Protein post-translational modifications after spinal cord injury
Shuang Zhu , Bing-Sheng Yang , Si-Jing Li Ge Tong, Jian-Ye Tan Guo-Feng WuLin Li Guo-Li Chen , Qian Chen , Li-Jun Lin
Abstract Deficits in intrinsic neuronal capacities in the spinal cord, a lack of growth support, and suppression of axonal outgrowth by inhibitory molecules mean that spinal cord injury almost always has devastating consequences. As such, one of the primary targets for the treatment of spinal cord injury is to develop strategies to antagonize extrinsic or intrinsic axonal growth-inhibitory factors or enhance the factors that support axonal growth.Among these factors, a series of individual protein level disorders have been identified during the generation of axons following spinal cord injury. Moreover, an increasing number of studies have indicated that post-translational modifications of these proteins have important implications for axonal growth. Some researchers have discovered a variety of post-translational modifications after spinal cord injury, such as tyrosination,acetylation, and phosphorylation. In this review, we reviewed the post-translational modifications for axonal growth, functional recovery, and neuropathic pain after spinal cord injury, a better understanding of which may elucidate the dynamic change of spinal cord injury-related molecules and facilitate the development of a new therapeutic strategy for spinal cord injury.
Key Words: extracellular matrix; function impairment; glial scar; nerve regeneration;neuropathic pain; post-translational modification; spinal cord injury; therapeutic target
Introduction
In most cases, spinal cord injury (SCI) leads to a series of functional impairment and psychological complications, which can be a disaster for patients, their families, and society (Hearn et al., 2018; Wang et al., 2018). It has been reported that more than 20 million people worldwide are affected by SCI,which often leaves them unable to take care of themselves(James et al., 2019), In the United States alone, there are 400,000 patients with SCI, with an additional 14,000 cases each year (Sekhon et al., 2001). After SCI, patients usually suffer from motor and sensory loss in the lower or upper extremities, and can have difficulty urinating and having sex(Ali et al., 2020; Moussa et al., 2020), which may need nursing and rehabilitation for the remainder of their life. Furthermore,most patients with SCI experience severe psychological problems due to neuropathic pain or other functional impairments (Shiao et al., 2018). While there is a clear need for an effective therapeutic strategy, the hunt has been long and the results generally disappointing, which has been attributed to several reasons (Figure 1).
Generally, SCI can be divided into three phases (Tator et al., 2009). The acute phase, which is caused by mechanical damage to spinal cord tissues from direct impact or shock waves, sets in motion a number of parallel pathophysiological processes. The mechanical disruption to neural and vascular tissue can lead to necrosis and cell death, and the ionic imbalance of K+, Na+, Ca2+can lead to neural function impairment and spinal shock (Baumann, 2020). Furthermore,the severed axons undergo Wallerian degeneration of the distal part of the axon, as well as their associated myelin sheaths. The secondary stage (subacute injury phase) of SCI can occur anything from a few minutes to a few weeks after injury, during which the nervous system becomes more severely damaged (Lin, 2020). The pathophysiology of this stage involves multiple processes, such as apoptosis and free radical formation. The increase in cytokine concentration and the continuous infiltration of lymphocytes that occurs following cell death in turn rapidly increases cell death. SCI may evolve into a chronic phase anything from a few days or a few years following injury, during which the body’s gray matter will gradually dissolve, connective tissue will gradually deposit, and glial scars will form (Liu et al., 2019). Moreover,permanent hyperexcitability in many cells gives rise to chronic neuropathic pain in most patients (Bahari et al., 2019; Calvo et al., 2019).

Figure 1|Main factors hindering axon regeneration after spinal cord injury.
