Synthesis of an Energetic Compound 1,3,5-Trinitrohexahydropyrimidine Based on Carbon-bromine Cleavage Reduction
YANG Kai-di, BI Fu-qiang, 2, ZHAI Lian-jie, ZHANG Jun-lin, HUO Huan, XUE Qi,WANG Bo-zhou,2
(1. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China; 2. State Key Laboratory of Fluorine & Nitrogen Chemicals,Xi′an 710065, China)
Abstract:Starting from 2-bromo-2-nitropropane-1,3-diol, tert-butylamine and 37% formaldehyde, an energetic compound of 1,3,5-trinitrohexahydropyrimidine (TNHP) was achieved with a total yield of 33% (or 21.11%) through a synthetic procedure including condensation-cyclization, nitration and NaBH4 (or KI) carbon-bromine cleavage reduction. The structure of TNHP as well as its synthetic intermediates were characterized by IR, NMR, elemental analysis and MS techniques. The single crystal of TNHP was prepared and determined via X-ray single crystal diffraction analysis, its physicochemical and detonation properties were further studied by experimental and theoretical methods. The results show that TNHP crystallizes in monoclinic with the space group of P2(1)/c with a crystal density of 1.787g/cm3. Its decomposition temperature is 150.6℃,and the calculated heat of formation, detonation velocity and impact activity are -50.49kJ/mol, 8388m/s and 22.5J, respectively, indicating a potential application prospect in the field of explosives.
Keywords:organic chemistry; 1,3,5-trinitrohexahydropyrimidine; carbon-bromine cleavage reduction; TNHP; energetic properties
Introduction
Energetic materials based on hexahydropyrimidine skeleton have attracted constant attentions from international researchers for their great chemical stability of six-member heterocyclic ring, detonation and safety performance[1-3]. Based on hexahydropyrimidine framework, a series of energetic compounds with excellent properties were synthesized, such as 1,3,5,5-tetranitro-hexahydropyrimidine(DNNC)[4-5],1,3-dinitrohexahydropyrimidin-5-yl nitrate(DNHM)[3],5-bromo-1,3,5-trinitrohexahydropyrimidine(TNBrP)[6]and so on.
Among these energetic materials,TNBrP is not only a promising energetic compound with good stability and higher detonation performance but also an important energetic intermediate. If TNBrP is treated with some effective reagent to make the carbon-bromine cleavage selectively, it will be of great significance to obtain a novel trinitro-substituted energetic compound and synthesize a series of hexahydropyrimidine energetic materials.
Referring to the relevant literatures[6-8], TNBrP was synthesized by heightening the condensation-cyclization reaction temperature and using crude nitric acid as nitration reagent and the reaction time was reduced from 10h to 4h, simplify the technology and increase the yield. Using 5-bromo-1,3,5-trinitro-hexahydro- pyrimidine (TNBrP) as starting materials, a new energetic compound 1,3,5-trinitrohexahydro- pyrimidine(TNHP) was designed and synthesized firstly via carbon-bromine cleavage reduction of NaBH4or KI, and the reaction mechanism of Carbon-Bromine cleavage reduction of NaBH4was discussed for the first time, and its physicochemical properties and detonation performances were also studied by the experimental method and theoretical calculation.
1 Experiment
1.1 Reagents and Instruments
All reagents and solvents were purchased from Aladdin Bio-Chem Technology Co., Ltd (Shanghai, China) and used without further purification unless otherwise indicated.
AV 500NMR spectrometer, Bruker, Switzerland. EQUINOX 55 Fourier transform Infrared spectrometer, Bruker, Germany. Vario EL cube elemental analyzer, Elemental, Germany. TG-DSC STA 499 F3 instrument, NETZSCH, Germany (Conditions: The experiment was carried out at dynamic nitrogen atmosphere, the temperature was between 25-300℃, the heating rate was 5℃/min, the sample scale was 0.5-1.0mg, and the plate was aluminum plate). Bruker Apex II CCD diffractometer.
1.2 Synthetic route of TNHP
The synthetic route of TNHP is as follow:

1.2.1 Synthesis of 5-bromo-1,3-di(tert-butyl)-5-nitro-hexahydropyrimidine (DBBrP)
To a stirred solution of 2-bromo-2-nitropropane-1,3-diol (18.00g, 0.090mol) in methanol(100mL) at 0℃,tert-butylamine(13.50g, 0.185mol) was added dropwise over 45min. The mixture was stirred at 0℃ for an additional 45min. Then, 37% formaldehyde solution(7.46g, 0.092mol) was added. The resulting mixture was stirred at 50℃ for 5h and cooled to 0℃,then 150mL water was added. The slight yellow solid (23.20g) was collected by filtration, recrystallized from water-ethanol and washed with a yield of 80.06%.
1H NMR(500MHz, Acetone-d6) δ:4.55(dd,2H), 5.15(dd,2H), 5.31(m,1H), 5.63(d,1H), 6.67(d,1H):13C NMR(125MHz , Acetone-d6) δ:47.42, 60.47, 75.31;IR(KBr),ν(cm-1):2971,1558,1365,1346,1031,948,935,762; Anal. Calcd. for C12H24BrN3O2(%):C 44.73, H 7.51, N 13.04; Found:C 44.57, H 7.448, N 12.93.
