Combined treatment with valproic acid and estrogen has neuroprotective effects in ovariectomized mice with Alzheimer’s disease
Yan-Zhen Li , Yuan-Jie Liu , Wei Zhang Shi-Fang Luo Xin Zhou ,Gui-Qiong He
Abstract Postmenopausal women with Alzheimer’s disease exhibit dramatically reduced sensitivity to estrogen replacement therapy, which is though to be related to an estrogen receptor (ER)α/ERβ ratio imbalance arising from a significantly decreased level of ERs of the brain. The aim of our study was to investigate whether valproic acid (VPA) can enhance the beneficial effects of estrogen on cognitive function through restoration of ERα and ERβ expression in the brain. We removed the ovaries of female APP/PS1 mice to simulate the low estrogen levels present in postmenopausal women and then administered VPA (30 mg/kg, intraperitoneal injection, once daily), 17β-estradiol (E2) (2.4 µg,intraperitoneal injection, once daily), liquiritigenin (LG) (50 µg/kg, intragastric infusion, once daily), VPA + E2, or VPA + LG for 4 successive weeks. Compared with treatment with a single drug, treatment with VPA + E2 or VPA + LG significantly increased the level of glycogen synthase kinase 3β, increased the expression of estrogen receptor α, reduced the expression of small ubiquitin-like modifiers, and increased the level of estrogen receptor β. This resulted in enhanced sensitivity to estrogen therapy, reduced amyloid β aggregation, reduced abnormal phosphorylation of the tau protein, reduced neuronal loss, increased dendritic spine and postsynaptic density, and significantly alleviated memory loss and learning impairment in Alzheimer’s disease. This study was approved by the Chongqing Medical University Animal Protection and Ethics Committee, China on March 6, 2013.
Key Words: 17β-estradiol; amyloid β; dementia; estrogen receptor α; estrogen receptor β; glycogen synthase kinase-3β; liquiritigenin;menopause; neuron loss; tau Chinese Library Classification No. R453; R741; Q579.1+3
Introduction
Alzheimer’s disease (AD) is a common form of degenerative neurological disorder resulting in memory loss and cognitive decline. Excessive deposition of extracellular senile plaques(SP), formation of intracellular neurofibrillary tangles, and loss of neurons are the predominant neuropathological features of AD (Goedert and Spillantini, 2006; Wang et al., 2020).Overexpression of glycogen synthase kinase 3β (GSK-3β) in patients with AD may promote amyloid β (Aβ) production, tau hyperphosphorylation, and neuronal degeneration (Hernández et al., 2010).
Valproic acid (VPA), a multifunctional drug used to treat epilepsy and bipolar disorder (Kostrouchová et al., 2007),plays important roles in promoting the release of neurotrophic factor and inducing neurogenesis (Qing et al., 2008; Hu et al., 2011). In addition, a recent study demonstrated that VPA inhibits GSK-3β activity by increasing p-Ser9-GSK-3β levels(Azab et al., 2017). Furthermore, we previously showed that VPA can reduce Aβ generation, decrease tau phosphorylation,and improve memory deficits, which is consistent with other studies (Qing et al., 2008; Hu et al., 2011; Xuan et al., 2015).
An epidemiological study indicated that AD affects postmenopausal women about 1.5-3 times more often than similarly aged men, suggesting that there is a link between menopausal estrogen loss and development of the disease(Gillies and McArthur, 2010). Clinical trials have reported that estrogen replacement therapy can effectively delay and reduce the risk of AD in women prior to or in the early stages of menopause, whereas postmenopausal women with AD have a dramatically reduced sensitivity to estrogen replacement therapy (Gleason et al., 2015; Kantarci et al.,2016).The mechanism underlying this phenomenon may be related to an imbalance in the estrogen receptor (ER)α/ERβ ratio owing to significantly lower expression of ERs in the brain (Sinopoli et al., 2006). Recent research has highlighted that GSK-3β may affect ERα phosphorylation and activity(Medunjanin et al., 2005; Fortunati et al., 2010). In addition, it can regulate ERβ expression and activity through the addition of small ubiquitin-like modifier (SUMO) proteins to this receptor (Picard et al., 2012).
In the brain, ERα is mainly expressed in the hippocampus,preoptic region, and hypothalamus. ERβ is mainly expressed in the olfactory bulb, cerebral cortex, and cerebral septum.Both ERα and ERβ play indispensable roles in the formation of learning and memory (Foster, 2012). ERα and ERβ nonselectively bind estrogens, including estrone, 17β-estradiol(E2), and estriol, of which E2 has the strongest estrogenic activity. Phytoestrogens, whose structure and function are similar to those of estrogens, are effective ERβ agonists and exhibit minor toxic side effects. The phytoestrogen liquiritigenin(LG) is a highly selective ERβ agonist that has neuroprotective and neurotrophic effects (Mersereau et al., 2008).