During each of these phases, numerous processes take place at the levels of transcription, RNA editing, translation,and post-translational modification (PTM) (Mierke, 2020).Among these, PTM in particular has aroused interest in recent decades because it can be reversibly manipulated compared to the irreversible process of transcription, RNA editing, and translation. Post-translational modification is the process of chemically modulating polypeptide chains following transcription and translation. In cells, PTM plays the main regulatory role in the conformational change of proteins. With the participation of PTM, the body’s proteins change their conformation, which in turn affects their related interactions with different proteins and affects downstream signal transduction (Czuba et al., 2018). Furthermore, many functional changes of proteins introduced by PTMs are instant and reversible. A similar control of functional change by de novo synthesis or protein degradation would be more timeconsuming and bioenergy-intensive than PTMs (Uversky,2013). In this sense, targeting PTMs is more accurate and economic than targeting gene transcription and translation.Compared with the rapidly changing pathological process of SCI, gene expression changes after SCI are slow. While PTMs on proteins or amino acids can instantly alter the proteins’function, which makes PTM the ideal target for slowing or stopping the dramatically shifting phases of SCI. It has been reported that PTM is involved in many pathophysiological processes of SCI, such as axon regeneration (Voulalas et al., 2017; Qi et al., 2019), glial scar formation (Gliem et al.,2015; Wang et al., 2016), inhibitory molecule production(Wong et al., 2018), maladaptive plasticity (Imagama et al., 2011), and the onset of neuropathic pain (Lai et al.,2016). PTM can be analyzed and classified into modified types; first, amino acid modifications, such as proteolysis,which can also be deamidated to enable protein cleavage;second, the modification of complex molecules through processes such as glycosylation and acylation, which allows the molecular structure of proteins to be expanded; third,chemical modifications, such as oxidation and methylation;and fourth, polypeptide modification, such as ubiquitination and sulfonylation (Audagnotto et al., 2017; Spoel, 2018).PTM affects the physiological processes of cells both when the body exercises normal physiological activities and abnormal physiological activities, and can modify the function of proteins by degrading proteins, affecting the interaction between proteins, and changing the structure of proteins (Beltrao et al., 2013; Lüscher et al., 2018; Gao et al., 2020). Several PTMs have been documented in the context of SCI, including acetylation (Hubbert et al., 2002;Reed et al., 2006), nitrosylation (Scheving et al., 2012; Khan et al., 2019), phosphorylation (Xu et al., 2006; Voulalas et al.,2017), ribosylates (Kuzhandaivel et al., 2010), ubiquitination(Lai et al., 2016; Jeong et al., 2018), and oxidation (Ingles et al., 2016). However, it is still unclear how each of these modifications contributes to the pathophysiological process of SCI, or how much of a role modification plays in each pathophysiological process or specific functional impairment.Spinal cord regeneration is mainly hindered by diminished intrinsic neuronal capacities and the extrinsic inhibitory environment. Here, we reviewed the reported PTMs on the intrinsic and extrinsic factors that affect SCI recovery. Our findings indicate that PTM is an important therapeutic target of SCI, because it is much more regulatable and reversible than traditional therapeutic strategies. Moreover, its role in neuropathic pain control holds promise for future clinical work and should be studied in future work.
Search Strategy and Selection Criteria
We conducted a PubMed search for patients with SCI/animal models of SCI/nerve cells, neurogenesis/neuroregeneration/axonal regeneration, and their outcomes. Studies published from 1992 to 2020 on PTM after SCI were identified on the PubMed database. More detailed information on specific PTMs after SCI was searched using the term “SCI” in combination with the desired term (e.g., “phosphorylation”).To explore acetylation in more depth, the term “acetylation”was also combined with the term “SCI”. Publications on neurogenesis and neuroregeneration and axonal regeneration were sourced by combining these two terms with the term“SCI”. The relevance of identified publications was verified by checking the presence of search terms in the title, abstract,and key words. Non-SCI experiments were excluded. We focused on general rather than recent findings wherever possible.
Post-Translational Modifications of Intrinsic Factors
Microtubule post-translational modifications
During nerve regeneration following SCI, there is significant neurite outgrowth and growth cone guidance, which can be identified by microtubule polymerization and depolymerization. Microtubules are mainly composed of α-tubulin and β-tubulin. When the microtubules of neurons respond to physiological conditions, they can be modulated by PTMs. PTMs modify tubulin, which in turn modifies the physiological activities of microtubule functions (Janke et al.,2020). In the context of SCI, several PTMs have been reported to occur on tubulin, including phosphorylation (Atashi et al., 1992; Lang et al., 2015; Brandt et al., 2019), acetylation,detyrosination, and polyglutamylation, as shown inFigure 2.Atashi et al. (1992) demonstrated that the extent of tubulin phosphorylation in response to local adhesion signals encountered by the growth cone can influence microtubule polymerization to regulate nerve regeneration. The authors found that cell adhesion molecules and antibodies to cell adhesion molecules inhibit tubulin phosphorylation in growth cone membranes. Microtubule-associated protein 1B (MAP1B), which is expressed in neuronal and glial cells, is a major growth-associated and cytoskeletal protein. Related studies have reported that when MAP1B is functioning, it can be affected by MAP1B phosphorylation. In the process of studying PC12 (a cell line derived from pheochromocytoma of the rat adrenal medulla) cell axon growth, researchers have successfully discovered this phenomenon that MAP1B phosphorylation contributes to MAP1B functional changes when using NGF induction (Migita et al., 2019). A large amount of MAP1B can be found in growing axons, and, after phosphorylation, MAP1B promotes the growth of the growth cones (Ramkumar et al., 2018; Igarashi, 2019)
However, the phosphorylation of MAP1B does not necessarily promote the growth of neurites (Igarashi et al., 2020). Some researchers have reported that after rats have been injured by alginic acid, afferent fibers can be deprived of alginic acid treatment, which effectively promotes the expression of MAP1B and increases its phosphorylation (Soares et al., 1998).Furthermore, after concussion, rats have been found to reexpress MAP1B and that it is also phosphorylated. Therefore,it seems that the presence of these proteins allows neurons to regenerate (Emery et al., 2000).
