Non-coding RNAs and stem cells: the dream team for neural regeneration in Parkinson’s disease?
Shubhra Acharya, Andrew I. Lumley, Yvan Devaux
Parkinson’s disease (PD) is a widely spread neurodegenerative movement disorder,affecting approximately 10 million people worldwide. It is primarily caused by the loss of dopaminergic neurons in the substantia nigra, which causes decreased secretion of dopamine leading to tremors, bradykinesia a n d r i g i d m u s c l e m o ve m e nt. T h e development of PD is complex and needs to be better understood. Current treatment strategies primarily involve targeting disease symptoms, however, since there is a continuous loss of dopaminergic neurons in the brain, PD appears to be incurable.Moreover, treatment strategies often carry severe side effects related to dopamine production, where too little or too much can cause debilitating issues such as dyskinesia.The pool of neural stem/progenitor cells(NSCs) located in sub-ventricular zone and hippocampal dentate gyrus, proliferate and are responsible to give rise to neurons and glia in response to any cellular damage.Though this activation of NSCs is highly regulated, it is insufficient to overcome the loss of dopaminergic neurons in PD. In this line, non-coding RNAs (ncRNAs) are involved in the underlying mechanisms of PD and are known to have important functional roles in neural regeneration (Acharya et al., 2020).Thus, the study of ncRNAs in NSC activation and adult neurogenesis post PD development is an extremely attractive area of research with significant clinical application potential.In 2006, Takahashi and Yamanaka discovered a remarkable method to be able to reprogram adult somatic cells back to the pluripotent state and called these cells induced pluripotent stem cells (iPSCs). iPSCs overcome the technical, ethical and religious challenges involved in use of embryonic stem cells (ESCs) and in addition carry ESClike properties. Since then, iPSCs have been differentiated into diverse cell types for the modelling and study of various disease states.iPSCs also promise to fill the gap between human and animal models in delineating potential mechanisms and innovative therapeutic strategies for neurodegenerative diseases, including PD. Studying brain disorders proved challenging for decades,mainly due to poor translatability of findings from clinical models to humans. Since PD is a polygenic disorder, by using different growth factors, iPSCs derived from patient’s somatic cells can be differentiated to neurons and glial cells that carry the exact genetic information/mutations present in the patients. These easily accessible ‘benchtop’neurons also follow the human brain development pattern, electrophysiological properties making them useful preclinical models to study age-related diseases such as PD. A subpopulation of PD patients carry mutations in the PD-related gene leucinerich repeat kinase 2 (LRRK2). Interestingly,it has been observed that iPSC and neurons derived from these patients are more sensitive to oxidative stress than the control neurons, illustrating the presence of a PD phenotype in these neurons (Nguyen et al.,2011). A recent preclinical study showed that functionally pure dopaminergic neuron populations derived from clinical grade human iPSCs were safe for transplantation in rats and improved their abnormal behavior induced by 6-OHDA (Doi et al., 2020). Thus,iPSC-derived neuronal models are being used in multiple pre-clinical transplantation trials to study the mechanisms underlying PD. However, more research is needed to improve their use and efficacy towards innovative treatment strategies.
The role of ncRNAs has been widely studied in many diseases, such as cancer,cardiovascular disease and in neurological and developmental studies. Non-coding RNAs are RNA molecules without known protein coding potential. They have been classified in two main categories based on their length: small ncRNAs (microRNAs,small nuclear RNAs, small nucleolar RNAs,tRNA derived fragments and Piwi-interacting RNAs) are shorter than 200 nucleotides and long ncRNAs (lncRNAs, including both linear and circular forms) comprise more than 200 nucleotides. While both categories regulate gene expression, microRNAs (miRNAs) ̶ the better characterized small ncRNAs ̶ downregulate messenger RNAs through base pairing (Beermann et al., 2016) and lncRNAs act at different epigenetic levels and through distinct molecular mechanisms (Devaux et al., 2015). Through their ability to regulate many different genes, lncRNAs are major players in physiological as well as pathological events. Several studies have highlighted that lncRNAs are implicated in adult neurogenesis. Long intergenic ncRNA TUNA,for example, has been shown to promote neural differentiation of mouse ESCs and causes locomotive defects in zebrafish (Lin et al., 2014). Similarly, increased expression of lncRNA brain-derived neurotrophic factor antisense (BDNF-AS) is associated with neuronal survival and growth (Modarresi et al., 2012). Another lncRNA, LncND, was found to be highly expressed in radial glia of the ventricular and sub-ventricular zones in human developing brain and plays a role in proliferation of these cells via miR-143-3p(Rani et al., 2016). In this way LncND could be a promising target to study the activation of endogenous stem cells. A recent study in rats subjected to focal cerebral ischemia showed that downregulation of H19 led to the suppression of proliferation and differentiation of NSCs, causing a reduction of motor and cognitive function (Fan et al., 2020). The effect on neurogenesis was shown to be via the H19/miR-675/TGF-β1 axis (Fan et al., 2020). This study highlights the protective actions of lncRNA H19 by fostering neurogenesis and limiting the development of cognitive disabilities.Similarly, lncRNA NEAT1-miR124-Wnt/β beta catenin signaling axis was shown to positively regulate neuronal differentiation, migration of spinal cord neural progenitor cells and recovery of locomotion deficits in mice(Cui et al., 2019). Encouragingly, strategies to deliver ncRNAs in patients using lipid or nanoparticle-based approaches have been developed. This bodes well for future novel therapies centered on these RNA molecules.Taken together, these studies demonstrate the important roles that ncRNAs,especially miRNAs and lncRNAs, can play in neurogenesis following brain disorders.This line of work should be extended to encompass ncRNAs in PD pathogenesis and progression.
