Arbutin effectively ameliorates the symptoms of Parkinson’s disease: the role of adenosine receptors and cyclic adenosine monophosphate
Jie Zhao, Manish Kumar, Jeevan Sharma, Zhihai Yuan
Abstract An antagonistic communication exists between adenosinergic and dopaminergic signaling in the basal ganglia, which suggests that the suppression of adenosine A2A receptors-cyclic adenosine monophosphate pathway may be able to restore the disrupted dopamine transmission that results in motor symptoms in Parkinson’s disease (PD). Arbutin is a natural glycoside that possesses antioxidant, antiinflammatory, and neuroprotective properties. The purpose of this study was to investigate whether arbutin could ameliorate the symptoms of PD and to examine the underlying mechanism. In this study, Swiss albino mouse models of PD were established by the intraperitoneal injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine for 4 successive days, with the concurrent intraperitoneal administration of arbutin(50 and 100 mg/kg) for 7 days. The results showed that arbutin significantly reduced lipid peroxidation, total nitrite levels, and inflammation in the substantia nigra and striatum of PD mouse models. In addition, arbutin decreased the activity of endogenous antioxidants, reduced the levels of dopamine, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and γ-aminobutyric acid, and minimized neurodegeneration in the striatum. Arbutin also reduced the abnormal performance of PD mouse models in the open field test, bar test, pole test, and rotarod test. The therapeutic efficacy of arbutin was similar to that of madopar. The intraperitoneal injection of the A2AR agonist CGS21680 (0.5 mg/kg)attenuated the therapeutic effects of arbutin, whereas the intraperitoneal injection of forskolin (3 mg/kg) enhanced arbutin-mediated improvements. These findings suggest that arbutin can improve the performance of PD mouse models by inhibiting the function of the A2AR and enhancing the effects of cyclic adenosine monophosphate. This study was approved by the Institutional Animal Ethics Committee (1616/PO/Re/S/12/CPCSEA) on November 17, 2019 (approval No. IAEC/2019/010).
Key Words: arbutin; CGS21680; dopamine; forskolin; GABA; inflammation; oxidative stress; Parkinson’s disease; striatum; substantia nigra Chinese Library Classification No. R453; R741; R363
Introduction
Parkinson’s disease (PD) is the second-most frequent progressive neurodegenerative disorder, affecting 1-3% of individuals older than 65 years. PD has a higher prevalence in males than in females (Hirsch et al., 2016). Although sporadic PD is more prevalent than familial PD (10%), both forms share common features, such as the intraneuronal accumulation of α-synuclein and ubiquitin in intracytoplasmic inclusions called Lewy bodies (Meredith et al., 2008; Bei et al., 2019).Reduced dopaminergic neuron density in the substantia nigra(SN) pars compacta leads to a dopamine deficit in the basal ganglia, which results in the typical clinical symptoms of PD(Sveinbjornsdottir, 2016). The degeneration of approximately 70% of the dopaminergic neurons in the SN pars compacta results in the development of motor symptoms, such as bradykinesia, rigidity, tremor, balance deficiencies, posture instability, and gait defects in PD (Hartmann, 2004). Nonmotor symptoms, which may appear long before motor symptoms, include constipation, sleep disturbances (e.g.,hyposmia and rapid eye movement), urinary incontinence,hallucinations, dementia, and hypotension (Radhakrishnan and Goyal, 2018; Seguella et al., 2020).
The current arsenal available for PD pharmacotherapy includes several drugs (e.g., levodopa/carbidopa, selegiline, and bromocriptine), which exclusively target the dopaminergic pathway for the symptomatic treatment of motor dysfunction,with limited long-term benefits and associated with a variety of adverse effects, such as dyskinesia, motor fluctuations,and psychosis (Abeliovich and Gitler, 2016). Several drugs are currently being examined in clinical trials: safinamide(NCT03881371), exenatide (NCT04232969), and ganoderma(NCT03594656) are in phase 3 trials; liraglutide (NCT02953665),cannabidiol (NCT03582137), bumetanide (NCT03899324),and nicotine (NCT03865121) are in phase 2 trials; and lithium(NCT04273932), rifaximin (NCT03575195), and flumazenil(NCT03462641) are in phase 1 trials. These drugs have been suggested to be able to improve the standard of living for PD patients (McFarthing et al., 2020). The pathogenesis of PD is multifaceted; however, none of the currently used therapeutic approaches are effective for the prevention of PD symptom relapse or are able to reverse or prevent the underlying neurodegenerative progression of the disease (Ravina et al.,2003). Therefore, a shift in focus toward the non-dopaminergic receptor and pharmacotherapies has occurred during recent years. The inhibition of the adenosine A2A-cyclic adenosine monophosphate (cAMP) pathway has gained much attention for the potential to improve the therapeutic outcomes of PD(Schwarzschild et al., 2006). The co-existence of adenosine A2Areceptors (A2ARs) and dopamine D2 receptors in the striatum (ST) allows for antagonistic communications between adenosinergic and dopaminergic signaling (Kulisevsky and Poyurovsky, 2012). Evidence has suggested that the concomitant activation of D2 receptors and the inhibition of A2ARs can substantially improve the motor functions in PD patients (Shen and Chen, 2009). Subsequently, computational strategies have been used to develop dual/multiple target ligands that act on heterodimeric A2AR/D2 receptor complexes (Shao et al., 2018),adenosine A2Areceptor (A2AR)/adenosine A1 receptor (A1R)/monoamine oxidase-B (MAO-B) (Mao et al., 2020), or D2/5-5-hydroxytryptamine 1A (HT1A) receptors (Zheng et al., 2019;Lv et al., 2020), which have transformed the approach to PD management. These dual-target-directed drugs, such as (E)-8-(3-Chlorostyryl)caffeine (A2AR and MAO-B antagonist), provide synergistic or additive effects during PD therapy (Petzer et al.,2009). Dual/multiple targeting agents eliminate many of the limitations (e.g., poor efficacy, safety, and drug resistance)associated with the use of an individual, single-target drug. A multi-targeted therapy (polypharmacology) is necessary for the successful treatment of complex diseases such as PD, which require the targeting of multiple pathways to achieve synergistic effects. In pre-clinical tests, A2AR antagonists (caffeine and preladenant) have been shown to reverse the mobility effects caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-and 6-hydroxydopamine-induced lesions and also reduced levodopa- and L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesia. As a monotherapy, the efficacy of A2AR antagonists have been shown to be slightly less than or equivalent to that of L-DOPA; however, the major benefits appeared when A2AR agonists were co-administered with L-DOPA. The combination of L-DOPA and A2AR antagonists demonstrated an enhanced therapeutic potential that can be harnessed for the treatment of advanced stages of PD (Shiozaki et al., 1999; Cieślak et al.,2008). Recently, istradefylline was approved by U.S. Food and Drug Administration (2019) as an A2AR antagonist and is now used in Japan, in combination with L-DOPA/carbidopa, to avoid“off” episodes in PD patients (Mao et al., 2020). In PD patients,“off” episodes are characterized by the worsening of motor and non-motor symptoms, despite continued L-DOPA therapy.The upregulation of A2ARs in response to L-DOPA/carbidopa therapy and the subsequent activation of the indirect pathway in the basal ganglia decreases motor activation, precipitating these “off” episodes (Berger et al., 2020). A2AR antagonists,such as istradefylline, can abolish the adenosinergic effects of intermittent L-DOPA/carbidopa therapy and reduce the occurrence of “off” phases in PD patients.
