Markers for neural degeneration and regeneration:novel highly sensitive methods for the measurement of thrombin and activated protein C in human cerebrospinal fluid
Alexandra Gerasimov, Valery Golderman, Shany Guly Gofrit, Shay Anat Aharoni,Daniela Noa Zohar, Ze’ev Itsekson-Hayosh, Tsviya Fay-Karmon, ,Sharon Hassin-Baer, , , , Joab Chapman, , , , Nicola Maggio, , , ,Efrat Shavit-Stein,
Abstract A novel method for measuring thrombin and aPC activity, using specific fluorogenic substrate, in CSF samples of neuroinflammatory diseases patients, can be used to monitor disease progression and evaluate of response to treatment
Abstract Inflammation and coagulation are tightly interconnected in the pathophysiology of neuronal diseases. Thrombin, a pro-coagulant serine protease is associated with neurodegeneration and its indirect inhibitor, activated protein C (aPC), is considered neuroprotective. While levels of thrombin and aPC activity are readily measured in the blood, similar assays in the cerebrospinal fluid (CSF) have not been described.The aim of this study was to establish a specific and sensitive enzymatic assay to measure both thrombin and aPC activity in the CSF. CSF was collected from 14 patients with suspected normal pressure hydrocephalus served as a control group, while seven patients with central nervous system infections served as an acute neuro-inflammatory study group and one sample of CSF following traumatic lumbar puncture served as a positive control. Thrombin and aPC activities were measured by fluorescence released by specific proteolytic cleavage in the presence of endopeptidase and amino-peptidase inhibitors to ensure specificity. Specificity of the method was verified by thrombin and serine-protease inhibitors N-alpha-((2-naphthylsulfinyl)glycyl)-DL-p-amidinophenylalanylpiperidine and phenylmethanesulfonyl fluoride.Inhibition of thrombin activity by CSF samples and levels of specific thrombin inhibitors were also assessed. Thrombin and aPC activities were reliably measured and were significantly higher in the CSF of patients with central nervous system infections compared to normal pressure hydrocephalus controls, suggesting the involvement of these factors in neuro-inflammation. CSF thrombin activity levels in the presence of known thrombin concentration were high in patients with central nervous system infections, and low in normal pressure hydrocephalus patients. Quantification of endogenous thrombin inhibitors protease nexin 1, amyloid precursor protein and anti-thrombin III in CSF by western blot indicated a significant elevation of amyloid precursor protein in infectious CSF. In conclusion, this study describes a novel and sensitive assay aimed at the detection of thrombin and aPC activity in CSF. This method may be useful for measuring these factors that reflect degenerative and protective influences of coagulation on neurological disorders. The study procedure was approved by the Ethics Committee of the Chaim Sheba Medical Center (approval No. 4245-17-SMC) on October 18, 2018.
Key Words: activated protein C; cerebrospinal fluid; infection; inflammation; normal pressure hydrocephalus; thrombin Chinese Library Classification No. R446; R364; R741
Introduction
Inflammation is a key process in a variety of brain diseases.It is found in epilepsy (Vezzani et al., 2013), following brain ischemia (Shichita et al., 2012), in autoimmune diseases(Correale et al., 2017) and in dementias (Parbo et al., 2017).Brain inflammation may originate from both central nervous system (CNS)-borne and systemic inflammatory processes(Varatharaj et al., 2017).
In the systemic circulation the well-established association between the inflammation and coagulation systems has been known for many years. These two systems modulate and affect each other. This tight association has also been found in the brain where the coagulation system triggers brain inflammation andvice versa(Chapman, 2013; Festoff and Citron, 2019). Interestingly, it has been found that direct coagulation inhibition may reduce the damage caused by inflammatory response (Shavit-Stein et al., 2019a).
Thrombin, a serine protease, and a central factor in the coagulation cascade, is a significant player in the inflammation-coagulation interface. Changes in thrombin activity levels are found in brains of animal models for inflammatory diseases (Beilin et al., 2005; Davalos et al., 2014;Bushi et al., 2017). Injecting mice with lipopolysaccharide,a bacterial component used to model sepsis (Remick et al.,2000), significantly increases levels of thrombin activity in the brain. Inhibition of thrombin prevents this effect and lowers levels of brain inflammatory factors (Shavit Stein et al., 2018).Endogenous inhibitors of thrombin such as protease nexin 1(PN1) and amyloid precursor protein (APP) are also known to play a major role in neurodegenerative and inflammatory diseases (Beilin et al., 2005, 2007; Gofrit and Shavit-Stein,2019). Taken together, these observations strongly support an involvement of thrombin in brain inflammatory responses.
Activated protein C (aPC) is also a serine protease with anti-coagulation and anti-inflammatory properties (Esmon, 2003;Festoff and Citron, 2019). aPC acts as a complementary factor for thrombin, down-regulating its activity.
