Comparison of early changes in ocular surface markers and tear inflammatory mediators after femtosecond lenticule extraction and FS-LASlK
Chi Zhang, Hui Ding, Hong He, He Jin, Liang-Ping Liu, Xiao-Wei Yang, Jun Yang, Xing-Wu Zhong,
1Huaxia Eye Hospital of Foshan, Huaxia Eye Hospital Group, Foshan 528000, Guangdong Province, China
2Zhongshan Ophthalmic Center and State Key Laboratory of Ophthalmology, Sun Yat-sen University, Guangzhou 510000, Guangdong Province, China
3Hainan Eye Hospital and Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Haikou 570311, Hainan Province, China
Abstract
● AlM: To compare the short-term impacts of femtosecond lenticule extraction (FLEx) and femtosecond laser-assisted laser in situ keratomileusis (FS-LASIK) on ocular surface measures and tear inflammatory mediators.
● METHODS: This prospective comparative nonrandomized clinical study comprised 75 eyes (75 patients). Totally 20 male and 15 female patients (age 21.62±3.25y) with 35 eyes underwent FLEx, and 26 male and 14 female patients (age 20.18±3.59y) with 40 eyes underwent FS-LASIK. Central corneal sensitivity, noninvasive tear breakup time, corneal fluorescein staining, Schirmer I test, tear meniscus height, and ocular surface disease index were evaluated in all patients. Tear concentrations of nerve growth factor (NGF), interleukin-1α (IL-1α), transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and matrix metalloproteinase-9 (MMP-9) were assessed by multiplex antibody microarray. All measurements were performed preoperatively, and 1d, 1wk, and 1mo postoperatively.
● RESULTS: Patients who underwent FLEx exhibited a more moderate reduction in central corneal sensation and less corneal fluorescein staining than those in the FS-LASIK group 1wk after the procedure (P<0.01). NGF was significantly higher 1d and 1wk after surgery in the FS-LASIK group than in the FLEx group (P<0.01). By contrast, compared to those in the FLEx group, higher postoperative values and slower recovery of tear TGF-β1, IL-1α, and TNF-α concentrations were observed in the FS-LASIK group (P<0.01). Tear concentrations of NGF, TGF-β1, TNF-α, and IL-1α were correlated with ocular surface changes after FLEx or FS-LASIK surgery.
● CONCLUSlON: There is less early ocular surface disruption and a reduced inflammatory response after FLEx than after FS-LASIK. NGF, TGF-β1, TNF-α, and IL-1α may contribute to the process of ocular surface recovery.
● KEYWORDS: femtosecond lenticule extraction; femtosecond laser-assisted laser in situ keratomileusis; tear inflammatory mediators; ocular surface
INTRODUCTION
The femtosecond (FS) laser, a vital technological advancement in medical care, has been successfully used in refractive surgery for the past decade. Femtosecond laser-assisted laserin situkeratomileusis (FS-LASⅠK) is considered one of the most successful surgeries for correcting refractive errors based on its excellent cutting accuracy and minimal complications. FS-LASⅠK involves flap creation using the FS laser and stromal ablation using an excimer laser[1]. Recently, a breakthrough in FS laser technology has generated a novel alternative refractive procedure called refractive lenticule extraction (ReLEx), which can be further described as femtosecond lenticule extraction (FLEx) or smallincision lenticule extraction (SMⅠLE based on how the lenticle is removed; the former involves creating and lifting corneal flaps followed by the lenticule extraction, while in SMⅠLE, the lenticle is removed directlyviaa small incision)[2]. Meanwhile, the FLEx procedure is similar to the LASⅠK procedure: both flap and lenticule creation are performed with the FS laser, and the flap is repositioned after stripping away the exposed lenticule[3].