Arbutin (4-hydroxyphenyl-β-D-glucopyranoside,Figure 1) is a hydroquinone that is widely found in several plant families,such as Lamiaceae, Ericaceae, Rosaceae, and Saxifragaceae(Pop et al., 2009). Arbutin suppresses various enzymatic activities, such as tyrosinase, α-amylase, and α-glucosidase,resulting in effects on melanin biosynthesis and hyperglycemia(Yousefi et al., 2013). Arbutin also inhibits the expression of genes associated with pro-inflammatory cytokines (e.g.,interleukins) and free radical biogenesis (Lee and Kim, 2012;Khadir et al., 2015). Arbutin has been associated with a variety of pharmacological activities, including anti-hypertensive, anti-diabetic, anti-seizure, anti-microbial, anti-tussive, anti-cystitis,anti-infective, diuretic, gastroprotective, hepatoprotective,and anti-hyperlipidemia activities (Myagmar et al., 2004;Shahaboddin et al., 2011; Dadgar et al., 2018; Ahmadian et al.,2019; Ye et al., 2019). Improvements in cognitive functions and motor performance following arbutin treatment have been demonstrated in pre-clinical tests in models of Alzheimer’s disease (streptozotocin), epilepsy (pentylenetetrazol), and PD(MPTP) (Dadgar et al., 2018; Ahmadian et al., 2019; Dastan et al., 2019). Furthermore, significant improvements in the motor functions of a Drosophila model of PD and the inhibition ofin vitrorotenone-triggered mitochondrial dysfunction have suggested that arbutin may potentially benefit PD therapy(Ding et al., 2020). Based on the existing literature, the arbutin-mediated protection from excitotoxic (Ahmadian et al., 2019) and autophagic (via 5′ adenosine monophosphateactivated protein kinase, AMPK) (Zhang et al., 2019; Ding et al., 2020) neurodegeneration indicates a potential role for A2ARs in the pharmacodynamics of arbutin. Therefore, the present study was performed to evaluate the effects of arbutin on the behavioral impairments and biochemical parameters in an MPTP-induced PD mouse model, in association with the effects of arbutin on A2ARs and the cAMP pathway.
Materials and Methods
Experimental animals and groups
All animal experimentation was performed according to the guidelines established by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA),Government of India, New Delhi. Fifty-six male Swiss albino mice (body weight 25-30 g; age 8-10 weeks) were procured from National Institute of Pharmaceutical Education and Research (NIPER), Mohali (India) after the authorization of the study design was obtained from the Institutional Animal Ethics Committee (1616/PO/Re/S/12/CPCSEA) on November 17,2019 (approval No. IAEC/2019/010). Mice were maintained in the Animal House Facility of the institute under a standard laboratory environment (temperature, 21-25°C; humidity,30-50%; light-dark cycle, 12 hours each). The animals were housed in cages made of polyacrylic material (size 44 × 29 ×16 cm3). Mice were fed a standard rodent pellet diet, and free access to water was allowed. Mice were allowed to acclimatize to the laboratory conditions for at least two weeks before experiments were initiated (experimentation was performed from 09:00 to 18:00). The caretakers were blinded to the different drug treatments.
The mice were distributed into 8 groups, in a single-blinded manner using the random allocation method (n= 7 each group): (i) the vehicle-control group was treated with saline (5

Figure 1|Chemical structures of arbutin and CGS21680.
Arbutin (IUPAC nomenclature: (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-(4-hydroxyphenoxy)oxane-3,4,5-triol) has 5 -OH groups that impart strong antioxidant properties. CGS21680 (IUPAC nomenclature: 3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxyoxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid) is a highly selective adenosine A2A receptor agonist with an inhibition constant (Ki) of 27 nM.mL/kg body weight each day, intraperitoneal injection [i.p.]);(ii) the Arbutin50 group was administered arbutin (50 mg/kg,i.p., 7 days; purity > 98%, Sigma-Aldrich, Mumbai, India);(iii) the MPTP group was administered MPTP (30 mg/kg,i.p.; Sigma-Aldrich), from days 1 to 4; (iv) the MPTP +Arbutin50 group was administered both MPTP (30 mg/kg for 4 days) and arbutin (50 mg/kg, i.p.) for 7 days; (v) the MPTP + Arbutin100 group was administered both MPTP and arbutin (100 mg/kg, i.p.); (vi) the MPTP + Madopar group was administered MPTP, and madopar (120 mg/kg,oral administration; levodopa and benserazide; Nicholas-Piramal, Mumbai, India) was administered orally, 30 minutes after MPTP injection for 7 consecutive days; (vii) the MPTP+ CGS21680 + Arbutin50 group was treated with MPTP,CGS21680 (0.5 mg/kg, i.p.; Sigma-Aldrich), and arbutin(50 mg/kg, i.p.); and (viii) the MPTP + Forskolin + Arbutin50 group was treated with MPTP, forskolin (3 mg/kg, i.p.; Sigma-Aldrich), and arbutin (50 mg/kg, i.p.). Hydrochloride has a molecular weight of 35.4, which corresponds to 17% of the MPTP hydrochloride weight. Therefore, 35.1 mg/kg (20 mg/kg× 1.17) of MPTP hydrochloride was used to deliver a dose equivalent to 30 mg/kg free MPTP, according to the procedure described by Jackson-Lewis and Przedborski (Jackson-Lewis and Przedborski, 2007). The quantity of MPTP necessary for the entire experiment was quantified based on the total body weight of the mice designated to receive MPTP treatment,and this quantity was dissolved in sterile saline so that each mouse was treated from the same batch. A subchronic dose(30 mg/kg per day, i.p.) of MPTP was prepared using normal saline (vehicle) and injected daily for 4 days (from day 1 to day 4, cumulative dose of 120 mg/kg). The arbutin dose was prepared by dissolving in normal saline, and arbutin was injected (Myagmar et al., 2004; Dadgar et al., 2018;Ahmadian et al., 2019; Ye et al., 2019) 30 minutes after MPTP administration for 7 consecutive days (Prema et al., 2015).The A2AR agonist CGS21680 hydrochloride (0.5 mg/kg, i.p.)and the adenylyl cyclase agonist forskolin (3 mg/kg, i.p.)were administered to separate groups of mice, 1 hour before behavioral studies were performed, to explore the roles played by adenosine receptors and cAMP in the mechanisms through which arbutin modulates PD pathology (Guzmán-Gutiérrez and Navarrete, 2009; Vuaden et al., 2011). Prior to the initiation of experiments, the mice were subjected to trials on all apparatus used for the behavioral studies to optimize their performances. Behavioral evaluations, including the open field test (OFT), catalepsy test, rotarod test, and pole test, were performed on days 6 and 7, in a blinded manner.The mean scores of three performances for each experiment were quantified during the behavioral assessments (Figure 2).