Both thrombin and aPC known to have cellular properties(neurodegenerative or neuroprotective, respectively) separate from their procoagulant or anticoagulant effects (Rajput et al., 2019). Thrombin activates a G-protein coupled protease activated receptor 1 inducing inflammation, vascular leakage,and cell death, while aPC activates the same receptor inducing cytoprotection. This phenomenon is known as ‘biased agonism’ and results due to separate intracellular signaling initiated by a differential proteolytic cleavage (Mosnier et al.,2012; Sinha et al., 2018).
Evaluation of both brain thrombin and aPC activities may be a useful tool for the diagnosis of inflammatory diseases as well as monitor disease progression, and evaluation of response to treatment. Thrombin and aPC activities in the cerebrospinal fluid (CSF) are therefore highly relevant measures as an insight into the degenerative/regenerative processes of the brain.
Sensitivity is limited for thrombin activity levels in assays used in clinical practice. Although prothrombin time (PT) and activated partial thromboplastin time (aPTT) tests are sensitive for the detection of very high levels of coagulation factors activity present in the blood, they fail in assessing coagulation activity in neural derived samples where very low levels of these factors are present. Indeed, thrombin levels in the brain are approximately 100-fold lower than its plasma levels (Bushi et al., 2013). Additionally, neural changes in thrombin activity are subtle, and are clinically insignificant in the context of plasma coagulation studies.
Specificity of PT and aPTT assay in evaluating thrombin activity is limited as well, due to their evaluation of the general coagulation cascade, which is useful in clinical practice, but fails to recognize a single coagulation factor.
Attempts to measure thrombin activity in the plasma using chromogenic and fluorogenic assays have been conducted successfully (Stief, 2006; Hao and Zhao, 2016). However, to the best of our knowledge, no diagnostic assay is available to date that enables the direct measurement of thrombin concentration in circulating blood (Müller et al., 2011).
Evaluation of aPC activity raises similar difficulties: assays are sensitive only for the high aPC levels present in human plasma, and with limited specificity due to the expensive and indirectly clinically used test (using snake venom for PC activation) (Asmat and Ramzan, 2018).
Ideally, measurements of thrombin and aPC activities should be conducted directly in brain tissue. Quantitative measurement of thrombin activity in murine brain slices has been described by our laboratory (Bushi et al., 2013;Golderman et al., 2020). However, while in human patients conducting measurements directly in the brain is unfeasible in most cases, CSF is an ideal window to the CNS for testing activity levels of coagulation factors. Previous attempts to measure thrombin activity directly in the CSF have been unsuccessful (Smirnova et al., 1997), perhaps due to the use of the less sensitive chromogenic assay. A novel method for evaluation of aPC activity in human CSF was recently developed in our laboratory (Golderman et al., 2020) and is presently being used for research purposes.
Here, we present novel and sensitive methods for the measurement of thrombin and aPC activities in human CSF using fluorogenic emitting substrates, one cleaved preferentially by thrombin and the other preferentially by aPC.The assays were verified by measuring activity levels in CSF taken from patients suffering from either CNS infections or normal pressure hydrocephalus (NPH).
Materials and Methods
Design and ethics
The study procedure was approved by the Ethics Committee of the Chaim Sheba Medical Center (approval No. 4245-17-SMC) on October 18, 2018 (Additional file 1) and conducted in accordance with theDeclaration of Helsinki. This work was reported according to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines(von Elm et al., 2014;Additional file 2). An informed consent form was signed by each participant before CSF collection(Additional file 3).
CSF collection
Human CSF was obtained from a total of 22 adult subjects undergoing lumbar puncture (LP), following explanation and after signing an informed consent. The samples were stored at -80˚C (no more than 6 hours following LP procedure) until use for the experiment. Except for a positive control used for evaluation of CSF taken from traumatic puncture (n= 1),all CSF samples (n= 21) included in our study were derived from non-traumatic LP and were clear and colorless on gross examination, thus minimizing the possibility of serumderived thrombin activity due to traumatic puncture. Our study included 6 patients with acute viral CNS infections,and 1 patient with chronic bacterial infection, and one with traumatic LP. None of the patients had received treatment prior to LP. The control group included 14 patients with suspected NPH according to the following criteria: the presence of a full or partial clinical triad of gait disturbance,urinary incontinence, and memory impairment in the presence of a radiologic finding of ventricular enlargement not attributable to atrophy or congenital enlargement; All patients went through LP, and the CSF opening pressure was normal.All displayed an improvement of gait after CSF drainage or ventricular shunting. Total number of patients included was 22. Study flowchart is shown inFigure 1. Our trial is not interventional, which is the reason that we were not obliged to register it on any clinical trial registry platform.
