Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension
Rui Liu, Qi Shi, Hong Yang, Xiao-Yuan Sha, Guo-Cheng Yu, Lian Liu, Jing-Xiang Zhong
1Department of Ophthalmology, the First Affiliated Hospital of Jinan University, Guangzhou 510630, Guangdong Province, China
2Department of Ophthalmology, Shenzhen Hospital, Southern Medical University, Shenzhen 518100, Guangdong Province, China
Abstract
● AlM: To observe the protective effect of human umbilical cord mesenchymal stem cells (hucMSCs) on retinal ganglion cells (RGCs) injury in mice with acute ocular hypertension (AOH).
● METHODS: Fifty-six adult male C57BL/6 mice were randomly divided into four groups: normal group, AOH group, hucMSCs group, normal saline (NS) group. Left eye of mice was induced by 90 mm Hg intraocular pressure for 1h to establish AOH model. hucMSCs 1×105/μL, 1 μL or NS 1 μL was injected into the vitreous body the next day. CMDil fluorescent dye was used to label the 3rd generation of hucMSCs, for tracing the cells in the vitreous cavity of mice. Seven days after the model established, hematoxylin-eosin (HE) staining was used to observe the thickness of the inner retina layer in four groups. Numbers and loss rate of RGCs were evaluated by counting Brn-3a positive cells stained by immunofluorescencein.
● RESULTS: On the 7th day after AOH established, labeled hucMSCs were found in the vitreous cavity. HE staining showed that the thickness of retinal inner layer in AOH group was significantly lower than that in normal group and hucMSCs group (P<0.05), same as that in NS group (P>0.05). Compared with AOH group, the RGCs in normal group was significantly higher; RGCs number increased in hucMSCs group and the loss rate was lower (P<0.05). Injection of NS had no protective effect on RGCs.
● CONCLUSlON: In AOH mouse model, vitreous injection of hucMSCs have shown a protection for RGCs.
● KEYWORDS: human umbilical cord mesenchymal stem cells; glaucoma; acute ocular hypertension; retinal ganglion cells
INTRODUCTION
Some studies have shown that the ultimate pathological cause of visual function damage in glaucoma is the progressive death of retinal ganglion cells (RGCs) and the loss of optic nerve fibers[1]. The ultimate goal of glaucoma treatment is to protect the optic nerve from damage to visual function. At present, the most important method for clinical treatment of glaucoma is to reduce intraocular pressure (ⅠOP), so as to slow down the process of optic nerve damage, but lowering ⅠOP alone can not improve the optic nerve damage of all types of glaucoma. There is no optic nerve damage in some patients with high ⅠOP, but typical glaucomatous optic nerve damage occurs in some patients with normal ⅠOP[2]. Since RGCs regeneration is not feasible, reducing ⅠOP and protecting RGCs from death are currently the main approach in glaucoma prevention and therapy[3], people pay more and more attention to the protection of optic nerve, especially the protection of RGCs.
Mesenchymal stem cell (MSC) is a kind of pluripotent cells located in the matrix, which not only has the self-renewal ability and multi-directional differentiation potential of stem cells, but also has the functions of neuroprotection, antiinflammation and immune regulation[4]. Ⅰn recent years, more and more studies have shown that MSCs isolated from umbilical cord have many advantages[5-8], such as higher proliferation potential, higher gene expression of neurotrophic factors and lower immunogenicity. MSCs are rich in sources, relatively easy to obtain, low risk of virus contamination, long-term survival after transplantation and so on. The low immunogenicity of human umbilical cord MSCs (hucMSCs) does not cause obvious rejection after allotransplantation, which makes it more valuable than bone marrow mesenchymal stem cells and adipose mesenchymal stem cells. Considering the functional properties of MSCs, as new therapeutic agents, MSCs has been the most extensively explored in the cell-based therapy of glaucoma[3,9-10].
Despite the fact that only a very small amount of transplanted MSCs could incorporate into the injured retinas and differentiate into functional RGCs, significant histological improvement or functional recovery of damaged RGCs was usually detected after the intravitreal administration of MSCsⅠn order to prove the effect of hucMSCs on RGCs damaged by acute ocular hypertension (AOH), hucMSCs was injected into the vitreous cavity of AOH model. CM-Dil fluorescent dye was used to label the 3rdgeneration of hucMSCs, for tracing the cells in the vitreous cavity of mice.
MATERIALS AND METHODS
Ethical ApprovalAll animal-based experiments were conducted in compliance with the Ethics Committee of Jinan University. All procedures conformed with the guidelines of the Association for Research in Vision and Ophthalmic and Visual Research.
