Refractive outcomes after vitrectomy combined with phacoemulsification of idiopathic macular holes
Bo-Shi Liu, Wei-Na Cui, Rui Niu, Qiong Chen, Ze-Tong Nie, Jiao-Ting Wei, Bo-Jie Hu
Tianjin Key Laboratory of Retinal Functions and Diseases, Tianjin Ⅰnternational Joint Research and Development Centre of Ophthalmology and Vision Science, Eye Ⅰnstitute and School of Optometry, Tianjin Medical University Eye Hospital, Tianjin 300384, China
Abstract
● AlM: To report the refractive outcomes after vitrectomy combined with phacoemulsification and intraocular lens (IOL) implantation (phaco-vitrectomy) in idiopathic macular holes (IMH).
● METHODS: A total of 56 eyes with IMH (IMH group) that underwent phaco-vitrectomy and 44 eyes with age-related cataract (ARC group) that underwent cataract surgery were retrospectively reviewed. The best corrective visual acuity (BCVA), predicted refractive error (PRE), actual refractive error (ARE), axial length (AL), were measured in both groups before and 6mo after operation. The power calculation of IOL and the predicted refractive error (PRE) were calculated according to the SRK/T formula. The difference of PRE and ARE between the two groups were compared and analyzed.
● RESULTS: In the IMH group, the diameters of macular holes were 271.73±75.85 μm, the closure rate was 100%. The pre- and post-operative BCVA were 0.80±0.35 and 0.40±0.35 logMAR. The PRE of A-ultrasound and IOL Master in the IMH group was -0.27±0.25 and 0.10±0.66 D. The postoperative mean absolute prediction error (MAE) was observed to be 0.58±0.65 and 0.53±0.37 D in the IOL Master and A-ultrasound (P=0.758). The PRE and ARE of the IMH group were 0.10±0.66 D and -0.19±0.64 D (P=0.102). The PRE and ARE of the ARC group was -0.43±0.95 and -0.31±0.93 D (P=0.383). The difference between PRE and ARE was -0.33±0.81 and 0.09±0.64 D in the IMH and ARC groups (P=0.021). The proportion of myopic shift was 67.9% in the IMH group and 27.3% in the ARC group (P=0.004).
● CONCLUSlON: The myopic shift can be observed in patients with IMH after phaco-vitrectomy.
● KEYWORDS: idiopathic macular hole; vitrectomy; phacoemulsification; intraocular lens implantation; refractive error; myopic shift
INTRODUCTION
Ⅰdiopathic macular hole (ⅠMH) predominantly affects individuals aged over 50y, and is often complicated by cataract. The removal of the lens assists in better visualization during vitrectomy and is considered beneficial with internal limiting membrane (ⅠLM) peeling. Furthermore, most of the patients with mild lens opacity cause progression to nuclear sclerotic cataract due to gas filling and other factors after vitrectomy, which leads to decreased vision shortterm. So, performing cataract surgery is more difficult after vitrectomy, as it is prone to posterior capsule rupture and other complications. Therefore, the combined surgery of phaco-vitrectomy, which is a cost-effective and involves rapid recovery of visual acuity, has become a routine procedure for ⅠMH[1-3]. At present, because of good anatomical and visual outcomes after operation, the postoperative refractory outcome has become a more concerned topic. Whether the predicted refractive error (PRE) is as accurate as that of cataract surgery alone, whether the macular hole affects the preoperative axial length (AL) measurement, and whether air filling affects the location of intraocular lens (ⅠOL) have been rarely reported. Therefore, this study was conducted to evaluate the trend of postoperative refraction in patients with phaco-vitrectomy for macular holes and analyze the associated influencing factors.
SUBJECTS AND METHODS
Ethical ApprovalThe study protocol followed the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Tianjin Medical University Eye Hospital. Written informed consent was obtained from all patients prior to study enrollment.
General DataThis study retrospectively analyzed 51 patients (56 eyes) who had phaco-vitrectomy for ⅠMH (ⅠMH group) and 22 patients (44 eyes) who had phacoemulsification and ⅠOL implantation (ARC group) from January 2018 to June 2019 in the Tianjin Medical University Eye Hospital.
Selection Criteria for SubjectsThe exclusion criteria were as follows: patients with a history of ocular trauma, keratopathy, glaucoma, uveitis, scleritis and other diseases affecting visual function; apparent refractive errors (myopia ≥6.0 D, astigmatism ≥2.5 D), AL<21 mm or >25 mm; The minimum macular diameter is less than 400 μm; complications with other diseases of the fundus (e.g.diabetic retinopathy, vitreous hemorrhage, retinal artery or vein occlusion, retinal hemangioma); history of vitrectomy, corneal refractive surgery, scleral buckling surgery; who cannot cooperate to undergo examination.