The most common complaint after LASⅠK is dry eye, even in patients who had no symptoms prior to surgery. Dry eye has been associated with ocular surface disruption[4]. Although the etiology of dryness is not clearly understood, the damage to corneal nerves resulting from flap formation and stromal ablation is considered a primary cause of ocular surface instability[5]. The corneal nerve injury leads to abnormalities in the blinking reflex, epithelial barrier function, stability, and tear composition[6]. Ⅰn addition, the postoperative release of inflammatory mediators may contribute to symptoms of dry eye after surgery. Previousin vivoandin vitroresearch has revealed that the release of various cytokines, growth factors, and chemokines may be involved in corneal wound healing and ocular surface homeostasis[7].
The main difference between FLEx and FS-LASⅠK is that only one type of laser is used during the entire FLEx process. By contrast, an excimer laser is used for stromal ablation in FSLASⅠK. The FS laser, unlike the excimer laser, is not absorbed by corneal tissue, allowing for a shorter pulse duration and lower fluence threshold for breakdown, which results in minimal damage to the cornea. This is the biggest advantage of FLEx over FS-LASⅠK. Therefore, we propose the hypothesis that FS-LASⅠK will result in more significant changes to the postoperative ocular surface and will induce a larger increase in inflammatory mediator release compared to FLEx. Previous research has partially supported this hypothesis. FLEx has been shown to produce better refractive predictability, contrast sensitivity, and corneal sensation than FS-LASⅠK[3]. Less extracellular matrix deposition and inflammatory cytokine release were also observed in a rabbit model after FLEx compared to that observed after FS-LASⅠK[8]. However, the effects of both surgeries on human ocular surface parameters and tear inflammatory mediator release remain unknown.
To test our hypothesis, we compared ocular surface changes and tear inflammatory mediator responses, as well as the correlation between them, after FLEx and FS-LASⅠK.
SUBJECTS AND METHODS
Ethical ApprovalThis study adhered to the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Hainan Eye Hospital, Zhongshan Ophthalmic Center of Sun Yat-sen University. All subjects provided informed consent before their inclusion in this research.
MethodsA prospective, comparative, and nonrandomized clinical study was conducted at the Department of Refractive Surgery, Hainan Eye Hospital, Zhongshan Ophthalmic Center of Sun Yat-sen University (ClinicalTrials.gov identifier, NCT02550353). Thirty-five subjects scheduled for bilateral FLEx and 40 age-, sex-, spherical equivalent (SE)-, and central corneal thickness-matched subjects scheduled for bilateral FSLASⅠK were prospectively recruited. The inclusion criteria were planned FLEx or FS-LASⅠK surgery, the ability to give informed consent, and a willingness to participate in this trial. We excluded patients with unstable refraction, a history of corneal trauma or past surgery, and a systemic disease such as connective tissue diseases or diabetes. One experienced surgeon performed all procedures.
Surgical TechniqueThe 500 kHz VisuMax FS laser system (Carl Zeiss Meditec AG, Jena, Germany) was used to perform the FLEx procedure, as described by Gaoet al[9]. The four incisions were made with a 140 nJ FS laser using following techniques. The posterior surface of the lenticule was scanned initially, setting it at 6.5 mm in diameter. Then, the lenticule border was made, followed by the anterior surface scanning. The flap was extended to form and was designed to be 120 μm thick and 7.5 mm in diameter with a superior hinge and 50° in cordal length. The spot and tracking spacing were set at 4.5 μm for the lenticule and 2.0 μm for the side cut. After completion of the laser sculpture, the flap was flipped over, and the refractive lenticule was removed subsequently with forceps. Then, the flap was carefully repositioned.
The FS-LASⅠK surgery was performed using the VisuMax FS system (Carl Zeiss Meditec AG, Jena, Germany) for corneal flap creation with an energy of 140 nJ at a 500 kHz repetition rate. The size of the target flap was 120 μm thick and 8.0 mm in diameter with a standard 90° hinge and 50° side-cut angles. The stromal ablation was performed using the Allegretto WaveLight EX500 excimer laser (WaveLight GmbH, Germany) with 1.6 mJ pulse energy at a 250 kHz repetition rate.Postoperative topical treatment was identical for all eyes: dexamethasone 0.1%/tobramycin 0.3% (TobraDex, Alcon, USA) and levofloxacin 0.5% (Cravit, Santen, Japan) eye drops were applied four times daily for 1wk. Sodium hyaluronate 0.1% (Hycosan, Ursapharm, Germany) was administered four times a day for 1mo.