Thus, given the evidence presented above, the aim of our study was to determine whether E2 or LG combined with VPA can ameliorate the cognitive impairment and neurodegenerative pathology observed in postmenopausal ovariectomized (OVX)APPswe/PSEN1dE9 double-transgenic (APP/PS1) mice through restoration of ERα and ERβ expression in the brain.
Materials and Methods
Animals
Eighty 3-month-old female APP/PS1 AD mice (weighing 23-27 g, specific pathogen-free) were obtained from the Biomedical Research Institute of Nanjing University (Nanjing,China; certificate No. 201800973). The animals’ ovaries were removed under anesthesia to simulate the physiological state of low estrogen levels in postmenopausal women. One month after surgery (at which time the mice were 4 months old, which is equivalent to 2 to 4 years after menopause in women), the mice were randomly divided into six groups with eight mice in each group: the single-drug groups (VPA, E2,or LG), the combined treatment groups (VPA + E2 or VPA +LG), and the control group. The mice received VPA (Sigma, St.Louis, MO, USA, 30 mg/kg per day, intraperitoneal injection,once daily), E2 (Sigma, 2.4 µg per mouse, once a day,intraperitoneal injection), LG (Shanghai Macklin Biochemical Co., Ltd., Shanghai, China, 50 µg/kg per day, intragastric infusion, once a day), or 0.9% sodium chloride (0.25 mL) for 4 weeks. All experiments were approved by the Chongqing Medical University Animal Protection and Ethics Committee on March 6, 2013. The mice were housed in cages in a laminar flow rack exposed to a 12 hours on/12 hours off light cycle and were given free access standard rodent chow and water,within a temperature-controlled room (20-25°C).
Morris water maze
The Morris water maze (MWM) apparatus (SLY-WMS 2.0;Shanghai Bio-will Co., Ltd., Shanghai, China) consisted of a circular tank 120 cm in diameter and 47 cm deep. The tank contained warm water that was maintained at 25 ± 0.5°C and was divided into four quadrants that were marked with cues on the exterior. A transparent escape platform (10 cm in diameter)was set 1 cm above the water level on day 1. Each mouse was allowed to freely explore the platform for 60 seconds. If the animal failed to locate the platform within 60 seconds,the mouse would be placed on it for 15 seconds. The hidden platform test consisted of four consecutive training days. The platform was submerged to a depth of 1 cm under the surface of the water. Spatial learning and memory were assessed by measuring escape latency and path length to reach the hidden platform in 60 seconds. During the probe test, the platform was removed from the pool, and the mice were allowed to swim freely for 60 seconds. All paths from all trials were analyzed using water maze software (Lyu et al., 2020).
Immunofluorescence and thioflavin S staining
Animals were euthanized by CO2inhalation after the behavioral experiments were complete. Next, the brains were removed, and half of each brain was dehydrated for 24 hours in a series of sucrose solutions (10%, 20%, and 30%), after which they were fixed in 4% paraformaldehyde overnight.These brain samples were then cut into 10-µm-thick coronal sections and used for immunofluorescence staining and thioflavin S staining (0.1%; Zhuhai Fei Yang Novel Materials Co., Ltd., Zhuhai, China). Immunofluorescence staining was performed by treating with 3% hydrogen peroxide and blocking in 10% fetal calf serum. The slides were incubated with a mouse anti-Aβ monoclonal 4G8 antibody (1:250; Cat#SIG-39240; Covance, Princeton, NJ, USA) at 4°C overnight.Subsequently, the slides were incubated with a goat antimouse IgG (H+L) CY3-conjugated secondary antibody (1:300;Affinity Biosciences, Changzhou, China) at 37°C for 1 hour,followed by a DAPI solution (Solarbio, Beijing, China). For thioflavin S staining, after being subjected to an ethanol gradient and 0.1% KMnO4treatment, sections were incubated with thioflavin S (0.1% in 80% of ethanol) for 8 minutes at room temperature, washed with ethanol (80% and 70%each for 30 seconds), and rinsed with distilled water twice.The slides were mounted with aqueous mounting media and dried in the dark overnight. Finally, all of the sections were visualized, and images were captured, using a digital fluorescence microscope (Leica Microsystems, Wetzlar,Germany), and the mean number of plaques per slice in the cortex and hippocampus of the six groups was quantified.
Enzyme-linked immunosorbent assay
The other half of each brain from each mouse was analyzed for Aβ40and Aβ42expression levels using an Aβ1-40and Aβ1-42Assay Kit (IBL, Minneapolis, MN, USA) according to the manufacturer’s instructions. The Aβ concentration in the braintissues was calculated from standard curves.