OFT
The OFT apparatus was made of plexiglass (75 × 75 × 75 cm3).

Figure 2|Experimental design of the study.
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) was administered(50 mg/kg, i.p.) to mice for 4 days, and arbutin (50 and 100 mg/kg, i.p.) was concurrently administered for 7 days. CGS21680 (adenosine A2Areceptor agonist, dose 0.5 mg/kg, i.p.) and forskolin (adenylyl cyclase agonist, dose 3 mg/kg, i.p.) were administered one hour before performing the behavioral studies, which were conducted on days 6 and 7 to assess the Parkinson’s disease-associated symptoms, such as effects on posture, movement, motor coordination, muscle strength, and rigidity. After the behavioral studies were performed, the striatum and substantia nigra regions were dissected from the brain, and biomarkers of oxidative stress, inflammation, and neurotransmitter levels were evaluated. DOPAC: 3,4-Dihydroxyphenylacetic acid; GABA:γ-aminobutyric acid; HVA: homovanillic acid; i.p.: intraperitoneal injection;NF-κB: nuclear factor kappa B; TNF-α: tumor necrosis factor-α.
The floor was divided into twenty-five 15 × 15 cm2squares,using blue lines, and a large central square (45 × 45 cm2) in the middle of the arena was defined using red lines (Brown et al., 1999). Behaviors in the OFT were scored by counting the number of times each mouse performed the following:traversed one of the grid lines with all four paws (line crossing); traversed one of the red lines with all four paws,entering into the central square (center square entries); raised the body using the hind legs (rearing); and displayed grooming behaviors, such as the licking of the fur, washing of face, or body scratching (grooming). Each animal was returned to its home cage after 5 minutes of behavioral assessment. Between each test, the OFT apparatus was thoroughly cleaned with 70% ethyl alcohol to eliminate any olfactory cues.
Catalepsy
Catalepsy refers to the freezing condition manifested by postural immobility (akinesia) and muscular rigidity. Cataleptic behavior was assessed using the bar test (Ferré et al., 1990).The animal was handled by its tail, with the forepaws placed on a horizontal wooden bar (4-cm height). The duration that the mouse maintained this forced position, with both front limbs outstretched and resting on the bar, was considered to serve as an index of cataleptic behavior. The removal of both front paws from the bar or any movement of the head in an exploratory position was considered to be the endpoint of catalepsy. The time period to the first movement of the front paws on the horizontal bar was reported as the cataleptic time(cut-off time of 180 seconds).
Pole test
The pole test is commonly used for the evaluation of bradykinesia. We used the standard method described by Ogawa et al. (1985). Briefly, each mouse was positioned headup at the apex of a wooden pole (height: 50 cm; diameter: 1 cm), which was placed vertically in an upright position in its home cage. The inherent tendency of a mouse positioned head-up on the top of a pole is to re-align to a head-down position and then to move down the length of the pole to return to its home cage. The following parameters were measured for each mouse: a) t-turn: the time taken to re-align to a head-down position on the pole; and b) t-total: the totaltime necessary to move down the length of the pole.
Rotarod test
The rotarod test is widely used to evaluate the muscle strength and motor coordination of rodents. The time that an animal remains on a rotating rod is a gauge of its overall physical state, balance, coordination, and ability to forecast motor activities. A digital rotarod apparatus (INCO, Ambala, India),consisting of 4 separate sections, allowed the examination of 4 animals simultaneously. Mice were handled by their tails and placed at the center of the rod in each compartment. The rotarod test was conducted at various speeds (5, 10, and 20 r/min), and the fall latency was determined for each mouse(Rozas et al., 1998).
Biochemical estimations
After behavioral testing, the mice were euthanized by cervical dislocation, and the brains were immediately isolated for biochemical analyses (n= 6). The isolated brains were rinsed with ice-cold isotonic saline, and the SN and ST were removed according to a mouse brain atlas (Kuan et al., 2015). A 10%w/v combined homogenate of the SN and ST structures was prepared in 50 mM phosphate buffer (ice-cold, pH 7.4) using a tissue homogenizer (Remi Motors, Remi-Electrotechnik,Vasai, India). The homogenized brain tissue was centrifuged(CPR-30 Remi Compufuge, Vasai, India) at 12,000 ×gfor 15 minutes (4°C), and the supernatant was obtained. The levels of oxidative stress biomarkers [thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), nitric oxide,superoxide dismutase (SOD), and catalase] and inflammation biomarkers [tumor necrosis factor-α (TNF-α), nuclear factor kappa-B (NF-κB)] were quantified. The levels of dopamine,3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid(HVA), and γ-aminobutyric acid (GABA) were estimated in the SN and ST homogenate using high-performance liquid chromatography or paper chromatography (Swamy et al.,2013). The quantification of TBARS is directly correlated with the lipid peroxidation product malondialdehyde (MDA).Briefly, a reaction mixture containing protein homogenate,glacial acetic acid (20%, pH 3.5; LobaChemie, Mumbai,India), thiobarbituric acid (TBA, 0.8%; LobaChemie), sodium dodecyl sulfate (SDS, 8.1%; LobaChemie), distilled water, and n-butanol (Merck, Mumbai, India):pyridine (LobaChemie)(15:1), was centrifuged (4000 ×gfor 10 minutes at 4°C). The supernatant, containing pink-colored MDA-TBA2 adducts,was removed to measure TBARS (nM/mg protein) using the molar extinction coefficient (ɛ) = 1.56 × 105M-1cm-1atλmax= 532 nm (Ohkawa et al., 1979). To measure GSH, the assay mixture (final volume 3 mL), containing supernatant and 4%sulfosalicylic acid (LobaChemie) was centrifuged (2000 ×gfor 10 minutes at 4°C), and the supernatant was combined with 5,5′-dithiobis-(2-nitrobenzoic acid) (0.1 mM, pH 8.0;LobaChemie, Mumbai, India) and phosphate buffer (0.3 M,pH 8.0). Glutathione (µM GSH/mg protein) was quantified spectrophotometrically (λmax= 412 nm), according to the method described by Ellman (1959), using a standard curve generated using 0.2, 0.4, 0.6, 0.8, and 1 mM GSH. SOD (E.C.1.15.1.1) activity (µM nitroblue tetrazolium reduced/min/mg protein) was estimated according to the method described by Winterbourn et al. (1975), using ɛ (formazan) = 15,000 M-1cm-1atλmax= 560 nm. Briefly, the reaction mixture (3 mL) containing supernatant, 1.5 mM nitroblue tetrazolium(Himedia Laboratories, Mumbai, India), riboflavin (0.12 mM;Himedia Laboratories), 0.1 M ethylenediaminetetraacetic acid(containing 0.0015% or 0.3 mM NaCN; Himedia Laboratories),and phosphate buffer (67 mM, pH 7.8) was illuminated for 15 minutes (60W Philips® fluorescent tube), and the change in absorbance was recorded. The presence of SOD in the sample inhibits the reduction of nitroblue tetrazolium by O2-and prevents formazan production. Catalase (EC 1.11.1.6) activity(µM H2O2decomposed/minute/mg protein) was measured (ɛ= 43.6 M-1cm-1atλmax= 240 nm) using the method described by Claiborne (1985). The change in the absorbance of the assay mixture (3 mL), containing supernatant (10%), H2O2(0.02 M, prepared in 0.05 M phosphate buffer), and phosphate buffer (0.05 M, pH 7.0), was recorded. The method described by Sastry et al. (2002) was used to calculate the total nitrite content (µmol/mg protein), using a standard curve generated using NaNO2(concentration range: 10-100 µM). Briefly,the assay mixture, containing carbonate buffer (pH 9.0),supernatant, Cu-Cd alloy (150 mg), NaOH (0.35 M), and ZnSO4solution (120 mM) was centrifuged (2000 ×gfor 10 minutes at 4°C). Supernatant was added to the Griess reagent (1:1 solution of 1% sulfanilamide in 3.0 M hydrochloric acid and 0.1% N-1-napthylethylenediamine·2HCl in H2O), and the absorbance was recorded atλmax= 548 nm. The total protein content was quantified using a standard curve generated using bovine serum albumin (concentration range: 0.2-2.4 mg/mL;Himedia Laboratories, Mumbai, India). The assay mixture was prepared using supernatant, Lowry’s reagent (1% w/v CuSO4solution, 2% w/v sodium-potassium tartrate, and 2% w/v Na2CO3in 0.1 M NaOH at a ratio of 1:1:98), phosphate buffer,and 1.0 N Folin-Ciocalteu reagent. Absorbance was recorded atλmax= 750 nm. The protein concentration was expressed as mg/mL of homogenate (Lowry et al., 1951).