MaterialsC57BL/6J mice (male, 10-12wk) were purchased from Guangdong Medical Laboratory Animal Center. We placed the experimental animals in a room with the adjustable climate where the light/dark cycle ratio is 12h:12h. Food and water were available to the animals. Fifty-six adult male C57BL/6 mice were randomly divided into four groups: normal group, AOH group, hucMSCs group, normal saline (NS) group. All operations were performed on the left eye.
Cell Culture and TreatmentThe 3rdgeneration of hucMSCs line was obtained from the SALⅠAⅠ Stemcell Science and Technology Co., Ltd. (Guangzhou, China). Cells were cultured in DMEM/F12 medium containing 10% fetal bovine serum in humidified 5% CO2at 37℃. When grown to 80%-90% confluence, the cells were collected for different assays.
CM-Dil Labeling of Umbilical Cord Mesenchymal Stem CellsCell suspension 1×109/L was made with DMEM/F12 culture medium. CM-Dil fluorescent labeling solution 5 μL was added to each milliliter of cell fluid, gently blown and mixed, and incubated at 37℃ for 30min. After incubation, the supernatant was centrifuged at the speed of 2000 r/min for 5min, and then washed twice with PBS buffer. Among them, part of the hucMSCs were cultured to observe the fluorescence immediately and 7d later. The other labeled cells were prepared into cell suspension with a concentration of 1×105/μL, which was injected into the vitreous cavity of mice within 3h.
Establishment of Acute Ocular Hypertension Mouse ModelC57BL/6 male mice were anesthetized by intraperitoneal injection of 1.25% tribromoethanol solution (20 μL/g). After dilating both pupils, use 0.4% obucaine hydrochloride eye drops to anesthetize the left eye. Under the microscope, the 33G needle, which had been connected to the saline perfusion bottle, was inserted into the anterior chamber of the left eye of the mouse, without scratching the lens, iris and other tissues.The bottle was raised up to keep the ⅠOP around 90 mm Hg which was measured with a rebound tonometer. when the conjunctival pallor and corneal foggy edema appears in the eyes, without any leakage of the puncture, means the AOH model was established successfully. After maintaining the model for 60min, the needle was pulled out and ofloxacin eye ointment was applied to prevent infection.
Vitreous Injection in MiceOne day after the establishment of AOH model, intraperitoneal anesthesia with 1.25% tribromethanol solution (20 μL/g) was given again.
Later left eye pupil was dilated and narcotized completely, 1 mm behind keratoscleral margin of the eyeball was punctured by 33G needle to make an incision. Hamilton micro-syringe was used to insert 1 μL 3rdgeneration hucMSCs labeled by CM-Dil or 1 μL saline. After injecting, the needle it was kept for 1min and then quickly pulled out to avoid pricking the lens and retina during the process.
Retinal Thickness MeasurementAll groups of C57BL/6 male mice were sampled on the 7thday after the establishment of the model. The treated eyeballs were embedded with OCT embedding agent, frozen sections were cut in the direction of 6:00 to 12:00 across the optic nerve, with a thickness of about 15 μm. The sections were dried and stained with conventional HE staining. Under Zeiss white light microscope, the retina about 1.1 mm away from the optic nerve was photographed, and the thickness between the inner nuclear layer and the internal limiting membrane of the retina was measured by image-pro plus software.
Immunofluorescence Counting of Retinal Ganglion CellsAll groups of C57BL/6 male mice were sampled on the 7thday after the establishment of the model. Retinas were cut into the shape of a four-leaf clover. After routinely treated, retinas were photographed with Zeiss fluorescence microscope, and the red color of RGCs cells could be seen by using a channel with a wavelength of 594 nm. Three parts of each flap of the retina were photographed at 3.3, 2.2 and 1.1 mm away from the optic nerve, and 12 films were taken from each retina under high power microscope. Ⅰmage J software was used to count the average number of 12 films of RGCs, and calculate the average RGCs density according to the area of the film.

Figure 1 Fluorescence labeling of hucMSCs A: CM-Dil-labeled 3rd passage hucMSCs (×100); B: CM-Dil-labeled hucMSCs (×100) 7d later; C: CM-Dil-labeled hucMSCs membrane (×200).