Patients with prominent features of dementia or extrapyramidal symptoms and signs, or a gait disturbance which is not typical for NPH were not included in order to reduce the likelihood of mixed neurodegenerative pathology such as Alzheimer’s disease or Parkinson’s disease; some displayed MRI ischemic findings on imaging yet the gait was typical for NPH gait was improved after CSF removal. Patients record assessment was conducted by three independent senior neurologists to reduce a biased diagnose. In addition,each computerizes patient records was assessed to ensure that patients received no antibiotic treatment prior to LP, in order to minimize potential differences.
Thrombin activity assay
Thrombin activity was assessed using a fluorogenic substrate cleaved by thrombin (Cat# I-1560; Bachem, Bubendorf,Switzerland; excitation 360 nm; emission 465 nm). The reactions were carried out in a black 96-well microplate using 60 µL of CSF. All reactions were performed in Tris buffer(in mM: 150 NaCl, 1 CaCl2, 50 Tris-HCl; pH 8.0). In order to exclude the effect of widely abundant CNS endopeptidases, all reactions were performed in the presence of endopeptidase inhibitors [bestatin hydrochloride (0.1 mg/mL), Cat# B8385,Sigma, St. Louis, MO, USA; 200 µM prolylendopeptidase inhibitor II, Cat# 537011, Merck Millipore, Burlington,MA, USA]. Controlled reactions were performed in each experiment in the presence of N-alpha-((2-naphthylsulfinyl)glycyl)-DL-p-amidinophenylalanylpiperidine (NAPAP), a specific thrombin inhibitor (1 µM; Cat# SC-208083; Santa Cruz Biotechnology, Dallas, TX, USA) to evaluate the specific contribution of thrombin to substrate cleavage. Calibration curve was created for each experiment using known bovine thrombin concentrations (Cat# T-4648, Sigma). Thrombin activity was normalized to total CSF protein concentration,which was measured by the Pyrogallol Red Molybdate protein dye-binding assay using Beckman Coulter au5822 (Beckman Instruments Inc., Fullerton, CA, USA).
Thrombin inhibition assay
The reactions were carried out using black 96-well microplate using bovine thrombin (0.0156 U/mL) in the presence of 60 µL CSF of selected patients, incubated for 1 hour at 37°C. Then,thrombin activity was measured as described above.
aPC activity assay
aPC activity was measured as previously described (Golderman et al., 2020), using a fluorogenic substrate (Pyr-Pro-Arg-AMC, 20 µM; GL Biochem Shanghai Ltd, Shanghai, China).The reactions were carried out in black 96-well microplates(Cat# 237108; Thermo Fisher Scientific, Nunc, Rochester,NY, USA). All reactions were performed in the presence of specific FXa and thrombin inhibitors, apixaban (1 µM; Cat#S1593; Selleckchem, Houston, TX, USA) and NAPAP (1 µM)respectively. Controlled reactions were performed in the presence of phenylmethanesulfonyl fluoride (PMSF, 2 mM,Cat# P7626, Sigma). The cleavage of the substrate was measured at 37°C every 2 minutes over 25 cycles (excitation 360 ± 9 nm, emission 465 ± 20 nm) using microplate reader(Infinite F NANO+; Tecan, Männedorf, Switzerland). The activity was calculated as the linear increase of fluorescence intensity over time. In order to ensure technical accuracy and method validation, a positive control was tested in each and every experiment.
Western blot assay
Proteins from selected CSF samples (30 µg/lane) were separated by polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes for western blot analysis. Membranes were incubated in blocking buffer (IB-500; Gene Bio-Application Ltd., Yavne, Israel) for 10 minutes at room temperature. Membranes were incubated with primary rabbit anti-APP antibody (1:500, Abcam, Cambridge,UK; Cat# ab32611 RRID:AB_778422), goat anti-PN1 antibody(1:100, Santa Cruz Biotechnology, Cat# SC-32454, D-18) or goat anti anti-thrombin III (ATIII; 1:500, R&D system, Cat#AF1267) overnight at 4°C, rinsed and incubated at room temperature for 1 hour with an appropriate secondary antibody, horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10,000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA), donkey anti-goat IgG (1:10,000, Jackson ImmunoResearch Laboratories), respectively. The bound antibodies were detected with enhanced chemiluminescence(ECL) assay kit (EZ-ECL, Biological Industries, Beit Haemek,Israel). Bands densities were quantified with ImageJ software(1.52a, NIH, Bethesda, MD, USA) and results were normalized to Ponceau (Cat# P3504, Sigma) levels. Measurements were conducted by a researcher blinded to the experiment.