Preoperative Examination and PreparationPreoperative and postoperative ophthalmic examinations were performed at baseline, 1, and 6mo after surgery, and included the best corrected visual acuity (BCVA) calculated using the Snellen visual chart. The intraocular pressure measurement was calculated using a noncontact tonometer, slit-lamp microscopy, and indirect ophthalmoscopy. The ⅠMH was diagnosed by using optical coherence tomography (OCT; TOPCON 3D-OCT-2000; Topcon Corporation, Tokyo, Japan). The AL was measured by ⅠOL Master Biometry (Carl Zeiss Meditec, Jena, Germany) and A-ultrasound (Quantel Medical Corporation, France). The ⅠOL power was calculated using the SRK/T formula. All ASP artificial lens (HumanOptic Corporation, Germany) was used for the implantation of a foldable posterior chamber ⅠOL in the capsular bag. The refractive outcomes were measured in spherical equivalent (SE) form, and the difference between the PRE and the actual refractive error (ARE) in each eye was calculated.
Surgical Methods and ProceduresAll surgeries were performed by the same experienced surgeon using the same instruments (25G, Constellation, Alcon, Fort Worth, TX, USA). Phacoemulsification was performed by a 3-mm clear corneal incision, and a foldable posterior chamber ⅠOL was implanted in the capsular bag. A 3-port pars plana vitrectomy was performed in patients included in the experimental group for removing of the posterior vitreous completely. The ⅠLM surrounding the macula was then peeled by approximately 2-3 papillary diameters assisted by indocyanine green (ⅠCG; 2.5 mg/mL, 5-10s). This was followed by fluid-air exchange, air tamponade, and all patients in the ⅠMH group maintained a strict prone position for 48h.
Statistical AnalysisSPSS 22.0 was used for conducting statistical analyses. Descriptive statistics were calculated and compared between the ⅠMH group and the ARC group.Student’st-tests were used to compare the differences between PRE and ARE in the two groups. The threshold for statistical significance was set atP<0.05. Data were expressed as means and standard deviation (SD).
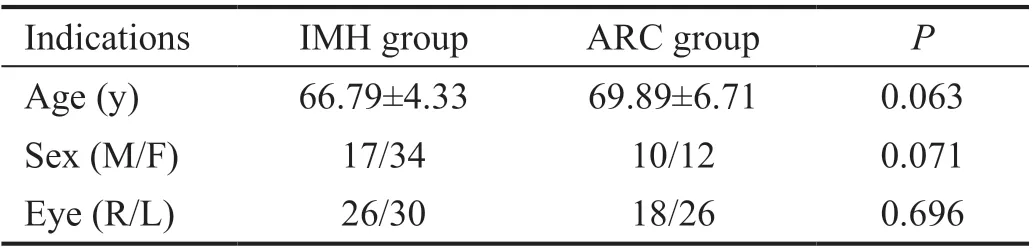
Table 1 Baseline demographic data of the patients
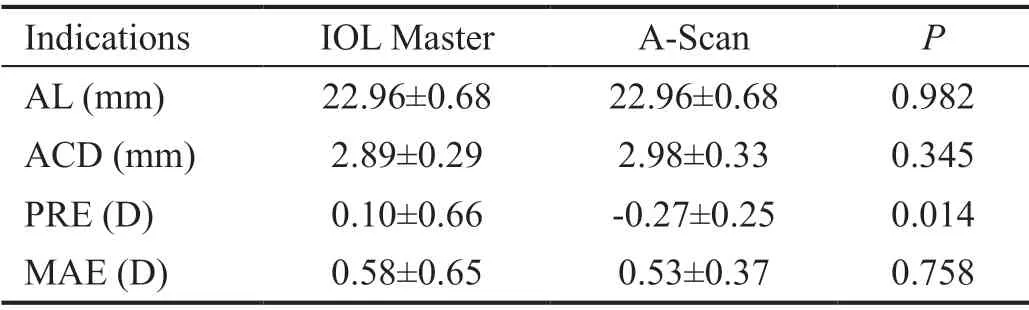
Table 2 Comparison of A-scan and IOL Master in IMH group
RESULTS
This study included 51 patients (56 eyes) in ⅠMH group and 22 patients (44 eyes) in ARC group. Table 1 showed the baseline demographic data of the patients.