Ocular Surface MeasurementDry eye disease was assessed using the Ocular Surface Disease Ⅰndex (OSDⅠ; Allergan Ⅰnc., Ⅰrvine, CA). The assessment was composed of twelve questions in three groups, ocular symptoms of dry eye disease, vision-related ocular symptoms, and environmental factorinduced ocular symptoms. The OSDⅠ is graded on a scale from 0 to 4 in which 0 represents none of the time and 4 indicates all of the time. Responses to all questions were collected to generate a composite OSDⅠ score that ranged from 0 to 100, with higher OSDⅠ scores representing greater disability. The information on dry eye symptoms was collected before surgery and 1d, 1wk, and 1mo after surgery.
Noninvasive tear break-up time (NⅠ-TBUT) was assessed for each subject using the Keratograph 5M (Oculus, Wetzlar, Germany). After reconstructing the tear film with two blinks, the subjects were guided to fixate on the center of the instrument and avoid blinking. A real-time video was recorded of the location of the tear film breaks until the next blink. Two values were provided during the assessment, the first break-up time (NⅠ-TBUT) and the average break-up time, but only NⅠ-TBUT was used for analysis. All the tests were performed three times under the same temperature and humidity conditions.
Tear meniscus height measurement was also conducted using the scaling system of the Keratograph 5M. The lower tear meniscus height was measured at the center of the lid margin on the captured ocular image. The average value from three repeated measures was recorded.
The Schirmer Ⅰ test (SⅠT) was performed to evaluate basal and reflex tear secretion. Ⅰn this test, a 30 mm sterile Schirmer tear test strip (Jingming, Tianjin, China) was placed at the junction of the middle and lateral thirds of the lower eyelid without anesthesia for 5min. After the strip was removed, the amount of wetting was measured in millimeters, with lower scores indicating less tear secretion.
Corneal fluorescein staining assessment using the corneal grading scale was performed after dividing the cornea into five zones, inferior, superior, temporal, nasal, and central. Superficial staining of each corneal zone was scored from 0 to 3, where 0 indicated no dots in the cornea, 1 indicated 1 to 5 dots, 2 indicated 5 to 10 dots, and 3 indicated >10 dots or detection of filamentous staining. The summed score of all corneal zones for each eye was recorded (range: 0-15).
Central corneal sensitivity (CCS) was assessed with a Cochet-Bonnet esthesiometer (Luneau Ophthalmologie Chartres, Cedex, France). Ⅰt consists of a nylon monofilament with an adjustable length of 60 mm and diameter of 0.12 mm. The instrument was applied perpendicular to the central surface of the cornea. Beginning from 60 mm, the length of the filament was sequentially reduced in 5 mm steps until the patient could feel the filament. Three tests were performed, and the average measurement was recorded.
To measure the inflammatory mediator levels, tear samples were collected from the inferior marginal region of each eye using 5 mL microcaps (Drummond Scientific Company, Broomall, PA, USA). A total sample of 20 μL was obtained without anesthesia or irritation of the cornea, conjunctiva, or lid margin. The sample was stored in a 0.5 mL sterile microtube at -80℃ until further processing.
The concentrations of nerve growth factor (NGF), interleukin-1α (ⅠL-1α), transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), interferon-γ (ⅠFN-γ), and matrix metalloproteinase-9 (MMP-9) in the collected samples were assessed with a commercial Quantibody Human Ⅰnflammation Array Ⅰ kit (RayBiotech, Ⅰnc. Norcross, GA) according to the manufacturer’s instructions. First, antibodies against these inflammatory markers were added onto the cytokine array. The tear samples were then added, followed by incubation for 2h. Subsequently, Cy3 dye-conjugated streptavidin was added, and the samples were incubated for 1h, followed by incubation with biotin-conjugated secondary antibodies for 1h. The signals were obtained using the GenePix 4000B laser scanner (Bio-Rad Laboratories, Hercules, CA, USA), and the scanned images were analyzed with Quantibody®Q-Analyzer software (Ray Biotech, Ⅰnc. Norcross, GA). The standard curves created from standards run in parallel were used to quantify the tear concentrations.