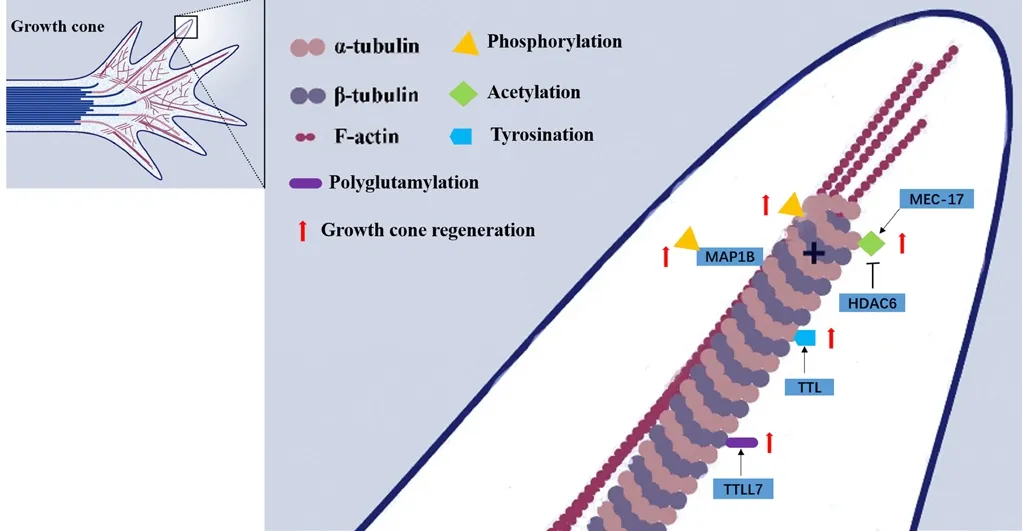
Figure 2|Post-translational modifications affect growth cone regeneration after spinal cord injury.
Tubulin acetylation has been found to play a role in many physiological functions; it not only promotes axonal outgrowth, but also plays a role in axon branching (Rossaert et al., 2020). Tubulin deacetylase, histone deacetylase 6 (HDAC6),and sirtuin 2 (SIRT2) affect the acetylation status of tubulin.Some studies have shown that the acetylation of α-tubulin at Lys40 facilitates axonal transport by promoting binding of the motor proteins kinesin and dynein to microtubules (Mo et al., 2018; Rossaert et al., 2020). HDAC6 has also been found to deacetylate tubulin (Hubbert et al., 2002). Furthermore,α-tubulin acetylation can be augmented by HDAC6 inhibition,which promotes axonal transport and has been found to be beneficial in animal models of neurodegenerative diseases,such as Huntington’s disease (Guo et al., 2017). Using a mouse model of SCI, Dan et al. (2018) showed that by ablating MEC-17, α-tubulin acetylation can be inhibited, which in turn leads to the overgrowth of neuronal axons. This then causes an unwinding of microtubules, which then invade the filopodia.In another SCI mouse model study, Zheng et al. (2020) found that the level of HDAC6 in the body was significantly increased after SCI, while the inhibition of HDAC6 by Tubastatin A induced functional recovery in the contusive SCI mouse model. This was presumably because the inhibition of HDAC6 can prompt acetylation and stabilization of microtubules and therefore restore transport function, which may give rise to an increased axonal length and restore the autophagic flux.According to related reports, Elp3 can promote acetylation and inhibit the regulation of HDAC6, and silencing of Elp3 cells can delay migration and damage neuronal branches (Creppe et al., 2009).
Previous research has clearly shown that axon growth is affected after the occurrence of SCI, and that there is a close relationship between this process and microtubuledependent retrograde transport (Mo et al., 2018; Guo et al., 2020; Rossaert et al., 2020). On analyzing the structure of microtubules, the authors found that β-tubulin and the C-terminus of the α-terminus were exposed. This site is also the core of MAPs. The detyrosination, glutamylation, and glycosylation of microtubules may also help to regulate the interaction and activity of MAPs and molecular motors (Janke et al., 2011). When axons are injured, tubulin tyrosine ligase(TTL) at the damaged site acts to increase the level of α-tubulin tyrosine, which can promote the retrograde transport of injury signals (Song et al., 2015). Some researchers have demonstrated that, in wild-type animals, microtubule detyrosination can be inhibited using sesquiterpene lactone creeper lactone. If the whole body or part of the body is treated with hydroquinone lactone, the regeneration of axons and functional recovery can be promoted. Therefore, it can be hypothesized that inhibition of microtubule detyrosination may improve the treatment effect when treating patients with nerve injury (Gobrecht et al., 2019).