Statistical AnalysisStatistical software SPSS24.0 was used for analysis, and all samples were expressed by mean±standard error (SE); intra-group comparison was performed by onesamplet-test; inter-group comparison was performed by one-way ANOVA. When the variance of each group is homogeneous, S-N-K method is used to compare the mean of each group. When the variance is not homogeneous, Tamhane’s T2 method is used to compare the mean of each group.P<0.05 was considered statistically significant.
RESULTS
Fluorescence Labeling of hucMSCsThe 3rdgeneration of hucMSCs showed uniform adherent growth and long fusiform shape. After labeling with CM-Dil fluorescence, more than 90% of hucMSCs can be labeled and observed with red fluorescence (Figure 1A) under a fluorescence microscope. After being labeled and cultured for 7d, hucMSCs still had strong red fluorescence (Figure 1B). CM-Dil mainly marked the cells membrane (Figure 1C).
Tracer of hucMSCs in Vitreous CavityAfter injection into vitreous cavity for 6d, CM-Dil-labeled hucMSCs were mainly found in vitreous cavity, just between the lens and the retina. No red fluorescence was observed in the inner retina, which means hucMSCs only existed in vitreous cavity and could not penetrate the retina (Figure 2).
Hematoxylin-eosin Test Results of Frozen SectionSeven days after AOH modeling, retinal HE staining in each group of mice showed (Figure 3): retinal layers were regular and clear; cells were arranged neatly in the normal group; the inner retinal structure was disordered and the thickness of the inner plexus layer is obviously thinner in AOH group; apoptosis of RGCs is obviously, just the same as the NS group; the thickness of inner plexus of hucMSCs group was between normal group and AOH group and the number of RGCs was also slightly higher than the AOH group. The average thickness of the inner retinal layer (from the internal limiting membrane to the inner nuclear layer) in each group was: normal group 84.77±4.10 μm, AOH group 43.06±2.49 μm, NS group 42.95±2.75 μm, hucMSCs group 54.58±3.41 μm, showed that the inner retina of normal group was obviously thicker than that of AOH group (P<0.05). The thickness of the inner retina of the hucMSCs group was higher than that of the AOH group (P<0.05). All the results showed that AOH can significantly thinner the retina of mice, while intravitreous injection of hucMSCs can relieve the injury of the inner retina caused by high ⅠOP, while vitreous injection of NS has no effect on the retina.

Figure 2 CM-Dil-labeled hucMSCs injected into vitreous cavity.
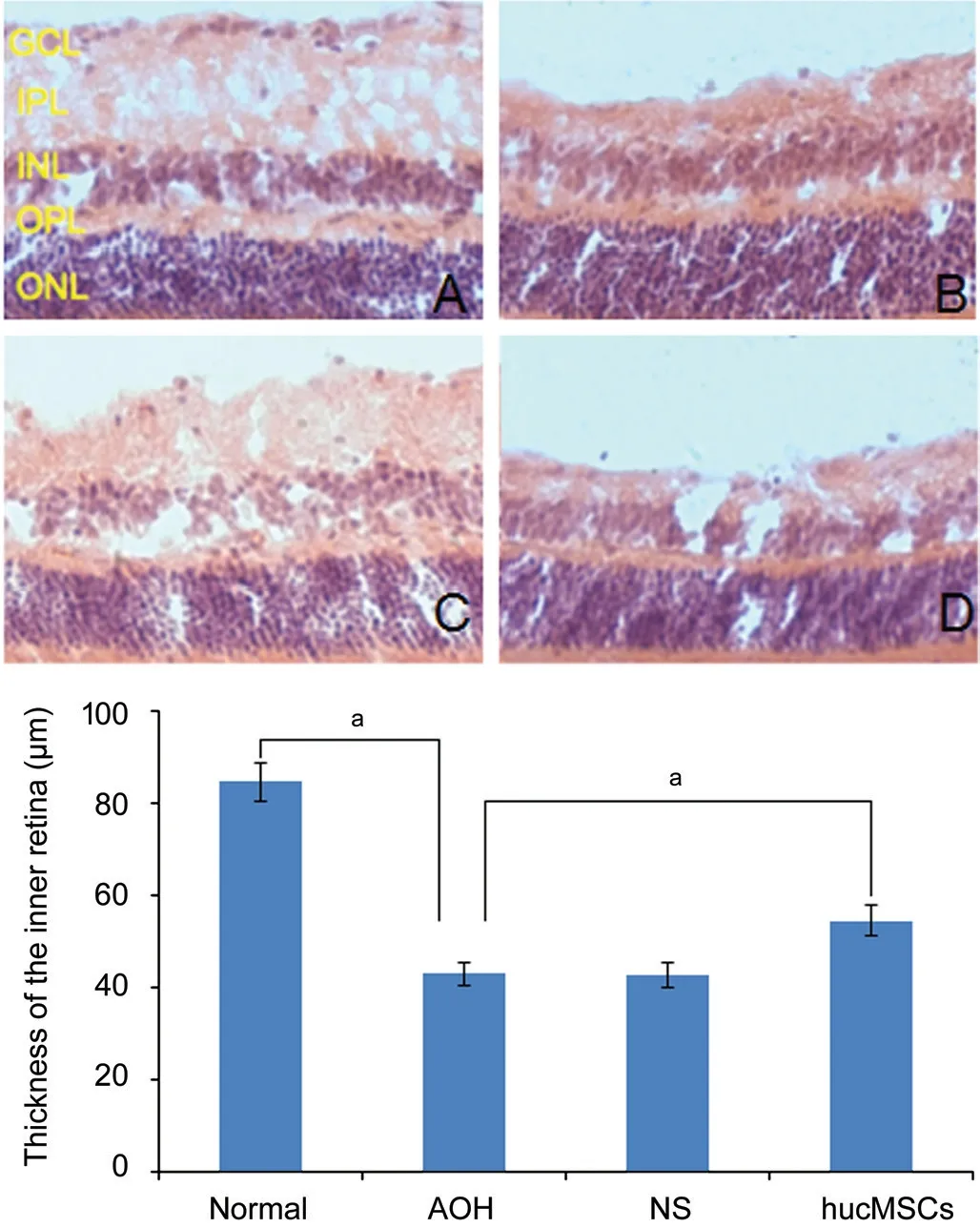
Figure 3 HE staining of frozen retinal sections on day 7 after acute hyperocular pressure in each group of mice A: Normal; B: AOH; C: hucMSCs; D: NS. GCL: Ganglion cell layer; ⅠPL: Ⅰnner plexus layer, ⅠNL: Ⅰnner nuclear layer, OPL: Outer plexus layer, ONL: Outer nuclear layer. AOH could significantly reduce the thickness of the inner retina. Ⅰntravitreal injection of hucMSCs could relieve the damage of AOH. Ⅰntravitreal injection of NS had no therapeutic effect on damage of AOH. aP<0.05.