Statistical analysis
Mann-WhitneyUtest was used specifically for comparison of CSF taken from a traumatic puncture (“bloody CSF”)with and without the thrombin inhibitor NAPAP. Aside from this specific analysis, used on CSF which was taken as a representative sample, unpairedt-tests, and one-way ANOVA with Tukeypost hocanalysis were conducted on the same,normally distributed data. Linear regression was conducted for thrombin versus aPC activities and for the PN1 level versus thrombin activity. Sample sizes were based on prior results in our laboratory, assuming power 60% and alpha 0.05. Results are expressed as the mean ± SEM,Pvalues < 0.05 were considered significant. All statistical analyses were conducted using Prism GraphPad (version 8.0.1 for Windows, GraphPad Software, La Jolla, CA, USA).
Results
Thrombin and aPC activities in representative CSF samples
We took CSF from a representative sampled patient with a CNS infection (meningitis,Figure 2A) and a representative sampled patient with a similar condition who had a traumatic LP (Figure 2B) both show measurable thrombin activity as indicated by the increased fluorescence with time. Thrombin activity was higher in the traumatic LP CSF sample compared to the respective non-traumatic puncture sample. Thrombin activity in both samples was undetectable in the presence of the selective thrombin inhibitor NAPAP.Figure 2CandDshows representative aPC activities in CSF samples taken from the same patients. The measurements in the presence of the non-selective inhibitor PMSF resulted in lower aPC activity in both patients. As expected, thrombin and aPC activities were higher in the traumatic LP, most likely due to the presence of coagulation factors derived from the systemic circulation.
Thrombin and aPC activities in CSF of patients with NPH compared to CNS infections
We measured changes in thrombin activity in the CSF during CNS infection (n= 7) and compared it with CSF taken from suspected NPH patients (n= 10) that served as noninflammatory controls. Thrombin activity was significantly higher in CSF taken from patients with various types of CNS infections compared to NPH controls (P< 0.0001;Figure 3A).
aPC activity measured in CSF of patients with infections was also significantly elevated compared to NPH controls (P<0.0001;Figure 3B). Interestingly, the linear regression analysis between thrombin and aPC activities was found to be nonsignificant (R2= 0.01,P= 0.8;Figure 3C). Temporal analysis showed two different patterns of thrombin and aPC activities.Patients sampled at early stages of the disease showed high thrombin activity levels, while patients sampled later in the course of the disease showed lower levels of thrombin activity.aPC activity pattern was found to be higher at later staged of sampling (Figure 3D).
Endogenous thrombin inhibition in human CSF
Thrombin activity is potentially affected by endogenous inhibitors in the CSF. We therefore measured thrombin inhibition indirectly by measuring thrombin activity of CSF samples applied on a known concentration of thrombin (0.015 mU/mL), in comparison with results of thrombin activity obtained in 0.015 mU/mL thrombin alone (n= 13), in the absence of CSF. CSF of patients with infection significantly increased the measured thrombin activity (n= 7,P= 0.02;Figure 4A), whereas CSF taken from NPH patients significantly inhibited thrombin activity (n= 8,P= 0.04;Figure 4A).Figure 4Bshows a representative fluorescence development pattern over time of thrombin, thrombin in the presence of CSF taken from a patient with infection and with NPH. Elevation of fluorescence over time was the highest in infectious CSF, and the lowest in CSF taken from NPH patients.These results suggest the presence of endogenous thrombin inhibitors which were subsequently measured using western blot. A trend toward increased PN1 levels was found in patients with infections (n= 6) compared to NPH patients (n= 9,P= 0.15;Figure 5A). APP levels were significantly higher in CSF taken from patients with infections (n= 6) compared to NPH control patients (n= 9,P= 0.01;Figure 5B). ATIII, a systemic derived essential thrombin inhibitor, was not found to be significantly different between CSF of infections (n= 6)and NPH patients (n= 8,P= 0.31;Figure 5C).

Figure 1|Study flowchart.