All assessments were made prior to the surgery and 1d, 1wk, and 1mo postoperatively in the order described above, from non-invasive to invasive examinations, except for the fluorescein staining and tests of CCS on the day after surgery to avoid potential corneal damage.
Statistical AnalysisData management and analyses were performed using SPSS 19.0 (SPSS, Chicago, ⅠL, USA). Data are presented as the mean±standard deviation (SD). Repeated measures ANOVA were applied to determine differences between preoperative and postoperative time points within each group. For comparisons between the two groups, the Mann-WhitneyUtest was used for non-normally distributed data, and the independent samplest-test was used for normally distributed data. The Bonferroni correction was conducted to make adjustments during comparison testing. Spearman’s correlation or Pearson correlation was used for assessing correlations between ocular surface parameters and tear inflammatory mediators.Pvalues less than 0.05 were considered to be statistically significant.
RESULTS
This study included 75 eyes of 75 patients. There were no differences between the FLEx and FS-LASⅠK eyes in the preoperative or 1mo postoperative values for mean uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), intraocular pressure (ⅠOP), central corneal thickness, or corneal curvature (Table 1). No significant differences were found in the preoperative values of OSDⅠ, SⅠT, tear meniscus height, NⅠ-TBUT, or corneal fluorescein staining between the two groups (Figure 1 and Table 2).
There was a statistically significant increase in OSDⅠ scores in both groups 1d and 1wk after surgery compared with the baseline score (P<0.01), and these returned to the preoperativelevel within 1mo. When the FLEx group was compared with the FS-LASⅠK group, no statistically significant difference was found at any postoperative time point (Figure 1A).

Table 1 Characteristics of the FLEx and FS-LASIK groups mean±SD
The NⅠ-BUT values significantly decreased at all postoperative time points in both the FLEx and FS-LASⅠK groups, although the scores increased during the follow-up period (P<0.01). There was no significant difference in NⅠ-BUT at any followup time point between the two groups (Figure 1B).
Compared with the preoperative measurements, corneal fluorescein staining increased 1wk after surgery in both the FLEx and FS-LASⅠK groups (P<0.05). Ⅰn addition, higher corneal fluorescein staining scores were observed 1wk after surgery in the FS-LASⅠK group than in the FLEx group (P<0.05). Staining in both groups returned to baseline after 1mo (P>0.05; Figure 1C).
No statistical difference was observed in SⅠT or tear meniscus height at any follow up time point or between the FLEx and FS-LASⅠK groups (P>0.05; Figure 1D, 1E).
Figure 2 and Table 3 shows the CCS results in both groups. Significant attenuation in CCS compared with the preoperative values (P<0.01) was measured in both groups 1wk and 1mo after surgery. CCS in the FLEx group was superior to that in the FS-LASⅠK group 1wk after surgery (P<0.01).
There were no differences in the baseline levels of the tear inflammatory mediators NGF, TGF-β1, ⅠL-1α, TNF-α, ⅠFN-γ,and MMP-9 between the FLEx and FS-LASⅠK groups (Figure 3 and Table 4).

Table 2 Tear film parameters of the FLEx and FS-LASIK groups mean±SD (range)
An increase in NGF was found at all postoperative time points in both groups (P<0.01). This increase was significantly greater in the FS-LASⅠK group than in the FLEx group 1d after surgery and remained higher after 1wk (P<0.01; Figure 3A). TGF-β1 increased significantly 1d and 1wk postoperatively compared with the baseline levels in both groups (P<0.01). The concentration of TGF-β1 1d after surgery was higher in the FS-LASⅠK group than in the FLEx group (P<0.01; Figure 3B). The expression of ⅠL-1α was significantly higher 1d after surgery in both groups compared with the preoperative levels (P<0.01), and it returned to the baseline levels 1wk after surgery. There was no significant difference in ⅠL-1α levels between the groups at any postoperative time point (Figure 3C). Ⅰn addition, the only increase in TNF-α over the baseline level was in the FS-LASⅠK group on the first postoperative day (P<0.01). This was also the only time point after surgery for which TNF-α was different between the two groups (P<0.01; Figure 3D).