Western blot analysis
Total protein was extracted from the collected mouse brain tissues using cold lysis buffer containing a protease inhibitor. The protein concentrations were determined by the bicinchonininc acid method (Beyotime Biotechnology,Shanghai, China). Equal amounts of protein from different samples were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and then electrotransferred onto a 0.45-µm polyvinylidene fluoride membrane (Millipore, Burlington, MA, USA). After the membrane was immersed in 3% bovine serum albumin for 2 hours, target proteins were detected using primary antibodies to amyloid precursor protein (APP; Cat# ab241592; 1:1000;Abcam, Cambridge, MA, USA), β-amyloid precursor protein cleavage enzyme-1 (BACE1; Cat# ab2077; 1:1000; Abcam),presenilin-1 (PS1; Cat# ab76083; 1:1000; Abcam), insulindegrading enzyme (IDE; Cat# ab133561; 1:1000; Abcam),neprilysin (NEP; Cat# ab58968; 1:1000; Abcam), tau-5 (Cat#ab80579; 1:1000; Abcam), paired helical filament-1 (PHF-1; Cat# ab184951; 1:1000; Abcam), neuronal nuclei antigen(NeuN; Cat# 24307; 1:1000; CST, Danvers, MA, USA), growth associated protein-43 (GAP43; Cat# ab75810; 1:1000;Abcam), postsynaptic density-95 (PSD-95; Cat# ab76115;1:1000; Abcam), ERα (Cat# ab32063; 1:800; Abcam), ERβ (Cat#ab3577; 1:1000; Abcam), GSK-3β (Cat# ab93926; 1:1000;Abcam), p-GSK-3β (Ser9) (Cat# b75814; 1:500; Abcam), small ubiquitin-like modifier 1 (SUMO1; Cat# sc-5308; 1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and β-actin (Cat#A5441; 1:1000; Sigma). The membranes were then incubated with goat anti-rabbit IgG (H+L) horseradish peroxidase(1:5000, Cat# S0001, Affinity Biosciences, Cincinnati, OH,USA) or goat anti-mouse IgG (H+L) horseradish peroxidase(1:5000, AS003, ABClonal, Wuhan, China) at 37°C for 40 minutes. Immunoreactive bands on the membrane were visualized by enhanced chemiluminescent detection (Beyotime Biotechnology). Relative expression was determined using Image Lab software (Bio-Rad, Hercules, CA, USA).
Golgi staining
Dendritic spine structure was observed by Golgi staining (He et al., 2020) using the FD Rapid Golgi Stain Kit (FD Neurotechnology,Inc., Columbia, MD, USA). All procedures were conducted in the dark. The brain tissues were immersed in a mixture of equal volumes of solutions A and B at room temperature for 2 weeks,after which the tissues were transferred to solution C for 72 hours. Next, 100-µm thick coronal sections were cut using a cryostat microtome at -22°C, and mounted on gelatin-coated microscope slides using solution C. A staining solution (one part solution D, one part solution E, and two parts DW) was used to stain the sections for 5 minutes. After the sections were dehydrated and cleared, coverslips were applied using neutral gum (Biosharp, Guangzhou, China). Golgi-stained sections were analyzed with an optical microscope (DFC425 C; Leica).
Transmission electron microscopy
One millimeter-thick sections were cut from the cortex and from hippocampal region CA1 of the mouse brains. The specimens were fixed in 3% glutaraldehyde for 2 hours,followed by 1% osmium tetroxide for 2 hours, and then dehydrated with a graded acetone series. Next, the tissues were embedded in epoxy resin and sliced into 100-nm-thick sections. Finally, the ultrathin sections were stained with uranyl acetate and lead citrate, and the synaptic ultrastructural changes were captured and observed with a transmission electron microscope (Philips, Amsterdam, The Netherlands).
Statistical analysis
Data are presented as the mean ± error of the mean (SEM).All quantitative results were analyzed using Graph Pad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA). The MWM data were analyzed by two-way analysis of variance with Student-Newman-Keuls tests. For all other data, one-way analysis of variance was used, followed by Tukey’spost hoctest. In all cases, aP-value < 0.05 was considered statistically significant.
Results
VPA combined with estrogen ameliorates memory and learning deficits in OVX AD mice
To test whether treatment with VPA and estrogen improves spatial memory and learning in OVX AD mice, an MWM test was conducted after 4 weeks of treatment. No statistically significant differences were found in escape latency (F=0.4247,P= 0.8255) or path length (F= 1.684,P= 0.1895)for the visible platform test among all groups (Figure 1AandB), which indicated that the treatment regimen did not affect vision or motility. For the hidden platform task, the combination treatment groups exhibited a considerable decrease in escape latency (F= 2.167,P= 0.0091) and path length (F= 1.165,P= 0.3029) compared with the singledrug groups and the control group (Tables 1and2). In the probe trials, the mice in the combination treatment groups traveled across the platform zone more often than those in the control group and single-drug groups. Furthermore, the VPA + LG group exhibited superior performance in the probe trial compared with the VPA + E2 group (F= 19.38,P< 0.0001;Figure 1C). These data indicated that the spatial memory and learning of OVX AD mice was improved after treatment, and that treatment with VPA and estrogen was more effective than treatment with VPA, E2, or LG alone.