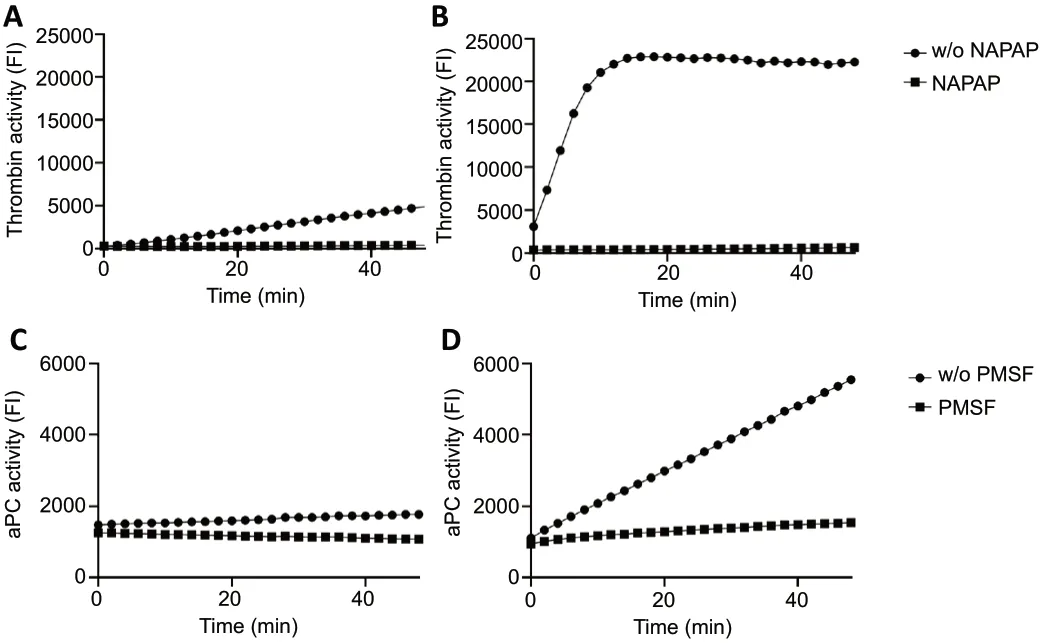
Figure 2|Increased thrombin and aPC activities in CSF samples following traumatic LP.
An inverse linear regression between thrombin activity and PN1 levels (R2= 0.8546,P= 0.024;Figure 6) was found in CSF taken from patients with infections.
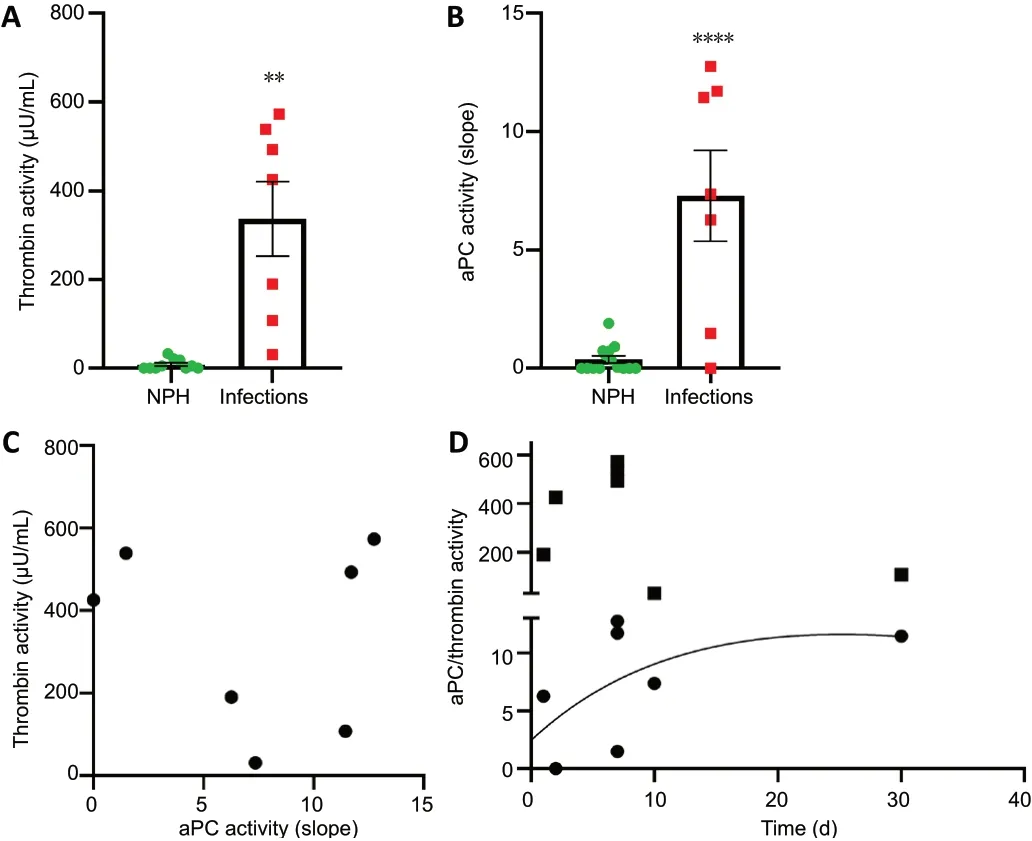
Figure 3|Elevated levels of thrombin and aPC activities in infections vs.NPH CSF.

Figure 4|Endogenous thrombin inhibition properties of CSF taken from central nervous system infections and NPH patients.