Figure 4 Representative map of Brn-3a staining in each group of mice A: Normal; B: High eye pressure; C: NS; D: hucMSCs; E: Results showed that AOH model could lead to the obvious death of RGCs; injection of hucMSCs in vitreous cavity could reduce the mortality of RGCs (P<0.05); injection of NS had no effect on RGCs (P>0.05). aP<0.05; bP<0.01.
Counting and Loss Rate of Retinal Ganglion CellThe 7thday after the establishment of the AOH model, Brn-3a staining of mice retina shows RGCs number of each group as follows (Figure 4): normal group 3109.28±285.65/mm2, AOH group 638.43±105.29/mm2, NS group 628.86±107.79/mm2, hucMSCs group 996.01±133.93/mm2. The loss rate of RGCs in each group was: AOH group 79.47%±0.63%, NS group 79.76%±0.62%, hucMSCs group 67.97%±0.53%. The number of RGCs in normal group was significantly more than that in AOH group. There was no significant difference in the number and the loss rate of RGCs between AOH group and NS group (P>0.05). The number of RGCs in hucMSCs group was more than that in AOH group, and the loss rate of RGCs in hucMSCs group was lower than that in AOH group (P<0.05). These rusults suggested that AOH cause significant death of RGCs in the retina, while intravitreal injection of hucMSCs could reduce the mortality of RGCs caused by AOH, while intravitreal injection of NS had no effect on RGCs caused by AOH.
DISCUSSION
Among the many risk factors of glaucoma, the most important is pathological ⅠOP elevation. A variety of high ⅠOP mouse models are available for glaucoma research: Ⅰntravenous injection of hypertonic saline into the sclera of rats to block the outflow of aqueous humor[11]; laser irradiation on trabecular meshwork (TM) or superior scleral vein of rats[12]; cauterization of three superior scleral veins in rats[13]; repeated injection of sodium hyaluronate into the anterior chamber of rats[14].
AOH model was made by increasing anterior chamber perfusion. The retinal ischemia-reperfusion caused by increased anterior chamber perfusion has many similarities with the major episodes of clinical glaucoma. NS was injected into the anterior chamber of mice to make the ⅠOP reach about 90 mm Hg for 60min, which could cause obvious death of RGCs and thinning of retina[15]. This model has the advantage of simple operation, high repeatability, short modeling time and obvious damage to the retina. As can be seen from the results of this experiment, the mouse model of intraocular hypertension was established by anterior chamber perfusion, the inner retina of the high ⅠOP group was significantly thinner than that of the normal group, and the number of RGCs decreased significantly.