Assays for the assessment of dopamine, DOPAC, HVA,GABA, TNF-α, and NF-κB levels
The supernatant from the SN and ST homogenate was combined with an equivalent amount of methanol to perform protein precipitation (4°C), which was filtered using a cellulose membrane with a pore size of 0.2 µm. The supernatant (20 µL)was injected into the column (C18 column; 5 µm, 4.6 × 250 mm) of a high-performance liquid chromatography system(Waters India Pvt. Ltd., New Delhi, India) with fluorescence detection (Agilent 1260 Infinity FLD G1321C). The mobile phase consisted of acetonitrile, water, and sodium potassium phosphate buffer solution (0.01 M, pH 4.1), delivered at a flow rate of 1.2 mL/min, and the samples were separated at 27°C. The fluorescence detector was programmed at an excitation wavelength ofλmax= 285 nm and an emission wavelength ofλmax= 333 nm. The concentrations of dopamine and its metabolites were quantified using external standards and the area under the peak technique. The peak areas were determined by injecting serial dilutions of the standards (0.5-100 ng/mL). Peak areas (vertical axis) were plotted against the corresponding concentrations (horizontal axis) of each individual monoamine standard to obtain a linear standard curve (linear regression equation for dopamine:y= 0.395x+0.059,r2= 0.993; DOPAC:y= 0.005x+ 0.163,r2= 0.998; HVA:y= 0.382x+ 0.073,r2= 0.997), which was used to quantify the concentrations in samples, with detection limits 0.01-0.04 ng/mL. The dopamine, DOPAC, and HVA concentrations were reported as ng/mg of protein. Paper chromatography was used to assess the GABA levels in the mouse brains. The sample(100 µL) from the SN and ST homogenate was combined with 1.5 mL of absolute alcohol. This mixture was centrifuged at 3000 ×gfor 15 minutes at room temperature (20-25°C).The obtained supernatant (50 µL) was used for paper chromatography, using a mobile phase consisting of n-butanol,glacial acetic acid, and water (1:5:10). The absorbance of the eluted solution was determined using a double-beam spectrophotometer (CyberLab Analytics®, Mumbai, India)atλmax= 509 nm and compared against a standard curve generated using a GABA solution at concentrations ranging from 31.25 to 2000 pmol/mL (Swamy et al., 2013). A doubleantibody, sandwich, enzyme-linked immunosorbent assay was used to quantify the TNF-α (Krishgen, Mumbai, India)and NF-κB levels (KinesisDX, Cerritos, CA, USA) in ST and SN structures, according to the manufacturer’s instructions.Briefly, brain tissue was homogenized as previously described and centrifuged at 2500 ×gfor 20 minutes. The supernatant(TNF-α,100 µL; NF-κB, 40 µL) was separated and added to mouse monoclonal antibody pre-coated wells (12 × 8 wells).After incubation (37°C) for 1 hour, biotin-labeled detection antibody was added (TNF-α, 100 µL; NF-κB, 10 µL), followed
by streptavidin-horseradish peroxidase (TNF-α, 100 µL; NFκB, 50 µL). The plates were covered incubated for 1 hour at 37°C. Subsequently, the chromogenic solution A/B or 3,3′,5,5′-tetramethylbenzidine substrate (TNF-α, 100 µL; NFκB, 50 µL) was added, resulting in the development of a bluish coloration. The reaction was stopped by the addition of stop solution (TNF-α, 100 µL; NF-κB, 50 µL), and the absorbance was measured immediately (within 15 minutes) atλmax= 450 nm, using an enzyme-linked immunosorbent assay microplate reader (iMARK, BioRad, Chennai, India). Standard curves were generated using mouse TNF-α (450, 225, 56.25, 28.13,14.06, 7.03, and 3.51 pg/mL) and NF-κB (12, 6, 3, 1.5, and 0.75 ng/mL). The concentrations of TNF-α (pg/mL) and NFκB (ng/mL) in each sample were quantified by comparison against the standard curve.
Histopathology measurement
The mice were decapitated, the whole brains were removed,and the ST regions were isolated. A 10% neutral buffered formalin solution containing 0.05% sodium azide (pH 7.0) was freshly prepared. The ST tissue was subjected to one week of fixation at 4°C in neutral buffered formalin fixative (10:1 ratio).Subsequently, the fixed brain tissue was preserved in 70%ethyl alcohol at 4°C until further processing. A microtome was used to generate 5-µm-thick sections, which were treated with hematoxylin and eosin stains. Permanent slides were prepared using coverslips, and a synthetic resin, dibutylphthalate polystyrene xylene, was used as the mounting medium. The slides were examined using light microscopy (INCO) at 100×magnifications.
Statistical analysis
The data were collected and analyzed by experimenters who were blinded to the treatments. All results are presented as the mean ± standard error of the mean (SEM). The data for all groups were analyzed using GraphPad Prism 5 software(GraphPad Software Inc., La Jolla, CA, USA). Intergroup variations were assessed using one-way analysis of variance,followed by Tukey’spost hoctest. AP-value of less than 0.05 was considered significant.