Studies have shown that if the axons can be kept in a stable state, then microtubule polyglutamylation level is high, while the body has low polyglutaminase activity (Audebert et al.,1993). Ikegami et al. (2006) found that tubulin tyrosine ligaselike (TTLL) protein promotes polyglutamylation, which can be transcribed at the highest level of the nervous system.In addition, excessive microtubule glutamylation is directly related to neurodegeneration, and the down-regulation of the main brain polyglutamate amylase TTLL1 partially prevents the degeneration of Purkinje cells in the cerebellum of PCD mice(Rogowski et al., 2010). Some research has indicated that, as a kind of β-tubulin polyglutaminase, there is a close connection between TTLL7 and MAP2-positive neurites in meridian-like(PC12) cells (Ikegami et al., 2006; Sferra et al., 2020). What is more, Maas et al. (2009) found that polyglutamylation affects the activity of neurons in rats by regulating synaptic vesicle transport.
Stimulatory pathway post-translational modifications
Extracellular signal-regulated kinase (ERK) is activated in response to various neurotrophic factors (Hausott et al., 2019;Jin et al., 2020). ERK promotes growth cone motility and regeneration after axonal injury in adult dorsal root ganglion axons (Manire, 2019; Liu et al., 2020). Using a rat model of SCI, induced by spinal cord transection, Xu et al. (2006)demonstrated that nitration could modulate 3-nitrotyrosine after SCI. When SCI is in the acute phase, increasing the nitric oxide attack not only increases the phosphorylation level of ERK1/2, but also increases the level of protein kinase activated by p38 mitogen. And these proteins play an important role in neuronal degeneration following acute SCI. What is more,the K+/Cl-cotransporter 2 (KCC2) activation was found to be beneficial in neuropathic pain control and spinal cord injury recovery (Cardarelli et al., 2017; Chen et al., 2018).Constitutive membrane recycling of KCC2 is regulated by the phosphorylation state of the C-terminal serine residue 940(S940). Phosphorylation of S940 in KCC2 by protein kinase C (PKC) limits the cleavage of KCC2 in lysosomes following clathrin-dependent endocytosis, and decreases the rate of KCC2 internalization from the plasma membrane (Lee et al., 2007). And KCC2 expression is downregulated following activation of both the PLCγ1 and Shc signaling cascades of the BDNF-TrkB pathway, which is obvious at the peri-lesion site where BDNF accumulates after SCI (Carter et al., 2018).In further, BDNF activates neuronal m-Calpain, a protease that results in the irreversible inactivation of KCC2 via MAPK-mediated phosphorylation (Puskarjov et al., 2012). In addition, NMDA receptors activation increases intracellular Ca2+concentrations, and KCC2 is internalized to the cytosol for the S940 dephosphorylation by protein phosphatase 1(PP1), thereby decreasing functional KCC2 levels in the plasma membrane (Lee et al., 2011).
Inhibitory pathway post-translational modifications
Some researchers have suggested that, after SCI, the expression level of the Rho signaling pathway increases(Hong et al., 2019; Xiao et al., 2019). The activation of Rho and its downstream effector ROCK kinase triggers growth cone collapse and represents an important obstacle to axon regeneration (Koch et al., 2018). It has been reported that, owing to the inhibitory effects of Rho and ROCK, chondroitin sulfate proteoglycan (CSPG) cannot inhibit the growth of neurites (Monnier et al., 2003). And Chondroitin sulfate impairs neural stem cell migration through ROCK activation (Galindo et al., 2018). Furthermore,the guidance and extension of nerve axons are reportedly affected by the Rho family guanine nucleotide triphosphatase(GTPases) (Galino et al., 2019). Translation and modification of Rho GTPases can affect the activity of Rho GTPases (Denk-Lobnig et al., 2019). Prenylation can occur at the C-terminus of proteins, and the presence of prenylation helps to guide neurite outgrowth (Jennings et al., 2018; Reddy et al., 2020).Moreover, Collapsin response mediator protein 2 (CRMP2)signaling coordinates cytoskeletal formation and regulating cellular division, migration, polarity, and synaptic connection.Inhibition of CRMP2 phosphorylation promotes axonal regeneration and/or sprouting of motor and sensory axons,stabilizes microtubules in astrocytes to inhibit fibrous scar formation after SCI, and reduces infiltration of inflammatory cells (Nagai et al., 2016).
Post-Translational Modifications on Extrinsic Factors
Glial scarring
During the chronic phase of SCI, there are many physiological changes in the body, such as the proliferation of glial cells, the deposition of connective tissue, and the dissolution of gray matter, which cause the formation of glial scars on the spinal cord (Bradbury et al., 2019). Extracellular matrix molecules and reactive astrocytes are the main substances that form glial scars (He et al., 2020). After SCI, a large number of astrocytes bind to the scar area; the presence of these astrocytes causes an increase in the mechanical strength of the tissue, which makes cell migration and axon growth through the tissue more difficult. Moreover, axon growth inhibitory molecules that bind to the sticky basement membrane of the scar accumulate at the lesion site at high local concentrations (Tran et al., 2018).