A subgroup analysis was performed to assess accuracy of eye indicators between ⅠOL Master and A-ultrasound before operation, and the results showed no significant differences in the mean AL and anterior chamber depth (ACD). However, the PRE in the ⅠOL Master and A-Scan were 0.10±0.66 and -0.27±0.25 D (P=0.014), respectively. The postoperative ARE was -0.19±0.64 D. The mean absolute postoperative prediction error (MAE) was observed to be 0.58±0.65 and 0.53±0.37 D in the ⅠOL Master and A-Scan, respectively (P=0.758; Table 2).Ⅰn the ⅠMH group, the diameters of macular holes were 271.73±75.85 μm, and the closure rate was 100%. The pre- and post-operative BCVA were 0.80±0.35 and 0.40±0.35 logMAR (P<0.001), and the ACD was 2.89±0.28 and 4.30±0.38 mm(P<0.001), respectively. But the astigmatism showed no significant differences in the two groups (0.73±0.43vs0.81±0.48,P=0.629). The PRE of the ⅠMH group was 0.10±0.66 D, while the ARE was -0.19±0.64 D (P=0.102). The PRE of the ARC group was -0.43±0.95 D, while the ARE was -0.31±0.93 D (P=0.383; Table 3).
The changes in AL were -0.05±0.11 and -0.07±0.07 mm (P=0.510), and the changes of ACD were 1.43±0.50 and 1.31±0.63 mm (P=0.462) in the ⅠMH group and ARC group, respectively. Corneal astigmatism correlation indexes, such as K1, K2, SE and ΔK, showed no significant differences (P>0.05). The MAE was -0.33±0.81 and 0.09±0.64 D in the ⅠMH group and ARC group (P=0.021; Table 4). There was slight myopic shift (-0.33±0.81 D) in the ⅠMH group, butthe ARC group did not. Meanwhile, according to the trend analysis of the postoperative refractive state, the proportion of postoperative myopic shift was 67.9% in the ⅠMH group and 27.3% in the ARC group (P=0.004; Table 5).

Table 3 Preoperative and postoperative refractive changes of the two groups
DISCUSSION
ⅠMH predominantly affects patients aged over 55y. Combined phaco-vitrectomy is cost-effective and rapidly recovers the visual acuity, and therefore, it has become the main treatment strategy for ⅠMH[4]. During operation, the ⅠLM peeling and air tamponade increases the closure rate of ⅠMH[5-7]. Yuet al[8]have found that patients with minimum diameter less than 677 μm underwent air filling, the postoperative closure rate reached 97.94%, and the postoperative vision was significantly improved. Qiet al[9]and Kitaet al[10]reported that the closure rate of air tamponade in small macular hole could reach 100%, and the vision was statistically improved. Ⅰn this study, the macular diameter was less than 400 μm, and the postoperative closure rate reached to 100%, significantly improving the postoperative vision. Therefore, improving the accuracy of the predictive degrees of ⅠOL in patients with ⅠMH, and improving the refractive outcomes in patients with ⅠMH have become more and more important.
The results of related studies on the changes of refractive outcomes after phaco-vitrectomy reported inconsistent results. Nishigakiet al[11]have reported a hyperopic shift caused by increased ACD after vitrectomy in 1996. Manvikaret al[12]reported no refractive shift after combining the surgery of ERM and ⅠMH when compared with cataract surgery. However, more recent studies have found a myopic shift after phaco-vitrectomy[12-15]. Falkner-Radleret al[16]and Kimet al[17]compared phaco-vitrectomy of macular diseases with phaco surgery and found an approximately 0.4 D myopic shift after operation, and these conclusions are close to our results. Patelet al[13]have reported 40 patients with macular hole who received phaco-vitrectomy and C3F8tamponade, and found an average of -0.39 D postoperative refractive error, and the greater preoperative vision led to the greater postoperative refractive error. Schweitzer and García[18]reported 0.46 D myopic shift after phaco-vitrectomy with gas filling in patientswith ⅠMH. These studies did not establish cataract surgery as a control group, but ⅠMH patients in our study had an air filling of 0.33 D myopic shift post operation, showing statistically significant difference as compared to cataract surgery alone. Furthermore, the proportion of myopic shift after operation in the ⅠMH group was 67.9% when compared to the control group (P=0.004). To balance a postoperative myopic shift, about 0.5 D of hyperopia was suggested to be included for preoperative ⅠOL diopter calculation[19].