VPA combined with estrogen decreases SPs and Aβ expression levels in the brain in OVX AD mice
Impairments in memory and cognitive functions are closely related to increased levels of Aβ (Selkoe and Hardy, 2016).To examine whether the combined treatment with VPA and estrogen alters APP processing and inhibits Aβ production,SPs were detected in OVX AD mouse brains. Immunostaining showed SP immunopositivity in the hippocampus and cortex of all of the treated groups (except for the group treated with E2 only) that was significantly reduced compared with the control group. The immunopositive SPs were noticeably smaller in the VPA + LG group than those in the control group and the single-drug groups (F= 26.18,P< 0.0001;Figure 2AandC). Moreover, thioflavin S staining also showed a noticeable reduction in SP formation in the hippocampus and cortex of mice in the two combined treatment groups (F=14.14,P< 0.0001;Figure 2BandD). However, no difference in SP formation was observed between the VPA + LG and VPA +E2 groups (P> 0.05).

Table 1 |Hidden platform test escape latency (in seconds) in an ovariectomized mouse model of Alzheimer’s disease
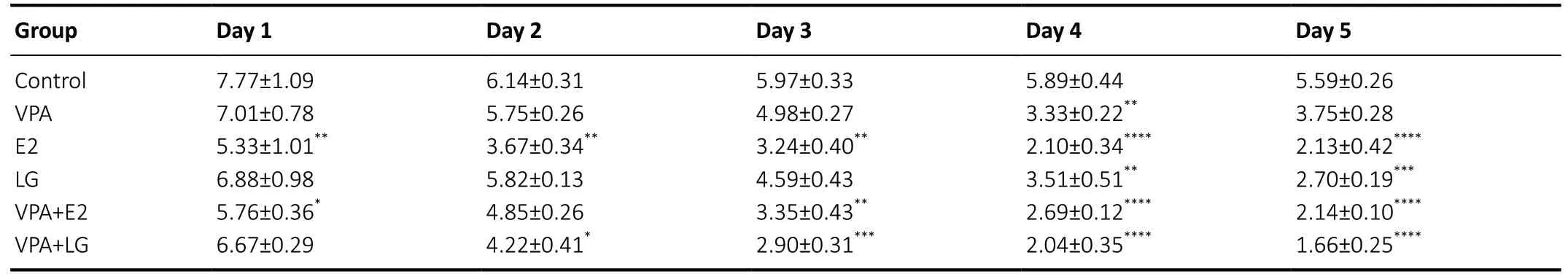
Table 2 |Hidden platform test change in path length (in m) in an ovariectomized mouse model of Alzheimer’s disease
To further elucidate the effects of the combination treatment on Aβ elevation, the expression levels of Aβ40and Aβ42in the hippocampus and cortex of the OVX AD mice were measured by enzyme-linked immunosorbent assay (Figure 2EandF).Aβ40expression was markedly decreased in the VPA + LG group compared with the control group (F= 16.74,P< 0.0001),and Aβ42expression was lower in all treatment groups (except for the E2 group) compared with the control group (F= 15.09,P= 0.0007). However, no difference in Aβ42expression was observed between the VPA + LG and VPA + E2 groups (P> 0.05).Taken together, these observations suggest that combined therapy is more affective at inhibiting Aβ production than single-drug treatment.
VPA combined with estrogen decreases Aβ formation and increases Aβ degradation in the brain in OVX AD mice
BACE1 and PS1 are the key enzymes involved in APP processing and Aβ production, while IDE and NEP are the main proteases involved in Aβ degradation (Murphy and LeVine,2010). To further explore whether combined treatment could decrease the Aβ burden by regulating APP processing in the brains of OVX AD mice, APP, BACE1, PS1, IDE, and NEP expression were analyzed by western blot was analyzed.No detectable difference was observed in APP expression among all groups (F= 0.7375,P= 0.6096). BACE1 (F= 11.24,P= 0.0003) and PS1 (F= 15.09,P< 0.0001) expression levels were significantly lower in the combination treatment groups compared with the control group (Figure 3AandC).Furthermore, IDE expression was significantly higher in the VPA + E2 and VPA + LG groups compared with the control and single-drug groups (F= 6.393,P< 0.0004). Similarly, NEP expression was higher in the VPA-only group and in both of the combined treatment groups than in the control group (F=41.95,P< 0.0001;Figure 3BandD). However, no difference in BACE1, PS1, or IDE expression was detected between the VPA+ LG and VPA + E2 groups.
VPA combined with estrogen prevents tau hyperphosphorylation in the brain in OVX AD mice
Abnormally hyperphosphorylated tau facilitates its aggregation into PHFs, which in turn become the main constituent of the neurofibrillary tangles that are characteristic of AD (Šimić et al., 2016). We examined expression of total tau (tau-5) and phosphorylated tau (PHF-1) by western blot. No difference in tau-5 expression was observed among all of the groups (F= 1.782,P= 0.1911). In contrast, all of the drug treatment groups exhibited a significant decrease in PHF-1 levels compared with the control group (F= 111.6,P< 0.0001;Figure 4AandB). However, no significant difference in PHF-1 expression was observed between the VPA + LG and VPA + E2 groups (P> 0.05).