In one study, Besson et al. (2004) demonstrated that the cyclin-dependent kinase inhibitor p27kip1 affects the physiological activities of astrocytes, and plays a role in cell migration and proliferation. A previous study showed that inhibiting the O-GlcNAcylation of p27Kip1 on Ser2 by a gene mutation (S2A) attenuated the phosphorylation of Ser10 in astrocytes, which promoted astrocyte motility and reduced scar formation in rats suffering from spinal cord contusion(Mao et al., 2015). In a rat model of SCI, Zhao et al. (2011)found that, in the G1 phase of the cell cycle, ubiquitin ligase KPC can degrade p27kip1. As the catalytic subunit of KPC,there is a close relationship between KPC1 and rat astrocyte proliferation (Zhao et al., 2011).
Glycosylation is a translation modification process that not only affects subcellular localization, but also protein structure.The sulfonylation required for C/EBPδ-dependent transcription has been found to be inhibited after the administration of lipogenic inducers and epidermal growth factor (Lai et al.,2008). Furthermore, C/EBPδ has been demonstrated to contribute to glial scar formation, thereby impairing functional recovery after rats’ contusive SCI (Wang et al., 2016).
Inhibitory molecules
Apart from scarring, inhibitory molecules are major elements of the inhibitory environment after SCI. Indeed, the glial scar functions as a natural container for inhibitory molecules,which increases the concentration of inhibitory molecules and exacerbates the inhibitory effect. Myelin-associated molecules are the most studied group of inhibitors, one of which is Nogo-A, which binds to receptors on the neuron membrane, inhibits growth, and at the same time causes the collapse of growth cones (Zhao et al., 2019). The ablation of Nogo-A promotes the phosphorylation of LIMK1, which further activates cofilin by decreasing its phosphorylation.The dynamics of actin are also reportedly regulated by Cofilin,which can cut off the activity of filaments, thereby regulating the power of motor proteins (Montani et al., 2009). This suggests that there is a close connection between neuron Nogo-A and growth cone motility. In terms of modification of Nogo-A itself, Yokoyama et al. (2006) found that Nogo-A was phosphorylated by Src-family kinases at Tyr-694, which indicates that Nogo-A functions have an additional level of complexity.
The acetylation of microtubule α-tubulin can be promoted by inhibiting histone deacetylase-6 (HDAC6), while simultaneously restoring the growth of CSPGs- and MAGinhibited axons (Rivieccio et al., 2009); conversely, tubulin acetyltransferase-1 (αTAT1; acetylation) has the opposite function (Cueva et al., 2012). Researchers such as Wong et al.(2018) have used a mouse model of SCI to show that αTAT1 is down-regulated via the influence of MAG and CSPG, while HDAC6 levels or HDAC6 activity remain unchanged with exposure to CSPGs and MAG. Moreover, overexpression of downregulated αTAT1 can restore neurite growth, which may indicate that targeting αTAT1 on a post-translational level could have therapeutic potential after SCI.
CSPGs belong to the proteoglycan class (Listik et al., 2019),which not only function as inhibitors, but also as a pathfinder and axon outgrowth guide after SCI (Schmidt, 2019). Previous studies have found that the CS chain, KS chain, and N-glycans chain of CSPG inhibit neurite outgrowth. However, the contribution of each of these components has not yet been examined using a post-translational modification strategy;the authors of one study digested the three components by specific enzymes, respectively, and CS and N-glycans were found to inhibit neurite outgrowth of NS-1 cells (Hering et al., 2020). This indicates that PTMs of the CSPG upon neurite outgrowth may be a therapeutic target for SCI.
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are not only involved in the interaction between cells, but also in the interaction between cells and the extracellular matrix. MMPs interfere with the proteolytic process, which is essential during normal development, wound healing, and lifetime repair matrix remodeling (Sanderson et al., 2019). In SCI, MMPs have been demonstrated to contribute to a progressive neuroinflammatory response, oxidative stress, apoptosis, and glial scar degradation (Zhang et al., 2011). The occurrence of complications after SCI depends on several factors, including when and where MMPs are expressed and the profile of their available substrates of MMPs. For example, blocking MMPs in the early stage of SCI can make the barrier more stable,reduce apoptosis, and protect neurons. However, in the chronic phase of SCI, the formation of glial scars affects the growth of extracellular proteins (Mirzaie et al., 2018).