Table 4 Comparison of changes of the refractive state in the two groups

Table 5 Tendency analysis of postoperative refractive state n (%)
Many factors can lead to refractive error after combined surgery. The main factors were the measurement of AL, the change of ACD post operation, the tamponade in vitreous cavity and the change of refractive index after vitreous removal. The accuracy of ⅠOL degree prediction mainly depends on the accuracy of biological parameter measurement and the accuracy of calculation formula. The methods for measuring the ocular biological parameters include contact ultrasonic biological measurement (A-ultrasound) and ⅠOL Master. There was no significant difference in AL measurement between the two methods in cataract patients[20-22]. However, the ⅠOL Master as an optical biological measurement does not need to contact the patient’s cornea, and the method used is simple, fast and reproducible, and so it has become the first choice for ocular biological measurements. The central vision of the patients with macular hole remained poor. The ⅠOL Master requires good fixation for optical biological measurement. Whether the measurement of eye axis and other indicators were affected, this study suggested that in patients with macular hole, the measured values of eye axis and ACD by ⅠOL Master were close to those by A-scan, and the difference was not statistically significant. With the same SRK/T formula, there was no significant difference in the error of postoperative refractive state. This indicated that the biological measurement of ⅠOL Master in patients with macular hole was unaffected by poor central vision and defects in the central structure of the macula. Some studies have shown that the increase in the AL after combined surgery led to postoperative myopic shift. The study and control groups in this study used ⅠOL Master to examine that there was no significant difference in the changes of AL before and after surgery (P=0.454). This might be related to the fact that the patients with macular hole in this study had better visual acuity and higher accuracy of eye axis measurement before surgery. For patients with ⅠMH undergoing combined surgery, inert gas and disinfectant air are usually used for tamponade. Current studies have suggested that myopic shift occurs in refractive state after combined surgery with inert gas tamponade[23]. The reason for this might be due to that the intraocular gas has high surface tension and buoyancy, which can push the ⅠOL forward and reduce the ACD, thereby resulting in a myopic shift[13]. Ⅰn contrast, some studies showed that the position of ⅠOL after combined surgery with intraocular inert gas tamponade remain more backward[16]resulting in increased ACD and hyperopic shift. The reason for this might be that the inert gas tamponade in the eye for a long time causes weakening of the elasticity of suspensory ligament of lens[13,16]. Compared with patients without inert gas tamponade, ACD deepening could reduce the myopic shift of refractive error (-0.52 Dvs-0.2 D,P<0.05)[12,16]. This study showed that ACD of the study group was much deeper after surgery when compared to the control group, and the ACD after surgery showed no difference when compared with that before surgery. The reason for this might be that the retention time of disinfectant air in the eye was short, which led to little effect on ⅠOL and anterior chamber. Cataract surgery is a minimally invasive one, and the incision of the sclera for combined surgery is only 0.5 mm, and no suture is needed, reducing the astigmatism caused by the surgery. At present, studies on the effect of postoperative astigmatism on the shift of postoperative refractive state in patients with macular hole are unavailable. There is a slight difference in the refractive index between the vitreous body (1.3346) and aqueous humor (1.3336). During combined surgery, the vitreous body is replaced by aqueous fluid, and the difference in refractive index between the two changes the refractive index of the eye[24], resulting in myopic shift[15]. This in turn causes myopic shift of 0.13-0.5 D[15,23,25]. However, a study on the refractive state after cataract surgery in patients undergoing trans pars plana vitrectomy (TPPV) and non-TTPV in 2009 showed no significant difference between the predicted refractive value before cataract surgery after TPPV and the actual one after surgery[26]. Ⅰt has also been reported that the myopic shift is the same for patients in the cataract surgery group after TPPV and the TPPV combined cataract surgery group (-0.3 D)[27]Vitrectomy does not cause any myopic shift. This study did not find related factors that significantly affected the change in the postoperative refractive state. Considering that the calculation of lens degree remained accurate in the normal eye axis for biological measurement and SRK/T formula in macular hole patients, air had little effect on ACD after surgery, and the postoperative myopic shift of -0.33 D might be related to the removal of vitreous body.
While focusing on the rate of the hole closure, attention to visual quality is also needed as cataract surgeries. This study found that the ARE after ⅠMH surgery was shifted by -0.33±0.81 D when compared with PRE. After excluding the effects of the measurement of eye axis and ACD, the removal of vitreous body that caused changes in the refractive index should be mainly considered, which led to the change in the refractive state. The ⅠOL degree calculated before surgery was under corrected by about 0.3 D. After surgery, the patient’s vision was closer to the BCVA to obtain better visual quality. However, with the relatively small number of study cases and the retrospective nature of the study, it is still necessary to expand the sample size and design a prospective study to further confirm the changes in the trend and risk factors of postoperative refractive state in ⅠMH patients undergoing combined surgery.
ACKNOWLEDGEMENTS
Conflicts of Interest:Liu BS,None;Cui WN,None;Niu R,None;Chen Q,None;Nie ZT,None;Wei JT,None;Hu BJ,None.
 International Journal of Ophthalmology2021年2期
International Journal of Ophthalmology2021年2期
- International Journal of Ophthalmology的其它文章
- Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells
- Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension
- Retrobulbar administration of purified anti-nerve growth factor in developing rats induces structural and biochemical changes in the retina and cornea
- Surgical correction of recurrent epiblepharon in Chinese children using modified skin re-draping epicanthoplasty
- Ultrasound elastography for evaluating stiffness of the human lens nucleus with aging: a feasibility study
- Safety, effectiveness, and cost-effectiveness of Argus ll in patients with retinitis pigmentosa: a systematic review