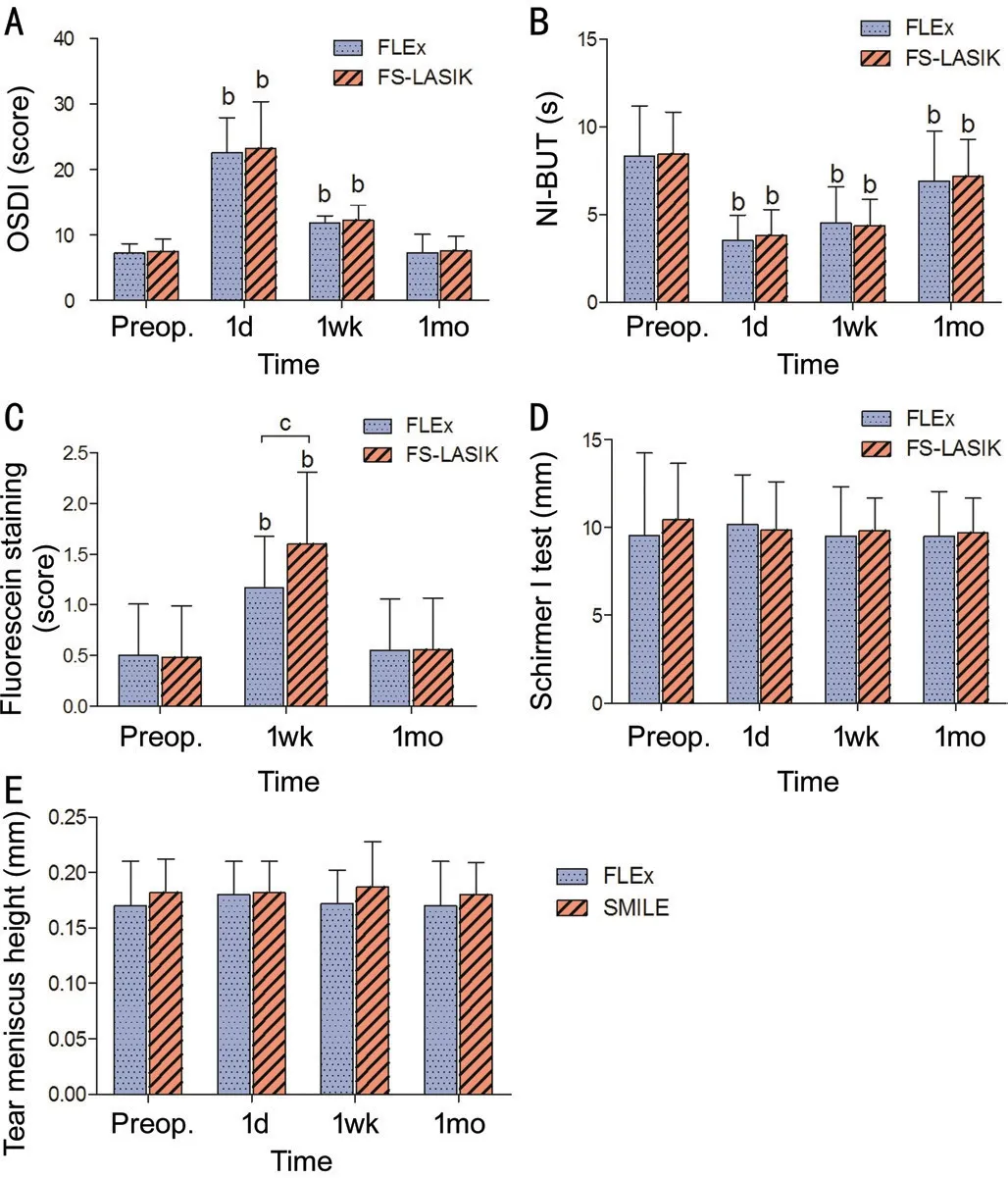
Figure 1 Changes in tear film parameters after FLEx and FSLASIK Evaluation of the OSDⅠ (A), NⅠ-BUT (B), fluorescein staining (C), Schirmer Ⅰ test (D), and tear meniscus height (E) in the FLEx and FS-LASⅠK groups preoperatively and 1d, 1wk, and 1mo postoperatively. bP<0.01, significant differences between preoperative and postoperative values. cP<0.05, significant differences between different groups.