Results
Arbutin ameliorates the locomotor and exploratory activities of MPTP-treated mice
OFT is used to assess locomotor and exploratory activities,which are key parameters of anxiety in rodents. Therefore,reduced OFT performances correlate with increased levels of anxiety in rodents. The administration of MPTP to mice caused a significant decline in OFT performance compared with vehicle-treated mice, which was demonstrated by a considerable decrease (P< 0.001) in the numbers of lines crossed, squares crossed, rearing events, and grooming behaviors. In the present study, the administration of arbutin(50 and 100 mg/kg) or madopar to MPTP-treated mice resulted in considerable improvements (P< 0.001) in OFT performances compared with those of mice treated with MPTP alone. The administration of CGS21680 (A2AR agonist)in mice treated with MPTP and arbutin 1 hour prior to OFT assessment substantially (P< 0.05) attenuated the arbutininduced improvements in OFT performance compared with that of mice treated with only MPTP and arbutin. In contrast,the administration of forskolin (adenylyl cyclase agonist)to mice treated with both MPTP and arbutin significantly potentiated the arbutin-mediated increase in the numbers of lines (P< 0.05), squares crossed, rearing events, and grooming behaviors (P< 0.01) compared with mice treated with only MPTP and arbutin (Figure 3).
Arbutin attenuates the symptoms of akinesia and bradykinesia in MPTP-treated mice
In agreement with previous findings (Prema et al., 2015), the administration of MPTP caused akinesia and bradykinesia in mice, as measured using the catalepsy test and pole test,respectively, compared with mice treated with vehicle,as indicated by a significant increase (P< 0.001) in the cataleptic score (Figure 4A) and in the t-turn (Figure 4B)and t-total times recorded in the pole-test (Figure 4C). The administration of arbutin (50 and 100 mg/kg) or madopar for 7 days to MPTP-treated mice substantially (P< 0.001)cataleptic behavior compared with that in mice treated with MPTP alone. Arbutin (50 and 100 mg/kg) administration to MPTP-treated mice significantly decreased the t-turn (P<0.01 andP< 0.001, respectively) and t-total (P< 0.01 andP< 0.001, respectively) time compared with mice treated with MPTP alone. The administration of madopar to MPTP-treated mice also decreased the t-turn (P< 0.001) and t-total (P<0.01) times compared with those in mice treated with MPTP alone. However, the administration of CGS21680 to mice treated with MPTP and arbutin abolished the arbutin-induced improvements in akinesia (P< 0.01) and bradykinesia (P< 0.05)compared with those in mice treated with only MPTP and arbutin. The administration of forskolin to mice treated with MPTP and arbutin resulted in the synergistic enhancement of the arbutin-induced decreases in the cataleptic score (P< 0.01)and t-turn (P< 0.01) and t-total (P< 0.001) times compared with mice treated with only MPTP and arbutin.
Arbutin improves motor coordination in MPTP-treated mice
Decreased muscle strength and motor planning are key features of PD (Rozas et al., 1998) and can be measured in small experimental animals using the rotarod test. A decrease in the mean fall latency at various rotating speeds (5, 10,and 20 r/min) indicates the loss of motor coordination in animals. In this study, the administration of MPTP resulted in a significant (P< 0.001) decrease in the fall latency of mice compared with that in mice treated with vehicle, which indicated a reduction in the muscle strength and motor coordination of MPTP-treated mice. Arbutin (50 mg/kg)administration to MPTP-mice significantly enhanced the fall latencies (5 r/min,P< 0.01; 10 and 20 r/min,P< 0.001)compared with those in mice treated with MPTP alone. The administration of arbutin (100 mg/kg) or madopar in MPTPtreated mice resulted in significantly (P< 0.001) increased fall latencies compared with those in mice treated with MPTP alone. CGS21680 administration to mice treated with MPTP and arbutin significantly attenuated the effects of arbutin on fall latency, whereas the administration of forskolin to mice treated with MPTP and arbutin potentiated the (P< 0.05) the arbutin-induced increase in fall latency compared with those in mice treated with only MPTP and arbutin (Figure 4D-F).
Arbutin reduces the oxido-nitrosative stress in the brains of MPTP-treated mice
Exposure to MPTP significantly (P< 0.001) enhanced lipid peroxidation (TBARS) (Figure 5A) and total nitrite levels(Figure 5E) and decreased GSH contents and SOD and catalase activities (Figure 5B-D) in mouse brain tissues (ST and SN)compared with those in vehicle-treated mice. However, the administration of arbutin (50 and 100 mg/kg dose) to MPTPtreated mice attenuated the TBARS (P< 0.001) and nitrite(P< 0.05,P< 0.01) levels, and increased the GSH levels (P< 0.05,P< 0.001) and SOD (P< 0.05,P< 0.01) and catalase(P< 0.05,P< 0.01) activities compared with those in mice treated with MPTP alone. Madopar administration to MPTPtreated mice decreased the TBARS (P< 0.001) and nitrite (P<0.01) contents and increased the GSH contents (P< 0.01) and SOD (P< 0.01) and catalase (P< 0.001) activities compared with those in mice treated with MPTP alone. Interestingly,the administration of CGS21680 to mice treated with MPTP and arbutin significantly enhanced the TBARS (P< 0.001) and nitrite (P< 0.05) levels and reduced (P< 0.05) the levels and activities of the antioxidants (GSH, SOD, and catalase) in the ST and SN regions compared with those in mice treated with only MPTP and arbutin. The administration of forskolin to mice treated with MPTP and arbutin reduced TBARS levels (P< 0.001) and enhanced the GSH levels (P< 0.001) and SOD(P< 0.01) and catalase (P< 0.001) activities in the ST and SN compared with mice treated with only MPTP and arbutin. The results of this study indicated that arbutin was able to restrain oxido-nitrosative stress in brain regions associated with PD pathogenesis (Figure 5A-E). Furthermore, the activation of A2AR by CGS21680 inhibited the antioxidative effects of arbutin, whereas the increase in cAMP associated with forskolin administration markedly potentiated the effects of arbutin.

Figure 3|Arbutin (50 and 100 mg/kg) ameliorates the open field test parameters in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice.
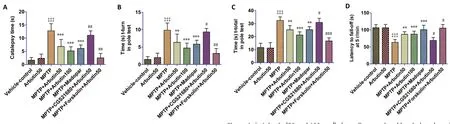

Figure 4|Arbutin (50 and 100 mg/kg) ameliorates the akinesia (catalepsy),bradykinesia (pole-test), and motor coordination (rotarod test) effects in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice.