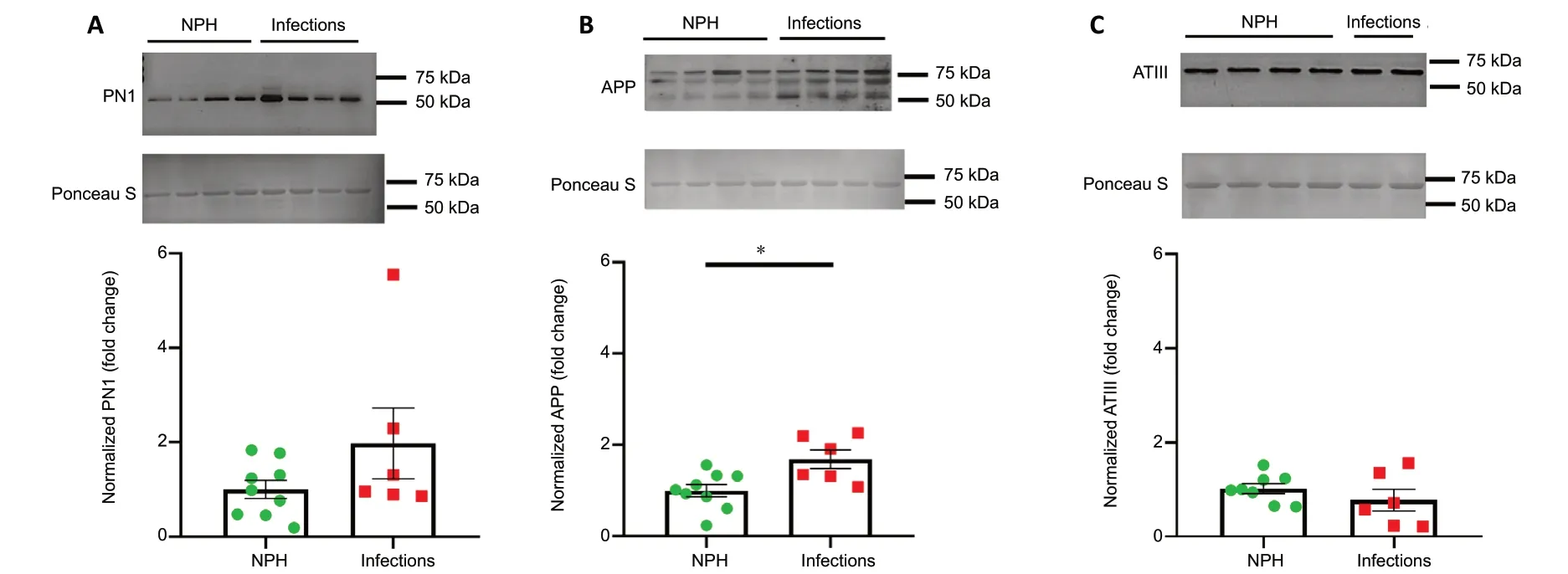
Figure 5|Levels of endogenous thrombin inhibitors (PN1, APP, ATIII) in CSF taken from patients with infections and NPH.

Figure 6|Inverse linear regression between PN1 and thrombin activity.
Discussion
CSF proteins have been studied to find biomarkers for diagnosis and prognosis in neurological diseases. Recently,proteomics analysis studies have identified more than 3000 proteins in the CSF (Bastos et al., 2017; Macron et al., 2018), where hundreds of them increase or decrease during different stages of neurological diseases. However,although proteomics is highly effective and sensitive for protein identification and changes in their expression levels,this method is not functionally informative since it does not measure protein-activity. Measurement of protein-activity levels is especially relevant in the field of enzymatically active proteins such as coagulation and inflammation proteins.Key proteases of the coagulation cascade are known to hold a role in brain regeneration and degeneration processes,respectively, as previously reviewed by our group (Gofrit and Shavit-Stein, 2019). Specifically, aPC and thrombin induces opposite inflammatory and anti-inflammatory responses in neural tissue, and therefore, measuring their activity levels in the CSF may indicate the neurodegeneration status. In the present study, we utilized novel and sensitive assays to show significantly elevated levels of thrombin and aPC activity in CSF of patients with various CNS infections compared to noninfectious NPH control patients. This was detected through highly sensitive and specific fluorogenic substrates (Bushi et al., 2013).