VPA combined with estrogen attenuates neuronal loss in the brain in OVX AD mice
Given that neuronal loss is a fundamental pathological feature of AD (Vasic et al., 2019), we next asked whether combined treatment could attenuate neuronal loss. To test this, we evaluated expression of NeuN, marker of mature neurons(Lorenz et al., 2005), by western blot. We found that NeuN was expressed at higher levels in all drug treatment groups(except for the VPA group) compared with the control group (F= 6.331,P= 0.0042;Figure 5AandB). Since dendritic spines are known to be synaptic sites on neuronal dendrites, and are closely related to learning and memory (Chidambaram et al.,2019), we used Golgi staining to detect whether treatment altered neuronal dendritic spine density. The density of the dendritic spines was increased in the LG group and the combination groups compared with the control group (F=7.043,P< 0.0001;Figure 5CandD). However, no difference in NeuN expression or dendritic spine density was observed between the VPA + LG and VPA + E2 groups (P> 0.05).
VPA combined with estrogen modulates synaptic structure in the hippocampus in OVX AD mice
Both biochemical and morphological changes in synapses are key factors affecting learning and memory (Spires-Jones and Hyman, 2014). Therefore, we analyzed synaptic ultrastructural changes by transmission election microscope. The results showed that the number of synapses in the hippocampus was increased in the combined treatment groups compared with the control and single-drug groups (F= 9.191,P< 0.0001;Figure 6AandB). Moreover, the PSD layer was significantly thicker in all of the drug groups (except for the E2 group) than in the control group (F= 4.797,P= 0.0023;Figure 6C). The synaptic cleft width and synaptic active zone length were also higher in the combined treatment groups than in the control group (F= 9.442,P< 0.0001;Figure 6DandE).
Synaptic function is associated with cognition and depends on the normal integration of glutamate receptors at the PSD(de Bartolomeis et al., 2014). Thus, western blot was used to investigate GAP-43 and PSD-95 expression. GAP-43 (F= 16.17,P< 0.0001) and PSD-95 (F= 11.51,P= 0.0003) expression levels were significantly higher in the combined treatment groups compared with the control and single-drug groups(Figure 6FandG).
VPA combined with estrogen inhibits GSK-3β and improves ER sensitivity in the brain in OVX AD mice
The GSK-3β kinase is rich in serine (Ser) and threonine (Tyr),and its activity is inhibited by Ser9 phosphorylation (Azab et al., 2017). Thus, we next sought to evaluate the relationship between drug treatment and the relative levels of total GSK-3β and p-GSK-3β (Ser9). No significant differences in GSK-3β expression was found among all of the groups (F= 2.141,P= 0.1299). However, p-GSK-3β expression was significantly increased in the treatment groups, especially the combined treatment groups, compared with the control group (F=16.32,P< 0.0001;Figure 7AandB).
ERα and ERβ are the two main subtypes of ER that are expressed in the brain (Foster, 2012). We examined ERα and ERβ expression by western blot and found that ERα expression was higher in all of the single-drug groups except for the VPAonly group, and was significantly higher in the combined treatment groups, than in the control group (F= 25.05,P<0.0001;Figure 7CandD). Additionally, there was a significant increase in ERβ expression in all of the drug treatment groups,except the VPA-only treatment group, compared with the control group (F= 138.9,P< 0.0001;Figure 7CandE).
Finally, given that change in ER expression levels may be associated with ER SUMOylation (Picard et al., 2012), we detected SUMO1 by western blot. A significantly lower level of SUMO1 was detected in the single-drug and combined treatment groups compared with the control group (F= 28.10,P= 0.0004;Figure 7CandF).
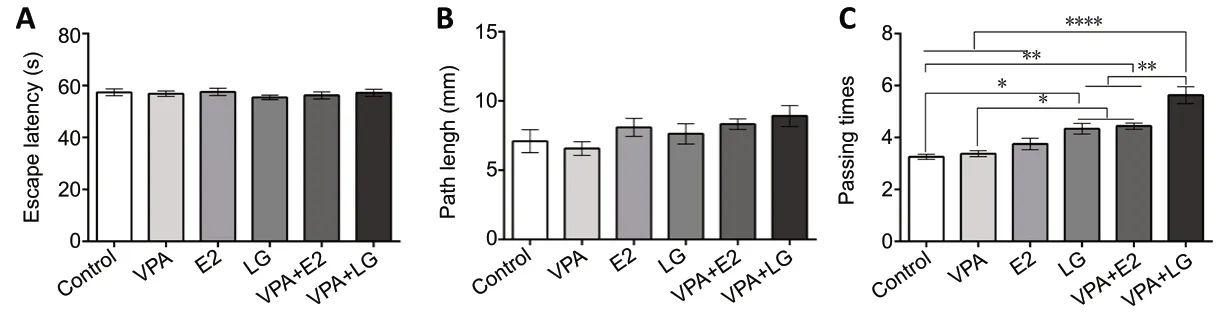
Figure 1|Spatial memory and learning as assessed by Morris water maze test in an ovariectomized mouse model of Alzheimer’s disease after 4 weeks of drug administration.