Arbutin improves the dopamine, DOPAC, HVA, and GABA levels in the brains of MPTP-treated mice
PD is characterized by significant decreases in dopaminergic(Hartmann, 2004) and GABAergic signaling in the basal ganglia(Swamy et al., 2013; Prema et al., 2015). In the present study, MPTP treatment decreased (P< 0.001) the levels of dopamine, DOPAC, HVA, and GABA in the examined brain regions (ST and SN) of mice when compared with those in mice treated with vehicle. The administration of arbutin (50 and 100 mg/kg) in MPTP-treated mice enhanced the levels of dopamine (P< 0.05,P< 0.01), its metabolites (P< 0.01,P<0.001), and GABA (P< 0.01,P< 0.001) compared with those in mice treated with MPTP alone. Madopar administrationto MPTP-treated mice also improved the dopamine (P <0.01), DOPAC (P < 0.05), HVA (P < 0.01), and GABA (P < 0.05)levels in the ST and SN compared with those in mice treated with MPTP alone. The administration of CGS21680 to mice treated with both MPTP and arbutin significantly inhibited the arbutin-induced increase in dopamine (P < 0.05), DOPAC(P < 0.01), HVA (P < 0.01), and GABA (P < 0.05) levels in the ST and SN compared with mice treated with only MPTP and arbutin. Forskolin administration in mice treated with MPTP and arbutin increased the dopamine (P < 0.001), DOPAC (P< 0.01), HVA (P < 0.001), and GABA (P < 0.01) levels in the ST and SN compared with those in mice treated with only MPTP and arbutin. Therefore, arbutin appeared to enhance dopaminergic and GABAergic signaling in the brain, despite MPTP treatment, which could be decreased by CGS21680 and potentiated by forskolin (Figure 6).
Arbutin attenuates neuroinflammation in MPTP-treated mice
MPTP treatment resulted in a substantial increase (P < 0.001)in the TNF-α and NF-κB levels in the examined brain regions(ST and SN) of mice compared with those in mice treated with vehicle. The administration of arbutin (50 and 100 mg/kg)to mice treated with MPTP significantly attenuated the MPTPinduced increase in TNF-α (P < 0.01) and NF-κB levels (P <0.05, P < 0.001) compared with those in mice treated with MPTP alone. Madopar administration to MPTP-treated mice significantly attenuated the MPTP-induced increase in TNF-α(P < 0.05) and NF-κB levels (P < 0.01) compared with those in mice treated with MPTP alone. The administration of CGS21680 to mice treated with MPTP and arbutin enhanced the TNF-α and NF-κB levels (P < 0.05) in the ST and SN compared with those in mice treated with only MPTP and arbutin. Forskolin administration in mice treated with MPTP and arbutin decreased the TNF-α (P < 0.01) and NF-κB levels (P< 0.05) compared with those in mice treated with only MPTP and arbutin (Figure 7).
Arbutin attenuates neurodegeneration in the striatum of MPTP-treated mice


Figure 5|Arbutin (50 and 100 mg/kg) reduces the levels of oxido-nitrosative stress in the striatum and substantia nigra brain regions of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice.

Figure 6|Arbutin (50 and 100 mg/kg) enhances the levels of neurotransmitters in the striatum and substantia nigra brain structures of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice.
The ST region of the mouse brain was subjected to histopathology analysis. Mice treated with vehicle or arbutin alone showed no signs of neurodegeneration, with normal brain tissue architecture (Figure 8AandB). The administration of MPTP caused neurodegenerative changes in the ST, which were characterized by the blebbing of the plasma membrane and chromatin condensation (Figure 8C). The administration of arbutin or madopar to MPTP-treated mice significantly attenuated the MPTP-induced neurodegenerative changes in the ST (Figure 8D-F). The administration of CGS21680 to mice treated with MPTP and arbutin attenuated neuroprotective effects of arbutin, whereas forskolin administration synergized these effects (Figure 8GandH).

Figure 7|Arbutin (50 and 100 mg/kg) decreases inflammation in the striatum and substantia nigra brain regions of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice.

Figure 8|Arbutin (50 and 100 mg/kg) improves the histopathology in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice(hematoxylin and eosin stain, original magnification, 100×).
Discussion
The mutual inhibitory signaling between A2ARs and dopamine receptors adversely affects the motor circuit and acts on both direct (monosynaptic GABA-regulated inhibitory pathway) and indirect (polysynaptic excitatory) pathways in the basal ganglia(Cieślak et al., 2008). The antagonistic A2AR-D1/D2 receptor heterodimeric complex in the basal ganglia has been shown to inhibit D1-mediated inhibitory GABAergic transmission(resulting in an excitatory effect on the direct pathway) or and to activate the D2-mediated indirect pathway (resulting in an inhibitory effect). The subsequent loss of inhibitory control over thalamocortical projection neurons results in motor dysfunctions (Kulisevsky and Poyurovsky, 2012) and the excitotoxic degeneration of dopaminergic neurons (Saransaari and Oja, 2005). Existing evidence suggests that thein vivogeneration of MPP+from MPTP by MAO-B causes the selective degeneration of dopaminergic nigrostriatal neurons (Huang et al., 2017). Decreased dopamine levels enhance the inhibitory influence of A2ARs on the existing dopamine nigrostriatal receptors, which aggravates the typical PD symptoms (Mori and Shindou, 2003). Furthermore, pre-clinical studies reported improved locomotor activity following the administration of A2Aantagonists (e.g., KW 6002), further supporting the ability to use the MPTP model to screen putative anti-PD drugs that may act via A2ARs (Shiozaki et al., 1999; Cieślak et al., 2008). The prevention of excitotoxic signaling and the inhibition of the AMPK-autophagy pathway associated with arbutin treatment suggests that arbutin may be able to provide PD symptom relief and indicates the potential role played by A2ARs in PD pathology (Ahmadian et al., 2019; Ding et al., 2020). Several studies have suggested that arbutin can modulate brain functions (Dadgar et al., 2018). Hydroquinone(Ha Park et al., 2016) and its derivatives, including arbutin,can penetrate through biological barriers, such as the plasma membrane (Gallo et al., 2015) and the blood-brain barrier,when administered either orally or systemically. The present findings indicated that arbutin protected dopaminergic nigrostriatal neurons and attenuated the MPTP-induced PDlike symptoms in mice (e.g., akinesia, bradykinesia, loss of motor coordination, postural imbalance, and reduced muscle strength). Interestingly, we observed that the activation of A2ARs by CGS21680 attenuated the anti-PD activities of arbutin in the MPTP model, whereas the activation of adenylyl cyclase-cAMP by forskolin enhanced these effects.