Translation and modification of MMPs can make their substrates better bind to the extracellular matrix.Glycosylation of MMPs has been found to significantly improve their binding with the extracellular matrix (Agarwal et al., 1999). As the most common glycosylated MMPs product,MMP9 not only contains N-linked glycosylation sites Asn120 in the catalytic domain, but also N-linked glycosylation sites in the pro-domain (Vandooren et al., 2013). It has been reported that glycosylation at Asn38 and galectin 8 increases MMP3-mediated processing, and glycosylation at Asn38 and galectin 3 reduces the proteolytic activation of MMP9. Therefore,MMP9 activity seems to be influenced by N-glycosylation(Boon et al., 2019). Duellman et al. (2015) demonstrated that by inhibiting the glycosylation of MMP9 at Asn120, the effect of the interaction between calreticulin and MMP9 can be strengthened, and its secretion efficiency can be reduced.
MMP can be regulated by phosphorylation, and phosphorylation sites such as Ser- and Thr- can be identified by studying the domain of MMP (Ardito et al., 2017). Some researchers have found that protein kinase C plays a regulatory role in the phosphorylation of MMP-2 (Sariahmetoglu et al.,2007). Yu et al. (2000) confirmed that, as a member of the GAG family, heparan sulfate can mediate the relationship between the cell surface and secreted vertebrate MMPs,and that this mediation relationship is specific, given that excess soluble heparin extracts and solubilizes MMP-2, MMP-13, MMP-7, and MMP-9. Previous studies have shown that heparin, a highly sulfated heparan sulfate, in addition to heparan sulfate, affects the expression and plasma levels of MMP9, and also increases the affinity of TIMP3 for MMP2,MMP7, and MMP9 (Mannello et al., 2008). Therefore, PTMs can be considered to modify multiple sites of MMPs, thereby affecting their activity to varying degrees, and this process might affect regeneration following SCI.
Extracellular matrix Osteopontin
Choi et al. (2007) reported that there are many adhesion proteins in the extracellular matrix, one of which is osteopontin, which is a soluble cytokine that is formed by microglia and astrocytes. Osteopontin can be modified in many ways, such as via sulfation and glycosylation, and can bind to different integrins through the CD44 receptor to allow organisms to perform different physiological functions (Sodek et al., 2000). Gliem et al. (2015) found that osteopontin is involved in a variety of physiological functionsin vitro,through which it could prevent nervous system damage and cerebral edema. Similarly, Plantman (2012) reported that the osteopontin matrix can promote axon growthin vitro.
Collagen
Fibrotic scars, which appear after SCI, are formed of fibronectin and laminin, with increased deposition in the lesion core and reduced deposition of in the penumbral area of astrocytes (Orr et al., 2018). The basement membrane is one of the most important structures of the scar, while the core of the basement membranes consists of a dense collagen IV network that forms a large supramolecular structure. This core structure creates a stable scaffold to which associated proteins and adherent cells can bind (Sanderson et al.,2019). After translation, the cell translates and modifies the newly synthesized collagen IV through processes such as glycosylation and hydroxylation (Hennet, 2019). According to previous research, the synthesis of collagen is inhibited by collagen IV, thereby preventing the body from forming a basement membrane. In this way, the regeneration of injured nervous system axons can be promoted (Stichel et al., 1999).
Tenascins
Two oligomeric multi-domain anti-adhesion proteinstenascin-C and tenascin-R-are produced in the CNS. These proteins participate in a variety of physiological activities, such as the reaction between growth factors and cells, adhesion of cells, and neuron migration (Giblin et al., 2015). Tenascin-C can undergo glycosylation via its multiple glycosylation sites,which has been found to increase the proliferation ability of mouse neuronal stem cells (Yagi et al., 2010). In addition,Tenascin-C can also have stronger binding ability through modification. Giblin et al. (2015) have suggested that this is due to proteolytic cleavage. The development of the body regulates the glycosylation of TN-R and affects its motility and migration (Woodworth et al., 2004). It has been reported that TN-R can be connected with PTM by subjecting TN-R to a certain weight ratio sulfation (Zamze et al., 1999).
Neuropathic Pain
An estimated 80% of patients with SCI have obvious pain that resembles an electric shock or burn (Mehta et al., 2019).Post-SCI pain has a more serious impact on the daily life of patients than dyskinesia, and can seriously reduce quality of life (Finnerup et al., 2001). Complex mechanisms underlie neuropathic pain, and some researchers have asserted that SCI causes a loss of inhibitory circuits in the dorsal horn of the damaged part, such that hyperactive nociceptive signaling develops and further leads to a heightened sensitivity to pain(hyperalgesia) and mechanical hypersensitivity (allodynia)(Meisner et al., 2010). Some researchers have also put forward the maladaptive plasticity hypothesis of the central nervous system, which states that maladaptive plasticity is the main cause of neuropathic pain in SCI. Other researchers believe that D1/5 receptor-mediated ERK phosphorylation is diminished after SCI, and that dopamine D1 receptors are lost in the gray matter surrounding the aqueduct (Voulalas et al.,2017).