To study and discover ncRNAs paramount to neurogenesis in PD, several public datasets from different sources associated with PD are already available and can be used to mine innovative candidates (Acharya et al., 2020).Once the candidate ncRNA is identified it is then important to study its functional role by knock-in and knock down trials.Fluorescent in situ hybridization can be used in order to determine cellular localization of candidate ncRNAs and to direct downstream silencing or overexpression experiments.For such work, powerful cutting-edge gene editing technologies such as CRISPR Cas9 could be harnessed for effective silencing or overexpression of ncRNAs, as previously demonstrated (Zhang et al., 2018; Fan et al., 2020). The overriding goal of such work would be to drive neurogenesis through ncRNA-mediated creation of new, or protection of pre-existing, neurons in the brain of PD patients. Therefore, there is hope that ncRNAs may be developed as either drug or drug targets for synchronizing the level of neurons for the treatment of PD.
To summarize, novel treatment strategies are required to enhance patient outcome and quality of life following PD diagnosis.Endogenous NSCs become impaired following PD onset and subsequently lose the ability of reactivation and self-renewal.IPSCs enable groundbreaking insights into how different populations of cells in the human body react during disease states.In this way, these cells represent the ideal platform to study disease progression as well as being able to provide feedback on the efficacy of novel treatment strategies.NcRNAs, which include miRNAs and lncRNAs,have been widely studied in human diseases.Novel research highlights their ability to act as tools, which can be manipulated to enable neurogenesis through their neuroprotective actions. Thus, ncRNAs may firstly be used to activate the endogenous NSCs to produce neurons and secondly, to prevent the apoptosis of mature neurons overcoming the initial loss in PD patients. This approach can also merit over the traditional dopamine recovery therapy as it will help overcome the initial loss of dopamine-producing neurons and in prevention of mature neurons from apoptosis, thereby targeting the root cause of the dopamine imbalances in the PD brain.We have the platform. We have the tools.The creation of a so called ‘dream team’combining and harnessing the respective strengths of ncRNAs and iPSCs might just be able to turn the tide in the battle against PD(Figure 1).
This work is supported by COST (European Cooperation in Science and Technology)
Action EU-CardioRNA CA17129, the National Research Fund of Luxembourg (grants # C14/BM/8225223, C17/BM/11613033 and AFR
14566210), the Ministry of Higher Education and Research of Luxembourg, and the Heart Foundation—Daniel Wagner.
Shubhra Acharya, Andrew I. Lumley,Yvan Devaux*
Cardiovascular Research Unit, Department of Population Health, Luxembourg Institute of Health,L-1445 Strassen, Luxembourg (Acharya S,Lumley AI, Devaux Y)
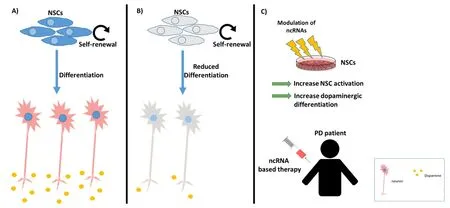
Figure 1|ncRNAs and NSCs - the dream team in the fight against PD?
Faculty of Science, Technology and Medicine,University of Luxembourg, L-4365 Esch-sur-Alzette,Luxembourg (Acharya S)
*Correspondence to:Yvan Devaux, PhD,yvan.devaux@lih.lu.https://orcid.org/0000-0002-5321-8543(Yvan Devaux)
Date of submission:September 22, 2020
Date of decision:November 27, 2020
Date of acceptance:December 25, 2020
Date of web publication:February 19, 2021
https://doi.org/10.4103/1673-5374.308090
How to cite this article:Acharya S, Lumley AI,Devaux Y (2021) Non-coding RNAs and stem cells: the dream team for neural regeneration in Parkinson’s disease? Neural Regen Res 16(10):2017-2018.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders
- The role of gap junctions in cell death and neuromodulation in the retina
- Don’t know what you got till it’s gone: microglial depletion and neurodegeneration
- Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia
- Protein post-translational modifications after spinal cord injury
- Axonal mRNA localization and local translation in neurodegenerative diseases