Figure 2|Combined treatment with VPA and estrogen decreases SP formation and Aβ levels in an ovariectomized mouse model of Alzheimer’s disease after 4 weeks of drug administration.

Figure 3|Combined treatment with VPA and estrogen decreases Aβproduction and increases Aβ degradation in the brain in an ovariectomized mouse model of Alzheimer’s disease after 4 weeks of drug administration.

Figure 4|Combined treatment with VPA and estrogen reduces neurofibrillary tangles in the brain in an ovariectomized mouse model of Alzheimer’s disease after 4 weeks of drug administration.
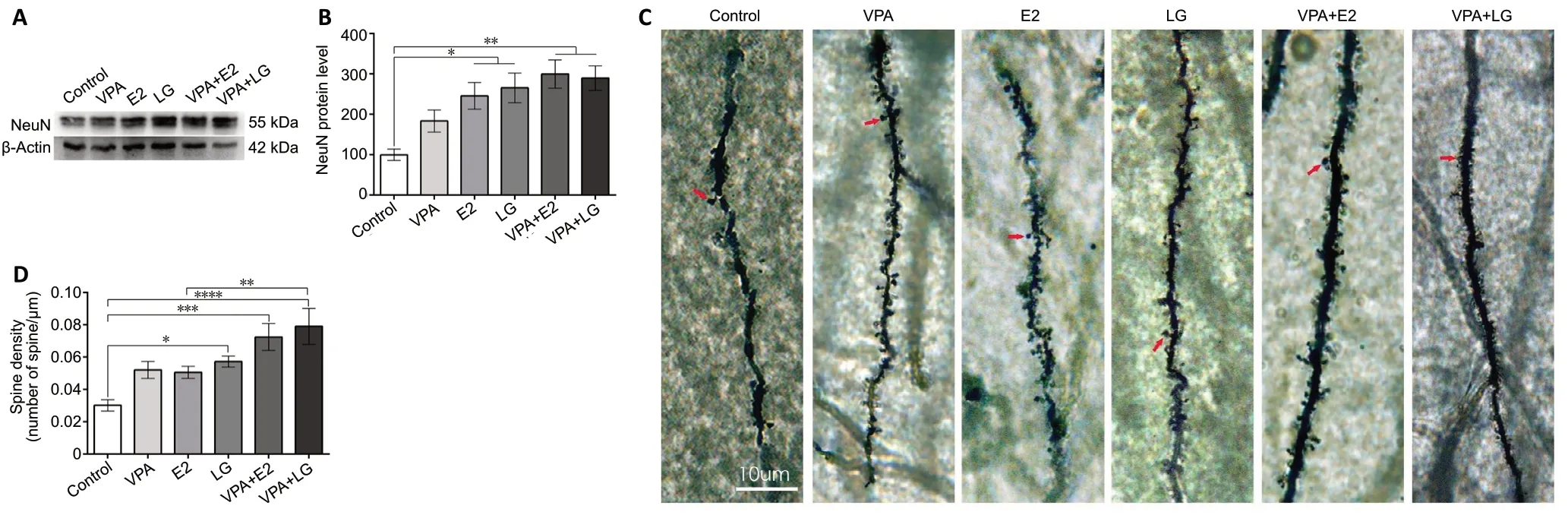
Figure 5|Combined treatment with VPA and estrogen attenuates neuronal loss in an ovariectomized mouse model of Alzheimer’s disease after 4 weeks of drug administration.