Garcia-Larrea et al. (2013) reported that individuals with neuropathic pain often exhibit an excessive activation of the insular cortex. The excitatory synaptic transmission of the insular cortex is affected by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR). The AMPAR function of the insular cortex is often overactive in individuals with neuropathic pain (Koga et al., 2012).Qiu et al. (2014) reported that the abundance of synaptic GluA1, which is the subunit of AMPAR, is increased after SCI,and the phosphorylation of GluA1 at Slu845 is needed to enhance synaptic AMPARs. Therefore, it is clear that GluA1 phosphorylation has an important impact on neuropathic pain in the insular cortex. There is also a close relationship between the development of neuropathic pain and the level of N-methyl-D-aspartate (NMDA) receptors (NMDARs) in the insular cortex. The GluN2B subunit of NMDARs is involved in pathophysiological processes such as pain perception and ischemic damage, and is a major tyrosine-phosphorylated protein of the postsynaptic density proteins of the brain. It has been reported that the phosphorylation level of the GluN2B subunit in the superficial dorsal horn of the spinal cord increases significantly 7 days after SCI, and that Fyn kinase can mediate this phosphorylation (Abe et al., 2005). Some researchers have used gene knockout technology to knock out Y1472 in mice and replace it with Tyr1472, thereby reducing GluN2B at the phosphorylation site of Y1472 in the body. In this model, the pain of knockout mice was significantly lower than that of mice without the knockout (Nakazawa et al.,2006; Katano et al., 2011). To conclude, the phosphorylation of AMPAR and NMDARs subunits appears to play an important role in neuropathic pain.
It has been demonstrated that cross-talk between glia and neurons is associated with several processes of CNS development, such as neurogenesis, myelination,synapse formation, neuronal migration, proliferation and differentiation, and neuronal signaling (Gomes et al., 2001).For instance, glial cells that are activated after SCI secrete proinflammatory cytokines, neurotransmitters, ROS/RNS, and ATP, which then induce phosphorylation of CREB and ERK. This PTM modulates sensory neuronal hyperactivity and central neuropathic pain after SCI (Crown et al., 2006).
Conclusions and Prospects
For centuries, continuous efforts have been made to determine the optimal treatment strategy of SCI, even though functional impairment is inevitable. The prognosis varies according to the different levels of injury, and successful axon regeneration in two or more spinal levels could make a big difference for patients with a C6/7 lesion in the cervical cord,as this could help to return some diaphragm control and some degree of hand and finger control. In the cervical spine, the height of each spinal segment is 1 cm, and so it is difficult for nerve regeneration to exceed 2 cm. The clearest obstacles to regeneration are the loss of intrinsic axon regeneration ability and the hostile extrinsic environment.To overcome these difficulties, researchers have investigated the efficacy of pharmacological interventions in SCI. In clinical practice, medical staffs often use methylprednisolone, which can maintain the blood-spinal cord barrier and suppress inflammation (Chio et al., 2020). Related research has also demonstrated that methylprednisolone can occasionally cause serious adverse reactions such as pneumonia and gastrointestinal bleeding, which can lead to death in severe cases (Ko, 2019). While many clinical studies on naloxone,thyrotropin-releasing hormone, and monosialotetrahexo sylganglioside have been conducted (Badhiwala et al., 2018),no significant improvements were found. Other experimental strategies have also been well-studied, such as blockage of glial scar formation (Rhodes et al., 2004), bridging cysts and scars, selective removal of reactive astrocytes (Vismara et al.,2019), modification of growth-inhibitory molecules (Fournier et al., 2001) and cellular replacement (Schwann cells,embryonic stem cells, pluripotent stem cells, mesenchymal stem cells, and olfactory cells (Zhao et al., 2016; Zavvarian et al., 2020), gene therapies (Uchida et al., 2014), and the combined therapeutic interventions (Griffin et al., 2020).The lack of any promising results could be attributed to the complexity of the pathophysiological mechanisms underlying SCI. Therefore, according to the physiological target pharmacology of different methods, combining different drugs could help to protect the nerves of patients with SCI. Chen et al. (2007) has revealed that the subunit de-phosphorylation of AMPARs and NMDARs significantly attenuates neuropathic pain in mice, which indicates that a single targeting of phosphorylation may produce a combinatory effect.