MPTP treatment induces mitochondrial dysfunction,particularly at complex I of the electron transfer chain,which causes energy failure and the generation of highly reactive species (e.g., oxygen, nitrite radicals, and aldehydes)(Huang et al., 2017), which can have deleterious effects on nigrostriatal dopaminergic neurons (Dauer and Przedborski,2003). MDA, 4-hydroxy 2-nonenal, and isoprostanes are highly immunogenic, lipid-peroxidizing, neurotoxic aldehydes that form complex bimolecular aggregates, deplete endogenous antioxidants, and disrupt neuronal membrane integrity (Guo et al., 2018). In the present study, the administration of MPTP considerably enhanced oxido-nitrosative stress in the brains (SN and ST) of mice. MPTP increased lipid peroxidation(TBARS) and nitrite levels and decreased the levels and activities of endogenous antioxidants, such as GSH, SOD,and catalase. The MPTP-induced increase in oxidative stress was effectively abolished, and the endogenous antioxidant levels were enhanced by the administration of arbutin (50 and 100 mg/kg) in MPTP-treated mice. The antioxidant effects demonstrated by arbutin (100 mg/kg) were similar to those of the standard PD-treatment drug (madopar) used in this study. The presence of five hydroxyl (-OH) groups in arbutin confers a robust free radical scavenging property.The classical structure-activity relationship theory directly correlates the number of oxidizable -OH groups in a molecule with its free radical scavenging efficacy. In previous studies,arbutin showed excellent 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activities because one molecule of arbutin can quench five radicals (Takebayashi et al., 2010). Recently reported data have reported that A2AR antagonists (e.g., SCH-58261, ZM-241385, KD-64, caffeine) protect against reactive oxygen species, nitric oxide synthase, cyclo-oxygenase, and microglia-mediated inflammation (Paterniti et al., 2011;Borea et al., 2018; Colella et al., 2018; Aires et al., 2019;Kotańska et al., 2020). A substantial increase in the expression of A2ARs in the basal ganglia neurons (Varani et al., 2010),commensurate with the detection of chronic oxidative stress and inflammation, has been observed in response to noxious stimuli, such as MPTP or 6-hydroxydopamine (Schwarzschild et al., 2006; Shen and Chen, 2009). The activation of A2ARs antagonizes the D2 receptor-mediated suppression of Ca2+influx through L-type voltage-dependent Ca2+channels (Shen and Chen, 2009). Excess intracellular Ca2+in the basal ganglia triggers the release of stored Ca2+from the endoplasmic reticulum, resulting in mitochondrial Ca2+overload, which triggers the opening of the mitochondrial membrane permeability transition pore, the release of cytochrome c,and the activation of apoptotic cell death pathways (Görlach et al., 2015). In agreement with these earlier findings, the administration of CGS21680 (A2AR agonist) one hour before behavioral studies in this study antagonized the antioxidative effects of arbutin, whereas the administration of forskolin(adenylyl cyclase agonist) potentiated the effects of arbutin in MPTP-treated mice. The literature reports have indicated that the activation of A2ARs by CGS21680 enhances the production of free oxygen and nitrogen radicals in brain tissues (Saura et al., 2005), whereas the activation of adenylyl cyclase by forskolin reduces these oxidative effects (Mehan et al., 2017).Free radicals and associated events (e.g., peroxidation of biomolecules, formation of neurotoxic aldehydes) activate the NF-κB-mediated transcription of pro-inflammatory cytokines(e.g., TNF-α, interleukins, nitric oxide synthase, and cyclooxygenase-2) in the striatum (Yan et al., 2014). Evidence has suggested that during the early prodromal phase of PD,the NF-κB-triggered inflammation initiates the retrograde degeneration of nigral dopaminergic neurons (Bellucci et al., 2020). The increased expression of pro-inflammatory cytokines, microglial activation, and the extravasation of T-cells (CD8+and CD4+) in the SN are consistent with PD pathogenesis (Tufekci et al., 2012; Zaitone et al., 2012). TNF-α activates pathways that trigger apoptosis and necrotic cell death (via caspases and p53), resulting in the gradual loss of dopaminergic innervations from the SN pars compacta to the ST. In the present study, MPTP significantly enhanced the TNF-α and NF-κB levels in the brains of mice. These findings support the findings of earlier studies, which showed the MPTP-induced activation of NF-κB, nitric oxide synthase,cyclo-oxygenase-2, and microglia (Khan et al., 2013) and the increased expression of pro-inflammatory cytokines and pro-apoptotic factors (e.g., Bax and Bad) in the SN and ST regions of mice (Meredith and Rademacher, 2011).However, the administration of arbutin (50 and 100 mg/kg)or madopar in MPTP-treated mice for 7 days decreased the TNF-α and NF-κB levels. Interestingly, CGS21680 abolished the anti-inflammatory activities of arbutin in MPTPtreated mice, whereas forskolin synergized these effects.The neuroprotective activity of arbutin was supported by histopathology studies. MPTP treatment caused the marked neurodegeneration of the ST regions in the brains of mice,which could be prevented by treatment with arbutin.CGS21680 attenuated the neuroprotective effects of arbutin in MPTP-treated mice, whereas forskolin enhanced these effects.
The levels of dopamine, its metabolites (e.g., DOPAC and HVA), and GABA were also evaluated in the SN and ST brain regions to assess the effects of arbutin on dopaminergic and GABAergic transmissions in MPTP-treated mice. In PD, the A2AR-mediated decrease in D2 receptor activation downregulates GABAergic inhibitory neurotransmission in the globus pallidus and SN, which triggers motor symptoms,such as gait abnormality, postural imbalance, rigidity, and tremors (Kulisevsky and Poyurovsky, 2012). The appearance of non-motor symptoms in PD has been attributed to a decrease in the GABA contents of the brain (Błaszczyk, 2016).In the current research, the administration of MPTP caused a significant decline in the dopamine, DOPAC, HVA, and GABA levels in the SN and ST regions. MPTP has also been shown to decrease dopaminergic and GABAergic signaling in the basal ganglia. In several previous studies, an increase in locomotor function following treatment with A2AR antagonists suggested control over MPTP-mediated dopaminergic loss (Shen and Chen, 2009). In the present study, the administration of arbutin or madopar to MPTP-treated mice significantly elevated the dopamine, DOPAC, HVA, and GABA levels compared with untreated mice treated with MPTP alone.The antioxidant and anti-inflammatory activities of arbutin(Dadgar et al., 2018) might be responsible for the protection of dopaminergic neurons against MPTP toxicity. Subsequently,GABA activity is enhanced due to the dopamine receptormediated increase in the firing rate of GABAergic neurons in the striatum (Schwarzschild et al., 2006). However, CGS21680 decreased the arbutin-induced increases in dopamine, DOPAC,HVA, and GABA levels in MPTP-treated mice, whereas forskolin treatment further enhanced these levels. Previous findings also indicate that A2AR transmission can inhibit dopaminergic and GABAergic signaling (Saransaari and Oja, 2005; Cieślak et al., 2008).
The biochemical findings supported the results of the behavioral studies. MPTP caused a significant increase in PD-like motor symptoms in mice, including the decreased performance in the OFT and reduced muscle strength and motor coordination, as measured by the rotarod test. MPTP triggered akinesia and bradykinesia in mice, as demonstrated by the increased cataleptic score and the t-turn and t-total times. The MPTP-induced motor dysfunction was effectively attenuated by treatment with arbutin (50 and 100 mg/kg). As expected, madopar also attenuated the MPTP-induced motor deficits in mice. However, CGS21680 antagonized the activities of arbutin, whereas forskolin potentiated the effects of arbutin in MPTP-treated mice. Although the A2AR-cAMP pathway is involved in a wide range of physiological activities (Pleli et al., 2018), in this study, the activation of A2ARs was found to aggravate the MPTP-induced PD-like pathology in mice,whereas forskolin (adenylyl cyclase-cAMP agonist) potentiated the anti-PD effects of arbutin. Although A2AR activation typically increases the activity of the adenylyl cyclasecAMP pathway, D2 receptors typically inhibit this pathway(Shen and Chen, 2009); however, in the present study,increased adenylyl cyclase-cAMP activity attenuated motordysfunction, which indicated a differential role for cAMP in the pathogenesis of PD. Although the absorption of arbutin from the gastrointestinal tract is excellent, the potential generation of D-glucose and hydroquinone by intestinal microflora under acidic conditions may limit the dose that can be administered orally. The systemic administration of arbutin avoids the potential for free hydroquinone production, with fewer side effects compared with oral administration (Schindler et al.,2002; de Arriba et al., 2013).
