å,α= 90°,β= 97.254 (2)°,γ= 90°,V= 5017.3 (2) Å3,T= 293 (2) K,Z= 2,Dcalcd= 1.135 g cm -3 ,F(000) = 1872, 20761 reflections measured (2.18° ≤θ≤ 73.86°), 13408 unique (Rint= 0.0389,Rsigma= 0.0574) which were used in all calculations.The finalR1was 0.0916 [I> 2σ(I)] and wR2was 0.2884 (all data).CCDC-1998470 contains the supplementary crystallographic data for this paper.These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_reque st/cif.
3.5 Antibacterial Activity Assay
Staphylococcus aureusATCC 43300 (methicillin-resistantS.aureus, MRSA),S.aureusATCC 700699 (vancomycinintermediateS.aureus, VISA),S.aureusATCC 25923,E.faecalisATCC 29212,P.aeruginosaATCC 27853,E.coliATCC 25922 andK.pneumoniaeATCC 700603 were standard isolates from ATCC (Manassas, VA, USA).The MIC values were measured using a previously reported method [7].Ciprofloxacin and vancomycin were used as positive controls.
AcknowledgementsThis work was supported by the National Key R&D Program of China (No.2017YFC1703800), the Program for the National Natural Science Foundation of China (Nos.81822042, 81630095), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Y036), Key-Area Research and Development Program of Guangdong Province (2020B1111110004), and the High-performance Computing Platform of Jinan University.
Compliance with Ethical Standards
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creat iveco mmons.org/licen ses/by/4.0/.
 Natural Products and Bioprospecting2021年1期
Natural Products and Bioprospecting2021年1期
- Natural Products and Bioprospecting的其它文章
- In Memory of the Late Professor Jun Zhou (1932-2020)
- In Mourning and Memory of Late Professor Zhou Jun
- Natural Products in Cancer Therapy: Past, Present and Future
- On the Famous Traditional Chinese Medicine “Fu Zi”: Discovery, Research, and Development of Cardioactive Constituent Mesaconine
- The Outlook of the Development of Innovative Products from Biocompatible Natural Spider Silk in the Beauty Thread-Lifting Industry
- Insight into Medicinal Chemistry Behind Traditional Chinese Medicines: p-Hydroxybenzyl Alcohol-Derived Dimers and Trimers from Gastrodia elata