1.2.2 Synthesis of 5-bromo-1,3,5-trinitrohexahydro-pyrimidine(TNBrP)
12mL 98% concentrated nitric acid was cooled to -5℃ using an ice water bath,and DBBrP (1.00g, 0.0031mol) was slowly added to the solution. When the addition was completed, the reaction mixture was warmed to 25℃,stirring for 4h, and then poured onto ice. The resulting precipitate was collected by filtration, washed with water, and dried to give a white solid(0.57g) with a yield of 61.19%.
1H NMR(500MHz, Acetone-d6) δ:5.02(d,2H), 5.41(d,2H), 6.14(d,1H), 6.29(d,1H):13C NMR(125MHz , Acetone-d6) δ:56.06, 61.22, 84.03;IR(KBr),ν(cm-1):2987,2941,2955,1583,1546,1486,1382,1342,1322,764,753,718;Anal. Calcd. for C4H6BrN5O6(%):C 16.00, H 2.00, N 23.33; Found:C 15.48, H 2.34, N 22.86.
1.2.3 Synthesis of 1,3,5-trinitrohexahydropyrimidine(TNHP)
Method1:To a solution of compound TNBrP (1.31g,0.0044mol) in 30mL of methanol,small portions of sodium borohydride (0.49g,0.013mol) was added in 5min at 25℃. After 50min, hydrochloric acid was added till pH=6, and the mixture was diluted with 15mL water.Then the precipitate was filtered off, dried, and recrystallized, white powder solid (0.65g) was obtained with a yield of 67.36%.
Method2:The solution of potassium iodide (0.63g) in methanol (10mL) with temperature at 0℃ was added to the cold solution of TNBrP (0.52g, 0.0024mol) dissolved in the mixed solution of methanol (8mL) and acetic acid (8mL).The resulting solution was stirred for 8h at room temperature. Water(100mL) was added and the resulting precipitate was collected by filtration and washed with water to give a white solid (0.20g) with a yield of 43.09%.
1H NMR(500 MHz ,Acetone-d6) δ:4.55(dd,2H), 5.15(dd,2H), 5.31(m,1H), 5.63(d,1H), 6.67(d,1H):13C NMR(125 MHz , Acetone-d6) δ:47.42, 60.47, 75.31.IR(KBr),ν(cm-1):3082, 2956, 1569, 1526, 1389, 1357, 981,761,575; Anal. Calcd. for C4H7N5O6(%):C 21.72, H 3.167, N 31.67; Found:C 22.06, H 3.295, N 30.86;m/z:220.03271.
2 Results and discussions
2.1 Proposed reaction mechanism of C—Br cleavage reduction
The NaBH4reduction process mainly depends on the H-in molecule, which attacks C5connecting with gem-nitro bromine making the C—Br bonded cleavage to yield target compound TNHP, sodium methoxyborate and hydrogen bromide. And the other H-in sodium methoxyborate still has reducibility which can make other carbon-bromine cleavage to yield the TNHP and sodium dimethoxyborate until there is no H-in borate. After the reaction, with the addition of water and acid, boron is present as boric acid in the system. The proposed reaction mechanism is shown in Fig.1.

Fig.1 Proposed reaction mechanism of C—Br cleavage reduction of NaBH4
2.2 Data of crystal structure
The crystal of TNHP was grown by slow evaporation method at room temperature and the X-ray single-crystal diffraction data for TNHP were collected on a Bruker SMART Apex Ⅱ CCD X-ray diffractometer equipped with a graphite monochromatized Mo Kα radiation (λ=0.71073Å, ω and φ scan mode). The data of crystal structure of TNHP were listed in Table 1.