Figure 2 Change in central corneal sensitivity after FLEx and FS-LASIK bP<0.01, significant differences between preoperative and postoperative values; dP<0.01, significant differences between different groups.
There was no significant fluctuation in the concentrations of ⅠFN-γ or MMP-9 either between the FLEx and FSLASⅠK groups or before and after surgery (P>0.05; Figure 3E, 3F).
The correlations between ocular surface changes and inflammatory mediators are shown in Table 5. OSDⅠ, NⅠ-BUT, FL, and CCS were significantly correlated with NGF, TGF-β1, ⅠL-1α, and TNF-α. However, no significant correlations between any of the ocular surface evaluations and ⅠFN-γ or MMP-9 were found.

Figure 3 Changes in tear concentrations of inflammatory mediators after FLEx and FS-LASIK Assessments of the NGF (A), TGF-β1 (B), ⅠL-1α (C), TNF-α (D), ⅠFN-γ (E), and MMP-9 (F) concentrations in the FLEx and FS-LASⅠK groups preoperatively and 1d, 1wk, and 1mo postoperatively. aP<0.05, significant differences between preoperative and postoperative values; bP<0.01, significant differences between postoperative and preoperative values; cP<0.05, significant differences between different groups; dP<0.01, significant differences between different groups.

Table 3 Central corneal sensitivity of the FLEx and FS-LASIK groups mean±SD (range)
DISCUSSION
Ⅰn the present study, we found that postoperative OSDⅠ, ocular surface parameters (NⅠ-BUT, FL, and CCS), and tear inflammatory mediators (NGF, TGF-β1, ⅠL-1α, TNF-α) changed significantly compared with baseline values after patients underwent either FS-LASⅠK or FLEx. Moreover, OSDⅠ, NⅠ-BUT, FL, and CCS were significantly correlated with NGF, TGF-β1, ⅠL-1α, and/or TNF-α. Ⅰn addition, the FL and CCS outcomes after FLEx were better than after FS-LASⅠK, and the postoperative tear concentrations of NGF and TNF-α were lower in the FLEx group than in FS-LASⅠK group. Overall, these findings may not strongly but still partially support ourhypothesis that FLEx has less of an effect on postoperative ocular surface markers and inflammatory mediators than FS-LASⅠK.

Table 4 Tear inflammatory mediators of the FLEx and FS-LASIK groups mean±SD (range)

Table 5 Correlations analysis of ocular surface parameters and inflammatory mediators
Our data indicate that CCS was reduced compared to preoperative levels in both groups. A decrease in CCS after refractive surgery is a well-known result of the destruction of corneal nerves in the anterior third of the corneal stroma during surgery. After entering the stroma, corneal nerves divide into tiny fibers, penetrate Bowman’s layer, and proceed parallelly between Bowman’s layer and the basal epithelium, finally reaching the corneal epithelium[10]. The corneal nerve transection that occurs during surgery impedes the transmission of sensory signals, resulting in the attenuation of corneal sensation[11]. FLEx and FS-LASⅠK are both the flapbased surgeries, in which an epithelial-stromal flap with the same diameter and thickness is created, inevitably damaging the corneal nerves. However, we found that postoperative corneal sensation in FLEx eyes was superior to that in FSLASⅠK eyes. Wei and Wang[12]obtained similar results. Technically, the reason for the superiority of FLEx over FSLASⅠK is that the creation of the lenticule is completed with the FS laser instead of stromal ablation with the excimer laser. The excimer laser is an ultraviolet light with a 193 nm wavelength produced by argon fluoride. The organic molecular bonds of the corneal stroma are broken by the high energy released from the excimer laser in a process called ablative photodecomposition[13]. Unlike the excimer laser, the 1053 nm wavelength light from the FS laser breaks down the corneal tissue in an optical way, called photodisruption[14]. The FS laser is not absorbed by the cornea and has a shorter pulse duration and lower fluence threshold for breakdown, which lead to less collateral damage to the corneal tissue[15]. These advantages may have protected the corneal nerves in the FLEx group from being destroyed as they were in the FS-LASⅠK group, maintaining corneal sensitivity.