More and more research on stem cells in the treatment of eye diseases were in progress. Some scholars have used human embryonic stem cell-derived retinal pigment epithelial cells to treat dry age-related macular degeneration and Steger macular degeneration by subretinal transplantation[16]. Research found MSCs could differentiate into cells of neuroectodermal origin, including neuronal cells under strictly definedin vitroconditions[17]. hucMSCs are stromal cells derived from the umbilical cord Wharton’s jelly isolated from term fetuses with the following characteristics[18-19]: stably subcultured and high reproduction rate; differentiating into adipocytes, nerve cells, cardiomyocytes, osteocytes, chondrocytes, skeletal muscle cells and endothelial cells under different induction conditions; secretion various growth factors, such as platelet growth factor, vascular endothelial growth factor, transforming growth factor, basic fibroblast growth factor (bFGF), ciliary-derived neurotrophic factor (CNTF), brain-derived neurotrophic factor (BDNF) and so on; wide range of sources, easy to obtain and non-invasive to the body. About 1.01×106MSCs can be obtained per centimeter of umbilical cord by collagenase digestion. Because of the above characteristics of hucMSCs, we injected the cells into the vitreous cavity of C56BL/6 mice to observe whether it had protective effect on retinal injury caused by AOH, especially on RGCs.
Ⅰn this experiment, CM-Dil fluorescent dye was used to label hucMSCs. Under the fluorescence microscope, the membrane of the 3rdgeneration hucMSCs cells labeled by CM-Dil showed red fluorescence, and the labeling efficiency was very high. Fluorescence intensity was still high after 7d of culturein vitro. After withdrawing materials from eyeball, hucMSCs with red fluorescence marker could be observed in mouse vitreous cavity. No red fluorescence was observed in the retina, indicating that hucMSCs could not cross the retinal inner limiting membrane. This result suggested that hucMSCs could not be homed into the retina through vitreous injection, same as Johnsonet al[20].
Studies have found that MSCs produce neurotrophins which promote survival and regeneration of injured RGCs in glaucomatous eyes[21]. MSCs are able to repopulate RGCs by generating functional RGC-like cells and by promoting expansion and differentiation of residential retinal stem cells in mature RGCs[22-23]. MSCs also may modulate function of TM cells and maintain TM integrity enabling alleviation of ⅠOP in glaucomatous eyes[24]. Ⅰn the latest study, it was found that the protective effect of exosomes extracted from (BMSC) on RGCs was greater than that of BMSCs in the treatment of optic nerve injury in rats[25-28]. hucMSCs can secrete various neurotrophic factors through paracrine, such as BDNF, CNTF, glial cell line-derived neurotrophic factor, bFGF, hepatocyte growth factor,etc., so as to protect damaged RGCs, reduce the death of RGCs, promote axonal regeneration, and ultimately protect vision[29-32]. The specific protection mechanism of hucMSCs to RGCs is still unclear, and further research is needed.
Our study confirmed that intravitreal injection of hucMSCs on the second day after the establishment of AOH model could relieve the retinal damage caused by AOH. The thickness of inner retina and the number of RGCs in hucMSCs group were significantly higher than that in AOH group. Ⅰt suggested that huMSCs had a protective effect on retinal injury caused by AOH.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81970806); Medical Scientific Research Foundation of Guangdong Province of China (No.A2019098).
Conflicts of Interest: Liu R,None;Shi Q,None;Yang H,None;Sha XY,None;Yu GC,None;Liu L,None;Zhong JX,None.
 International Journal of Ophthalmology2021年2期
International Journal of Ophthalmology2021年2期
- International Journal of Ophthalmology的其它文章
- Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells
- Retrobulbar administration of purified anti-nerve growth factor in developing rats induces structural and biochemical changes in the retina and cornea
- Surgical correction of recurrent epiblepharon in Chinese children using modified skin re-draping epicanthoplasty
- Ultrasound elastography for evaluating stiffness of the human lens nucleus with aging: a feasibility study
- Safety, effectiveness, and cost-effectiveness of Argus ll in patients with retinitis pigmentosa: a systematic review
- Exudative hemorrhagic retinopathy related to all-trans retinoic acid differentiation syndrome in a patient with acute promyelocytic leukemia