The high sensitivity of the presented method was due to several reasons. Primarily, the use of fluorogenic moiety over a chromogenic one, as it has been used in previous attempts to measure thrombin activity in CSF (Smirnova et al., 1997).Furthermore, the fluoresce reader device used in this study is highly advanced and enables detection at extremely low fluorescence levels (amol/well compare to fmol/well of the competitors). Together, this may explain the difference between previous attempts to measure thrombin activity in the CSF and the current findings. During recent years,technology advancement has allowed a fast and relatively cheap detection of brain-borne molecules that are known to be present at very low concentrations in human tissues such as CSF, plasma, and serum. The best example is the detection neurofilaments (NF), known to be a byproduct of axonal damage, released to the CSF and plasma (Rossi et al., 2018;Zmira et al., 2020). NF were undetectable in CSF and plasma for many years using ELISA. Recently, single molecule assay(SIMOA) has allowed their detection in plasma (Hendricks et al., 2019). Though using the same antibodies for the immunodetection, the advanced magnetic beads method and instrument allow greater and sensitive detection level.We believe that the method and results of this current study present a conceptual and technology advancement in the field of CSF degenerative/regenerative assessment in neurological disease. The specificity of the present assay is achieved with the use of a unique peptide sequence cleaved preferentially by the targeted serine protease. Specificity for the thrombin activity assay is further improved using endopeptidase inhibitors combination and is further supported by the reduction of the measured fluorescence following the addition of NAPAP, a selective thrombin inhibitor. Although the thrombin inhibitor hirudin was previously used by our group(Beilin et al., 2001), NAPAP was chosen over other thrombin inhibitors in the present work due to its high specificity,small size, and rapid activity. Similarly, the specificity of the aPC activity assay is further improved using the factor Xa inhibitor apixaban, and NAPAP, as described in previous report (Golderman et al., 2020). Measured aPC activity was abolished with the non-specific inhibitor PMSF. We used CSF of traumatic puncture as a technical positive control since in these settings of blood leakage it is assumed that bloodderived coagulation factors levels in the CSF will be increased.Indeed, measurements of both proteases’ activity are higher and show different pattern in these traumatic puncture CSF samples.
Thrombin activity elevation in inflammatory neural tissue is well known and described in several disease models, including Streptozotocin model for diabetic neuropathy (Shavit-Stein et al., 2019b), experimental autoimmune neuritis (Shavit-Stein et al., 2019a), SOD1 model for amyotrophic lateral sclerosis(ALS) (Shavit-stein et al., 2020), and ischemic stroke (Bushi et al., 2013). Inflammation of neuronal tissue is accompanied by changing of CSF cytokine profile, as described in various CNS disorders, including Alzheimer’s disease (Mattsson et al., 2009), amyotrophic lateral sclerosis (Almer et al., 2002),bipolar disorder and Schizophrenia (Wang and Miller, 2018).Coagulation activation, increased thrombin-antithrombin complexes level (Weisfelt et al., 2007) and fibrinolysis inhibition were previously reported in CSF of infectious CNS disease patients, in addition to inflammatory cytokine elevation (Weisfelt et al., 2007). Elevated aPC levels in infectious CSF strengthens previously described results from our laboratory, which was tested in glia cell lines, in mice brains, and in human CSF, and further validate our method for aPC activity measurement in CSF (Golderman et al.,2020). Elevation of aPC may represent a protective feedback response to rising thrombin levels.
Temporal analysis shows that thrombin and aPC levels change over time. Thrombin activity is elevated early at the course of the disease, followed by a later reduction, whereas aPC levels rise during disease course. CSF taken from infected patients late in their disease course showed relatively lower levels of thrombin activity, possibly explained by consumption of thrombin during a later stage of inflammation. The rise in aPC levels may represent a counter feedback loop aimed at limiting the destructive potential of thrombin (Mosnier et al.,2012).
The possible mechanism of a feedback loop is also supported by the elevation of intrinsic thrombin inhibitors (PN1 and APP also known as PN2) in the CSF of infectious patients.This elevation is not measured in ATIII levels, a key thrombin inhibitor of the systemic circulation, which supports a local,brain induced response to high thrombin levels. This is in line with previous findings in the brain of experimental autoimmune encephalomyelitis (EAE) mice (the animal model of multiple sclerosis) indicating increased PN1 and APP levels during the inflammatory phase of the disease (Beilin et al.,2005, 2007). Indeed, association between levels of PN1 and thrombin activity show an inverse linear correlation.Interestingly, thrombin inhibition assay showed inhibited thrombin activity in the presence of NPH patients CSF and elevated thrombin activity when CSF of infectious patients was applied, despite higher levels of intrinsic thrombin inhibitors. This data suggests that these intrinsic inhibitors are blocked by the excess thrombin levels in the CSF, and thus cannot be detected using thrombin inhibition assay.However, it should be noted that APP effects and participates in multiple neurological processes. APP elevation may be part of other unrelated effect, and a mere coincidental finding.The interaction between APP and thrombin activity should be interpreted with caution.