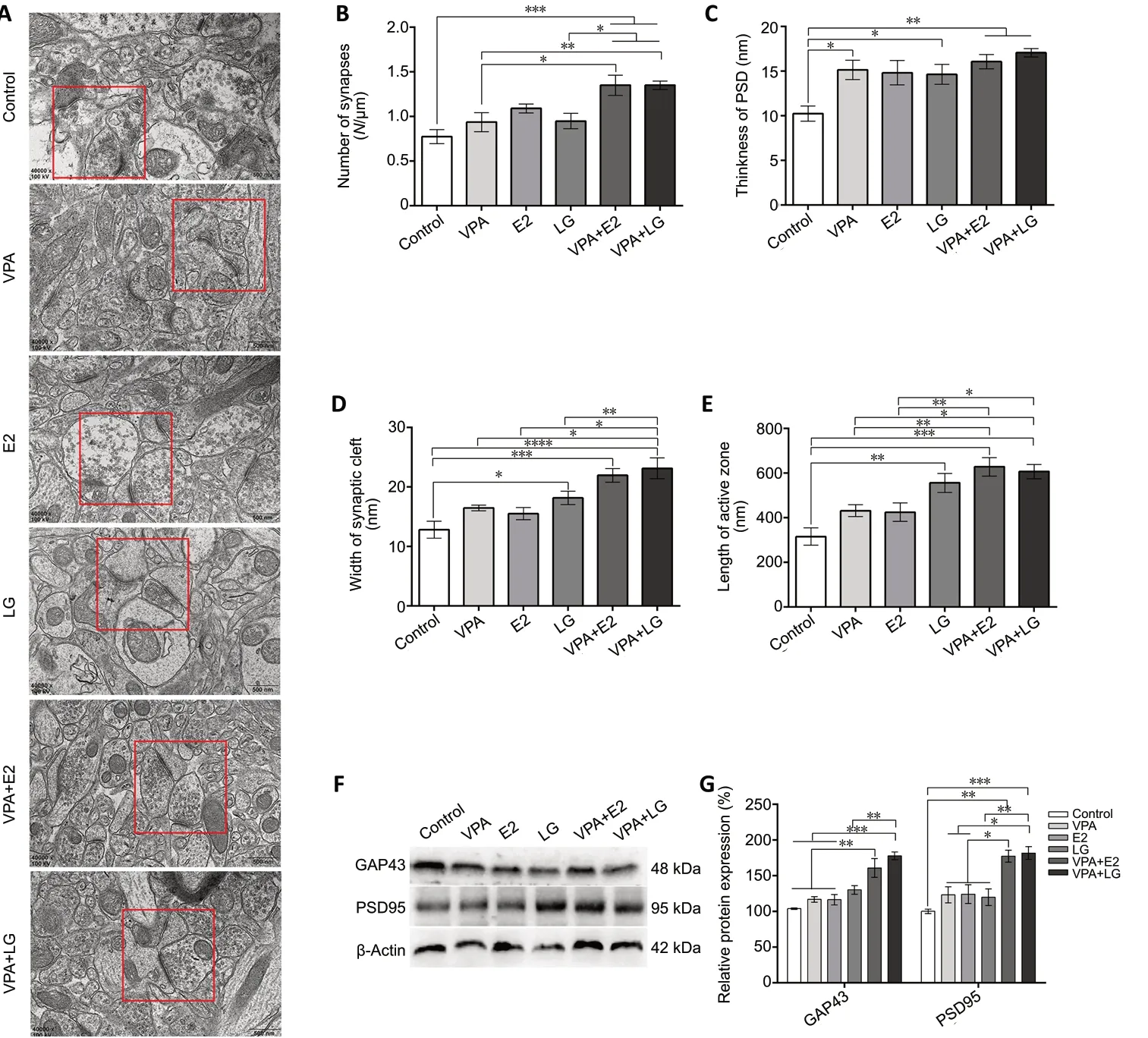
Figure 6|Effect of combined treatment with VPA and estrogen on synapses in the brain in an ovariectomized mouse model of Alzheimer’s disease after 4 weeks of drug administration.
Discussion
VPA can reduce Aβ generation and SP formation by inhibiting GSK-3β-mediated β-secretase cleavage of APP, decrease tau hyperphosphorylation through GSK-3β signaling, and improve memory deficits in AD mice (Chen et al., 2018; Das et al., 2019). Interestingly, we previously found that the protective effects of VPA are greater in male AD mice than in female AD mice due low estrogen levels in the brains of the female mice. Estrogen can reduce Aβ production and tau hyperphosphorylation in the brain (Brann et al., 2007).However, women with advanced menopausal AD suffer from altered ERα and ERβ expression in the brain, resulting in an ERα/ERβ ratio imbalance, which dramatically reduces sensitivity to estrogen and renders estrogen replacement therapy treatment largely ineffective.

Figure 7|Combined treatment with VPA and estrogen up-regulated GSK-3β expression in the brain in an ovariectomized mouse model of Alzheimer’s disease after 4 weeks of drug administration.
In this study, we found that treatment with VPA and estrogen is more effective than treatment with estrogen intervention alone, and can significantly improve spatial memory and learning in OVX AD mice. The progressive cognitive impairment seen in AD is closely correlated with neural plasticity exhaustion and synaptic loss (de Calignon et al., 2010; D’Amelio et al., 2011), which are early events in AD development (Scheff et al., 2007). Modification of synaptic connectivity correlates with a change in synapse number and density, which causes cognitive deficits and neural system dysfunction (Citri and Malenka, 2008). Here,TEM analysis revealed that the thickness of the PSD layer was increased when mice were treated with VPA and estrogen compared with the control group. PSD, which is an electrondense material that is uniformly distributed across the postsynaptic membrane, plays important roles in postsynaptic signaling transduction and assembly (de Bartolomeis et al.,2014). Combined treatment with VPA and estrogen not only significantly increased the number and density of presynaptic vesicles, but led to upregulation of GAP-43 and PSD-95 (the synaptic protein markers), which are distributed on preand post-synaptic membranes and play a role in axonal regeneration and synaptic remodeling. Thus, treatment with VPA plus estrogen resulted in striking rescue of early synaptic impairment in OVX AD mice.