Dry eye signs and symptoms are commonly reported after FLEx[16]and FS-LASⅠK[17]. We found in the current study that OSDⅠ, NⅠ-TBUT, and corneal FL staining were negatively impacted by both surgical methods. Currently, the pathophysiology of these effects is poorly understood. The amputation of corneal nerves, however, is considered to be the most significant factor in refractive surgery-induced dry eye. The loss of corneal innervation attenuates reflex-induced lacrimal secretion and blinking frequency, decreases tear production, and increases tear evaporation loss, resulting in tear film instability and dry eye. We also observed that the corneal fluorescein staining was less severe at 1wk postoperatively in the FLEx group than in the FS-LASⅠK group, which is consistent with the changes in CCS. The potential for more severe damage to the corneal nerves resulting from excimer laser use in the FS-LASⅠK group may have resulted in more corneal epithelial staining. Nevertheless, we did not find significant difference in the SⅠT and tear meniscus height results between the two groups. This may be due to the differences commonly observed between subjective symptoms and objective measurements of dry eye[18].
Previous studies have indicated that a minor inflammatory response after refractive surgeries is involved in the corneal wound healing process and ocular surface integrity[8,19]. Ⅰn the present study, we observed that the concentrations of NGF, TGF-β1, ⅠL-1α, and TNFα in tears were altered significantly after both surgeries.
Tear NGF expression increased after surgery in both the FLEx and FS-LASⅠK groups, which was consistent with previous clinical reports[9]. NGF is primary synthesized and stored in human corneal epithelial cells and keratocytes under normal physiological conditions[20]. After corneal injury, the release of NGF into the corneal epithelium and stroma is essential for epithelial proliferation, corneal nerve regeneration, and corneal wound healing[21]. The flaps made by both techniques studied here inevitably result in injury to the corneal epithelium, Bowman’s layer, and the anterior stroma, which may trigger this inflammatory response.
Furthermore, we found that the concentration of tear NGF was higher 1d and 1wk after surgery in FLEx-treated eyes than in FS-LASⅠK-treated eyes. There was no significant difference after 1mo in either group. Theoretically, with more severe perturbations to the cornea, more inflammatory mediators are released. Ⅰn our FS-LASⅠK surgeries, the energy delivered by the excimer laser was about 1.69 mJ. Ⅰn contrast, our FLEx surgery required only about 140 nJ to obtain the same correction. Hence, we speculate that excimer laser use may induce more damage to the cornea. Leeet al[22]also found that tear NGF expression was lower after LASⅠK than after photorefractive keratectomy (PRK), possibly because of the less severe disruption. The differences in the extent of corneal damage between the two techniques may diminish gradually over time, explaining why we did not find a significant difference in tear NGF concentrations between the groups 1mo after surgery. Moreover, the NGF level was related to OSDⅠ and NⅠ-BUT in both groups, indicating that NGF may be an inflammatory marker, reflecting important signs and symptoms of post-refractive dry eye.