Our study group include seven patients with CNS infections.It is interesting to note that the patient diagnosed with neurosyphilis had a below-average level of thrombin activity,and relatively high level of aPC activity. This patient was sampled relatively late (30 days after symptoms appearance).Neurosyphilis is different in its course compared to the other diagnoses in our study group. Although traditionally defined as a late sequela of syphilis infection (tertiary syphilis), recent studies suggest the presences of the causative agent in the CSF early in the disease (Ghanem, 2010). Therefore, the inflammatory CNS process is present for many months prior to the diagnosis of neurosyphilis and CSF sampling. This lowgrade progressive inflammatory process may represent a later pseudo-chronic stage in the disease following the earlyacute phase. We can hypothesize that in the early stage high thrombin activity levels would have been measured as part of neuroinflammatory intrinsic and extrinsic processes,which may induce a feedback loop with compensatory effects. Among them, increased endogenous brain-derived thrombin inhibitors together with elevated aPC activity and thrombin consumption by its cellular targets and extracellular inhibitors. The source of thrombin in the CSF is a matter of debate. Previous work by Lewczuk et al. evaluating a number of neurological diseases suggests that the presence of prothrombin in these pathologies is blood derived, supporting the hypothesis that CSF measured thrombin activity is partially extrinsic (Lewczuk et al., 1999). However, neuronal cells are known to express prothrombin mRNA supporting an intrinsic source for thrombin (Dihanich et al., 1991). We hypothesize that thrombin activity measured is a combination of both,with a different contribution according to disease stage.
The present study has several limitations. Ideally, control group for this study would have included healthy volunteers.Since NPH is not an acute inflammatory disorder, the inflammatory process is secondary to damage caused by neuronal tissue repression. Since LP is a part of the diagnostic process, CSF taken from NPH patients is more accessible.These patients had no apparent clinical or laboratory signs of underlying inflammatory CNS process. Several studies have examined the composition of cytokines present in the CSF of NPH, with mixed results. Although interleukin 6 was elevated in the CSF of NPH patients in most of the studies, levels of other cytokines were not consistently elevated (Jeppsson et al., 2013; Pyykkö et al., 2014; Sosvorova et al., 2014,2015). Thus, we assume that the use of NPH as a control group is reasonable. Further research in healthy control is needed. Due to the need of human tissue and its limited accessibility, our study included only 22 patients. However, the significance of the results suggests a robust effect. Patients in CNS infection group suffered different type of infections in various parts of the CNS, including neurosyphilis, meningitis,enterovirus myelitis, and thick borne encephalitis. This may affect the results, due to changes in timing and magnitude of the inflammatory response. A further research that will be conducted in larger groups is needed and may indicate differential results between types of infections, and may allow increased temporal and spatial resolution.
In conclusion, in the present study, we describe novel and sensitive methods for the measurement of thrombin and aPC activities in human CSF. By applying these methods, we were able to measure an early elevation in thrombin activity, and a later elevation in aPC activity in the CSF of patients with infections of the CNS. This was accompanied by elevated expression of endogenous brain-derived thrombin inhibitors.Our results support this new tool for the evaluation of CNS degenerative regenerative balance, and potentially for monitoring treatment response.
Author contributions:Formal analysis, investigation, conducted most of the enzymatic activity experiments, original draft: AG. Formal analysis,conducted a large portion of the enzymatic activity experiments,and investigation: VG. Formal analysis, original draft, visualization:SGG. Investigation, conducted most of the biochemistry experiments,visualization: SAA. Data acquisition and approved manuscript: DNZ, ZIH,TFK, and SHB. Conceptualization, formal analysis, review and editing:JC. Conceptualization, review and editing: NM. Conceptualization,methodology, formal analysis, review and editing, and supervision: ESS.
Conflicts of interest:A provisional patent (“Methods of determining activity of activated protein C” # 63/001,349) on the highly sensitive method for the measurement of activated protein C has been submitted to the US patent authorities.
Financial support:None.
Institutional review board statement:The study procedure was approved by the Ethics Committee of the Chaim Sheba Medical Center(approval No. 4245-17-SMC) on October 18, 2018 and conducted in accordance with the Declaration of Helsinki.
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the forms the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names andinitials will not be published and due efforts will be made to conceal their identity.
Reporting statement:This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Sheba Medical Center in Israel.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Anonymized individual data will be available immediately after study publication upon request from those who wish to access the data for 5 years, as well as study protocol and statistical analyses. If anonymized data is provided, it should be done so after proposals to efrat.shavit.stein@gmail.com. Raw data (including personal information and participant codes) will be stored in a locked cabinet at Sheba Medical Center for this time period before being destroyed.Personal results will also be available to participants upon request. If requested, study protocols and outputs of statistical analysis will be available.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional file 1: Ethical Approval Documentation.
Additional file 2: STROBE checklist.
Additional file 3: Model consent form.
- 中国神经再生研究(英文版)的其它文章
- Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders
- The role of gap junctions in cell death and neuromodulation in the retina
- Don’t know what you got till it’s gone: microglial depletion and neurodegeneration
- Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia
- Protein post-translational modifications after spinal cord injury
- Axonal mRNA localization and local translation in neurodegenerative diseases