Aβ is generated from APP by a sequential limited proteolytic process mediated by β- and γ-secretases. This process is regulated by various enzymes, among which BACE1 and PS1 are the key enzymes involved in Aβ production, and IDE and NEP are the two main proteases responsible for Aβ degradation. Under normal circumstances, Aβ is constantly produced from its precursor and immediately catabolized(Selkoe and Hardy, 2016). When the balance of Aβ production and clearance is disrupted, Aβ accumulates in the brain. The results from our study show that the combined use of VPA and estrogen reduces BACE1 and PS1 levels in the brain of OVX AD mice and significantly up-regulates IDE and NEP expression,indicating that the combined treatment regimen can reduce SP formation. Tau, a predominantly axonal microtubuleassociated protein, promotes microtubule formation and maintains microtubule stability. Hyperphosphorylated tau is the principal component of neurofibrillary tangles, which interfere with normal neuronal function and eventually lead to neuronal degeneration and death (Avila et al., 2004).We found no difference in Tau-5 expression among the groups tested in this study; however, expression of PHF-1, which targets pSer396/pSer404 (Neddens et al., 2018),was significantly reduced in the brains of mice in all of the treatment groups, indicating that both VPA and estrogen can reduce tau hyperphosphorylation.
In this study, we investigated the effect and possible mechanisms of VPA combined with estrogen treatment in OVX AD mice. GSK-3β is major kinase associated with tau hyperphosphorylation and Aβ generation. GSK-3β inhibitors(lithium and tideglusib) failed to show significant cognitive or clinical benefits in AD patients in controlled trials (Matsunaga et al., 2019). However, other studies suggest that GSK-3β inhibitors might be beneficial for AD therapy (Maqbool et al.,2016; Saraswati et al., 2018; Jaworski et al., 2019). GSK-3β can affect ERα phosphorylation, thereby regulating its expression and activity (Medunjanin et al., 2005; Fortunati et al., 2010).In the present study we found that that the covalent binding of ERβ and SUMO1 can significantly inhibit ERβ transcriptional activity and its response to estrogen (Picard et al., 2012).SUMO is closely related to the progression of AD (Takahashi et al., 2008). For example, SUMO1 binding to AD-related proteins(e.g., tau, APP, and BACE1) (Dorval and Fraser, 2006; Zhang and Sarge, 2008; Yun et al., 2013) promotes Aβ production.Furthermore, ERβ binding to SUMO1 is regulated by GSK-3β:GSK-3β overexpression increases ERβ-SUMO1 expression,and decreased GSK-3β expression reduces ERβ-SUMO1 expression (Picard et al., 2012). We found no difference in GSK-3β levels among all of the groups tested, but did observe a significant increase in p-GSK-3β (ser9) content in all groups,especially in the group that was treated with both VPA and estrogen; phosphorylation of the GSK-3β ser9 residue inhibits its catalytic activity (Azab et al., 2017). In addition, SUMO1 expression was significantly reduced, and ERα and ERβ levels were markedly higher, in the combined group compared with the control group.In summary, the current study confirms that VPA combined with estrogen is better than VPA or estrogen intervention alone in treating AD in a late menopausal mouse model.Although combining E2 or LG with VPA achieved similar results in terms of attenuating neuropathological features, the VPA+ LG group demonstrated superior improvements in spatial memory and learning compared with the VPA + E2 group. VPA could enhance ER sensitivity to estrogen by inhibiting GSK-3β activity, consequently improving spatial memory and learning,reducing Aβ deposition and tau hyperphosphorylation,promoting neuronal maturation, and improving synaptic structure and function. However, morein vitroandin vivostudies are needed to identify the interactions between and regulatory mechanisms of GSK-3β and ERs in AD.
Author contributions:Study concept and design: GQH, XZ; manuscript writing and language polishing: YZL, YJL; experiment implementation: YZL,YJL, WZ, SFL; data analysis: YZL. All authors approved the final version of this manuscript.
Conflicts of interest:The authors declare that they have no conflict of interest.
Financial support:This study was supported by the National Natural Science Foundation of China, Nos. 81671257, 81371221, 31600825 (all to GQH). The funding sources had no role in study conception and design,data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement:The study was approved by the Chongqing Medical University Animal Protection and Ethics Committee on March 6, 2013.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Bruno P. Imbimbo, Chiesi Farmaceutici, Italy.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders
- The role of gap junctions in cell death and neuromodulation in the retina
- Don’t know what you got till it’s gone: microglial depletion and neurodegeneration
- Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia
- Protein post-translational modifications after spinal cord injury
- Axonal mRNA localization and local translation in neurodegenerative diseases