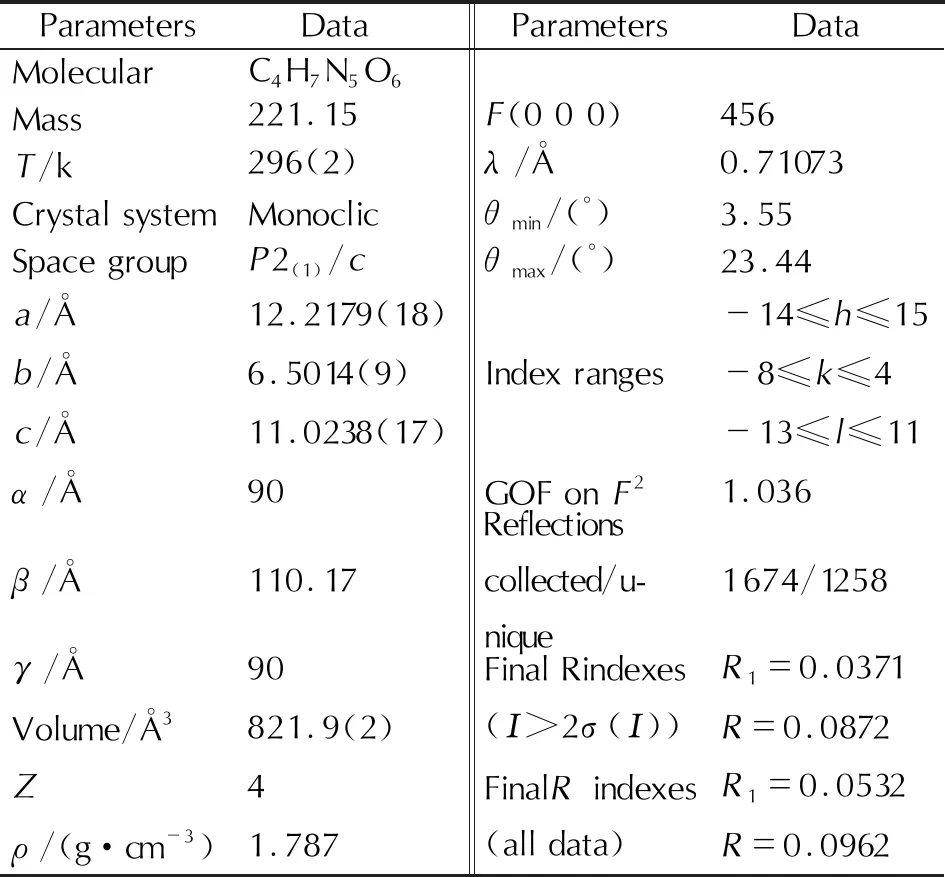
Table 1 Crystal structure data of TNHP
We chose a crystal of TNHP with the volume of 821.9Å3, and found that the crystal system of TNHP is monoclic with the space group ofP2(1)/c.
2.3 Thermal behaviors of TNHP
The thermal behaviors of TNBrP and TNHP were studied by DSC-TG, and the results are shown in Fig.2. The patterns show that TNBrP has an endothermic peak at 154.5℃ which belongs to its melting process and two exothermic peaks at 173.5℃ and 222.8℃ which caused by its decomposition processes. While TNHP only has a lower decomposition point at 150.6℃ and there is no melting process.
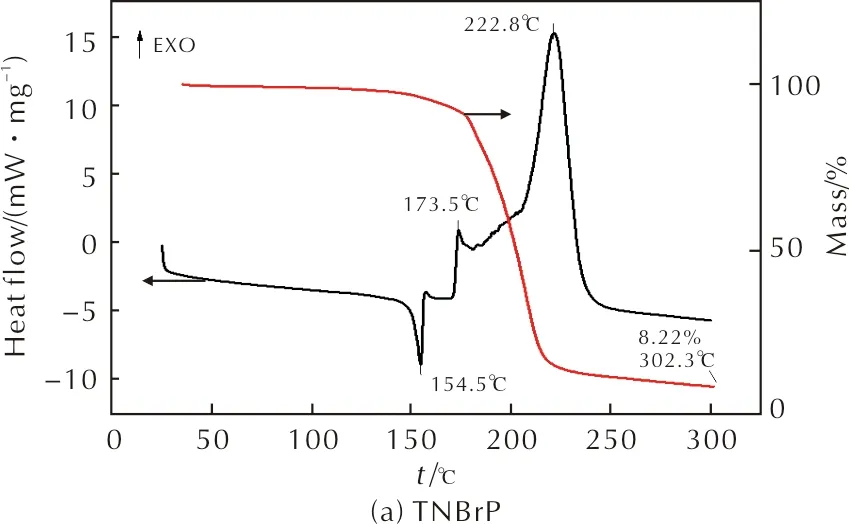
Fig.2 DSC curves of TNBrP and TNHP
2.4 Detonation performance of TNHP
The molecule of TNHP was optimized with the help of Gaussian 09 based on B3LYP/6-311G level of theory[9]. And the enthalpy of formation of TNHP was obtained and corrected via CBS-4M[10]level of theory. Then, the detonation performance of TNHP was determined via density of crystal and calculated enthalpy of formation by Explore[11]. Meanwhile, compared with the similar energetic materials DNHM[3,13]and TNT[12], the results were listed in Table 2, and show that the real density of TNHP is 1.787g/cm3, the calculated detonation velocity is 8388m/s, the detonation pressure is 29.88GPa, and the Enthalpy of formation is -50.49kJ/mol. The detonation performance of TNHP is slightly lower than that of DNHM but higher than TNT, and TNHP has great extensibility to synthesize other compounds like DNNC, considering that TNHP has great application potential.

Table 2 The physicochemical properties and detonation performances of TNHP
3 Conclusions
(1) A novel energetic compound TNHP was synthesized via condensation-cyclization, nitration of crude nitric acid and carbon-bromine cleavage reduction of NaBH4with the total yield of 33.0%, and its structure was confirmed by IR,1H and13C NMR, elemental analysis, MS and x-ray single crystal diffraction. It crystallizes in monoclinic with the space group ofP2(1)/cand the crystal density of 1.787g/cm3.
(2) The physicochemical properties and detonation performance of TNHP were studied by the experimental method and theoretical calculation with a decomposition point of 150.6℃, detonation velocity of 8388 m/s and the impact sensitivity of 22.5J, indicating that TNHP has a potential application prospect in the fields of explosive.