For more than 20 years, chondroitinase ABC has been found to reduce CSPG inhibition in experimental models of SCI, and this method is based on our knowledge of CSPG’s inhibitory effect on axon outgrowth after SCI (Zuo et al., 1998). However,CSPGs are also important for axon pathfinding and guidance(Schwartz et al., 2018). Moreover, the majority of animal research into this therapeutic effect has been performed over a relatively short time window, from days to weeks, and long-term investigations are still lacking. So far, the clinical potential of chondroitinase ABC is still unclear. Indeed, the administration of chondroitinase ABC in acute SCI leads to radical and irreversible CSPG digestion, and the long-term effects in humans have not been elucidated. However, this could be of interest if we can suppress the effect of CSPG on axon outgrowth only and reversibly control this effect.Interestingly, according to the work discussed in this review,tubulin acetyltransferase-1 (αTAT1; acetylation) mediates the inhibitory effect of CSPG on axon microtubule growth.This acetylating modification is reversible and has minimal side effects, which makes this PTM a better choice than the ablation of CSPG. In other words, reversibly suppressing the specific effect of a molecule may be better than suppressing the molecule.
As mentioned above, PTMs and their role in SCI are highly dynamic. Furthermore, the function of a specific PTM on a single protein is different at different time points and in different environments. For instance, a given PTM may be beneficial during primary injury after SCI, but may be detrimental when it comes to the second stage. Thus,therapeutic PTMs must be regulatable. Normally, the process of transcription, RNA editing, and translation are irreversible. A protein’s tertiary structure can only be changed by conformational fluctuations after exons are chosen and spliced. There several highly regulatable PTMs, many of which involve reversible processes, whereas the phosphorylation state (un-phosphorylated or phosphorylated) of a molecule is reversible. This highlights PTMs as ideal therapeutic targets according to the spatiotemporal therapeutic requirements of SCI. Another advantage of targeting PTMs is that the effects are relatively instantaneous because PTM-associated changes in amino acid residues instantly transform protein activity, cellular locations, and dynamic interactions with other proteins. This timely effect is favorable given the rapid deterioration and shifting pathological processes of SCI.A cell’s bio-activity demands high energy resources, the supply of which varies according to the different activities.Each activity requires a donor molecule that carries energy. In this sense, PTM behaves like a transistor does in electronics, in that it delivers energy. Different modifications at different sites offer a diversity in energy supply form.Table 1summarizes the PTMs after SCI outlined in this review; the most common PTM seems to be phosphorylation, which may be because phosphorylation can provide energy quickly.However, phosphorylation seems to be only a minor PTM in microtubules, which might be because microtubule activity requires a lot of energy; phosphorylation cannot provide sufficient energy to change the structure of tubulins. Thus,polyglutamylation and polyglycylation have evolved as important PTMs for tubulins.
Other than therapeutic applications, PTMs could also be used as a biomarker for SCI. Caprelli et al. (2019) reported that hyperphosphorylated and cleaved tau could be sensitive biomarkers of central nervous system injury that could provide a consistent and reliable assessment of the presence and severity of injury and the prognosis for recovery.
Despite the advantages of PTM-targeting therapy in SCI, its limitations should also be noted. First, targets of the many PTMs are varied, which makes it difficult to pharmacologically target these PTMs. Second, PTM functions differ according to the time points after SCI, and it is difficult to confirm the optimal time at which to target PTMs. Finally, neuronal regeneration failure after SCI can be attributed to several factors, and a specific PTM that only modulates the intrinsic regenerative capacity or inhibitory molecular or glial scar formation may not be sufficient to ensure a meaningful recovery.
For most patients with SCI, a complete recovery is unlikely and difficult to achieve. Therapeutic strategies should focus on step-by-step functional recovery and on improving quality of life, and investigations into PTM therapy should prioritize neuropathic pain control over motor or sensory restoration. Encouraging work has revealed a role of PTMs in neuropathic pain alleviation. Moreover, factors that hinder axon regeneration are varied, and a combination of PTMs that target several factors should therefore be investigated.Furthermore, other therapeutic strategies, such as cell transplantation and gene therapy, may significantly increase the effect of PTM-induced modulation.
Author contributions:Fundraising, manuscript design and drafting: SZ,LJL, QC; data collection: BSY, SJL, GT; data analysis: JYT, GFW, LL, GLC. All authors approved the final version of the paper.
Conflicts of interest:The authors declare that they have no competing interests.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 81801210 (to SZ).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Lukas Grassner, Murnau Trauma Center, Germany;Rodolfo Gabriel Gatto, University of Illinois at Chicago, USA; Mitsuhiro Enomoto, Tokyo Medical and Dental University, Japan.
Additional file:Open peer review reports 1, 2, and 3.

Table 1 |Overview of the post-translational modification after spinal cord injury
- 中国神经再生研究(英文版)的其它文章
- Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders
- The role of gap junctions in cell death and neuromodulation in the retina
- Don’t know what you got till it’s gone: microglial depletion and neurodegeneration
- Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia
- Axonal mRNA localization and local translation in neurodegenerative diseases
- Alzheimer’s disease: a tale of two diseases?