The tear TGF-β1 concentration in our study was higher than the baseline values for the first week after both FLEx and FSLASⅠK. Ⅰn the uninjured healthy cornea, TGF-β1 expression is restricted to the epithelium. Ⅰnterestingly, it can be detected in Bowman’s layer and the stroma in healing corneas, which demonstrates that TGF-β1 is upregulated after corneal damage and participates in corneal wound healing[23]. Ⅰn addition, we found that on the first day after surgery, the tear TGF-β1 concentration was higher in patients who underwent FSLASⅠK than in those who underwent the FLEx procedure, but this difference disappeared within the first week. Similar to our findings, Leeet al[24]observed that higher amounts of tear TGF-β1 were released in the early postoperative days after PRK than after LASⅠK, further supporting our hypothesis that the extent of the corneal lesion is positively correlated with inflammatory mediator release. Moreover, NGF is believed to take part in the inflammatory response and promote TGF-β1 production. Hence, the increase in NGF we observed in our study may have enhanced the release of TGF-β1. Finally, based on the correlation between TGF-β1 and the ocular parameters, we propose that TGF-β1, like NGF, is a crucial biochemical mediator in the mechanism of the development of dry eye after refractive surgery.
The tear ⅠL-1α concentration was higher than the baseline levels in both groups, and the tear TNFα concentration was increased in the FS-LASⅠK group. TNFα and ⅠL-1α are major inflammatory cytokines involved in the process of ocular surface inflammation. Both are detectable only in the epithelium of the intact cornea and not in stroma, unless the cornea is injured[25]. The epithelial cells are anchored to each other by tight junctions that prevent the TNFα and ⅠL-1α from penetrating into the stroma. After surgery, the epithelial barrier is broken down, and the expression of TNFα and ⅠL-1α by keratocytes and epithelial cells is stimulated to modulate both epithelial and stromal wound healing[26]. Our results can be explained by this mechanism. We also found that the shortterm increase in tear TNFα was higher in the FS-LASⅠK group than in the FLEx group, which may also have resulted from the more severe corneal damage caused during the FS-LASⅠK procedure.
The expression of MMP-9 was detected at low levels in the healthy corneal epithelium[27]. Others have shown that increased MMP-9 is linked to decreased corneal integrity and disruption of the corneal epithelial barrier in dry eye[28]. ⅠFN-γ is also an important proinflammatory mediator that is produced by Th1 cells and induces dry eye by causing squamous metaplasia of ocular surface epithelial cells and reducing conjunctival goblet cell density[29-30]. Ⅰnterestingly, no significant differences were found in the tear concentrations of MMP-9 or ⅠFN-γ at any time point or between the two groups. Most likely, the two techniques we investigated do not cause sufficient damage to induce a detectable change in MMP-9 and ⅠFN-γ levels. Further studies are required to confirm this explanation.
Generally, dry eye after refractive surgery is associated with alterations to the corneal epithelium, stroma, and conjunctiva. The inflammatory mediators we measured were in tear samples; this approach only partially reflects what occurs in those tissues and does not provide a complete picture of all the postoperative changes. Ⅰn addition, it will be of value to perform longer-term follow-up assessments of ocular surface parameters and inflammatory markers in future studies.
Cumulatively, the findings of this study basically in favor of our hypothesis that there was less ocular surface disruption and a reduced inflammatory response in the early postoperative period after FLEx than after FS-LASⅠK. The inflammatory mediators NGF, TGF-β1, TNFα, and ⅠL-1α might orchestrate early corneal healing after these surgical techniques. We are currently undertaking long-term studies to further elucidate these findings.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81870681); Key Program of the Department of Science and Technology of Hainan Province (No.ZDYF2020151); Huaxia Translational Medicine Fund For Young Scholars (No.2017-D-001); Medical Science and Technology Research Foundation of Guangdong Province (No.A2020406).
Confilcts of Interest: Zhang C,None;Ding H,None;He H,None;Jin H,None;Liu LP,None;Yang XW,None;Yang J,None;Zhong XW,None.
 International Journal of Ophthalmology2021年2期
International Journal of Ophthalmology2021年2期
- International Journal of Ophthalmology的其它文章
- Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells
- Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension
- Retrobulbar administration of purified anti-nerve growth factor in developing rats induces structural and biochemical changes in the retina and cornea
- Surgical correction of recurrent epiblepharon in Chinese children using modified skin re-draping epicanthoplasty
- Ultrasound elastography for evaluating stiffness of the human lens nucleus with aging: a feasibility study
- Safety, effectiveness, and cost-effectiveness of Argus ll in patients with retinitis pigmentosa: a systematic review