Data obtained from human studies have revealed that in addition to the loss of dopaminergic neurons in the SN,several other neuronal types (e.g., adrenergic, cholinergic,glutamatergic, serotonergic, and GABAergic) are also adversely affected by the progression of PD (Hartmann, 2004). Significant increases in the levels of oxidative stress biomarkers (e.g.,8-hydroxyguanosine, carbonyl, 4-hydroxy 2-nonenal, and MDA) and inflammatory markers (e.g., TNF-α, interleukin 1,interleukin 1β, interleukin 6, and interferon γ) and decreased GSH-dependent antioxidants in the SN region and cerebrospinal fluid have consistently been observed in PD patients (Farooqui and Farooqui, 2011). These findings indicated that oxidative and inflammatory insults are the primary factors that contribute to the degeneration of dopaminergic neurons and the formation of toxic protein aggregates in the brain. Abnormalities in the ubiquitin-proteasome and autophagy-lysosome pathways may hamper the removal of misfolded proteins, such as α-synuclein,from the brain (Toulorge et al., 2016). In this study, arbutin ameliorated the motor functions in MPTP-treated mice by protecting dopaminergic neurons against oxido-nitrosative and inflammatory damage, in addition to restoring the levels of dopamine and GABA transmitters in the striatum. The therapeutic benefits of arbutin in the MPTP model can be extended to the other models of PD as several studies have reported that chronic exposure to chemicals such as MPTP,rotenone, paraquat (herbicide), and maneb (fungicide) can initiate a vicious cycle of self-replenishing neurotoxins (e.g.,free radicals, lipid peroxidation products, and pro-inflammatory cytokines) that specifically decrease the dopaminergic neuron density in the striatum (Sherer et al., 2003; Meredith et al.,2008; Prema et al., 2015) and initiate Lewy body pathology(Jagmag et al., 2015). Therefore, the findings of the present study revealed that arbutin targeted multiple neurotoxic events that suggest that arbutin may be suitable as an anti-PD drug, with the potential to enhance therapeutic outcomes in PD patients. Arbutin has been reported to modulate several pathways (e.g., AMPK, nuclear factor erythroid 2-related factor 2/heme oxygenase-1, Wnt/β-catenin, and Sirt1/NFκBp65), biomolecules (e.g., free radicals, TNF-α,interleukins, cardiac troponin-I, glial fibrillary acidic protein,α-glucosidase, α-amylase, and tyrosinase), and cell activities(e.g., autophagy, myelination, proliferation, and differentiation),which ameliorate brain (Dadgar et al., 2018), bone (Man et al.,2019), cardiac (Zhang et al., 2019), retinal (Ebrahim-Tabar et al.,2020), lung (Ye et al., 2019), hepatic (Mirshahvalad et al., 2016),and metabolic functions (Yousefi et al., 2013). Furthermore,previous studies have indicated that arbutin can alleviate the pathogenic events associated with a variety of toxic molecules,such as cyclosporine (Khadir et al., 2015), MPTP (Dadgar et al.,2018), streptozotocin (Dastan et al., 2019), carbon tetrachloride(Mirshahvalad et al., 2016), lysolecithin (Ebrahim-Tabar et al.,2020), γ-radiation (Nadi et al., 2016), lipopolysaccharide (Zhang et al., 2019), pentylenetetrazol (Ahmadian et al., 2019), and rotenone (Ding et al., 2020). These findings have suggested that arbutin can fulfill the characteristics of a multi-target drug; however, additional investigations (e.g., receptor-binding studies) remain necessary to correlate these relationships.Furthermore, the present study indicated that A2AR activation and the adenylyl cyclase-cAMP pathways might be involved in the mitigation of PD-like symptoms following arbutin treatment in the MPTP mouse model. However, additional studies (e.g.,radioligand binding assays) remain necessary to elucidate the anti-PD mechanism of arbutin and the involvement of adenosine receptors.
In this study, the anti-PD effects observed in the MPTP mouse model can be attributed to the protective effects of arbutin on the existing neurons. The extent of neurodegeneration tends to be high by the time that clinical symptoms manifest in PD patients. However, the present results suggest that the therapeutic effects of arbutin might be correlated with the upregulation of dopamine and GABA levels, in addition to antioxidant and anti-inflammatory effects in the ST and SN regions of the brain. Arbutin treatment might be able to rescue neuronal viability before irreversible damage occurs in the basal ganglia. The findings of the present study favor the pro-survival and regenerative capabilities of arbutin, which will be evaluated in future studies. In this study, CGS21680 and forskolin were used as pharmacological interventions to explore the influence of A2ARs and cAMP on the effects of arbutin on PD symptoms. Interestingly, we observed the involvement of A2AR-cAMP in the anti-PD effects of arbutin in the MPTP mouse model. However, further studies are required to establish the direct associations between the A2AR-cAMP pathway and the therapeutic effects of arbutin against PD.
In summary, arbutin improved various motor functions,including posture, movement, and rigidity in MPTPtreated mice. Arbutin exhibited potent antioxidant and anti-inflammatory activities and was able to restore the neurotransmitter levels (e.g., dopamine and GABA) in the striatum and protect neurons against degeneration. The findings of this study indicated that the inhibition of A2ARs and increased adenylyl cyclase-cAMP activity in the brain might be involved in the observed therapeutic benefits associated with arbutin treatment in the MPTP model of PD. Arbutin can be used as an alternative or co-adjuvant drug in the therapy of PD.
Acknowledgments:The authors are thankful to the management of SwiftSchool of Pharmacy, Ghaggar Sarai (Rajpura) for providing the necessary research facilities.
Author contributions:Study conception and design: MK, ZY; literature search:JZ, JS; experiment implementation: JS; data analysis: MK, JZ; manuscript draft:MK. All authors read and approved the manuscript.
Conflicts of interest:None declared.
Financial support:None.
Institutional review board statement:The study protocol was approved by the Institutional Animal Ethics Committee (1616/PO/Re/S/12/CPCSEA)on November 17, 2019 with approval No. IAEC/2019/010. All the animal experimentation was performed as per instructions of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA),Government of India, New Delhi.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders
- The role of gap junctions in cell death and neuromodulation in the retina
- Don’t know what you got till it’s gone: microglial depletion and neurodegeneration
- Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia
- Protein post-translational modifications after spinal cord injury
- Axonal mRNA localization and local translation in neurodegenerative diseases

