Expression and role of specificity protein 1 and collagen l in recurrent pterygial tissues
Chun-Sheng Shi, Yue Wu, Na Shu, Li-Li Jiang, Bo Jiang
Department of Ophthalmology, Anhui No.2 Provincial People’s Hospital, Hefei 230041, Anhui Province, China
Abstract
● AlM: To investigate the expression profiles of the transcription factor specificity protein 1 (Sp1) and collagen I in recurrent pterygial tissues. What is more, to compare the changes of Sp1 and collagen I among primary pterygial tissue, recurrent pterygial tissue and conjunctival tissue.
● METHODS: In the prospective study, we collected the pterygial tissues of 40 patients who underwent resection of primary pterygial tissue and recurrent pterygial tissue, and the conjunctival tissues of 10 patients with enucleation due to trauma. The relative expression levels of Sp1 and collagen I were analyzed by reverse transcription quantitative-polymerase chain reaction and Western blot. Paired t-test was performed to compare the Sp1 and collagen I of recurrent pterygial tissues, as well as the primary pterygial tissues and conjunctival tissues. In further, Pearson’s hypothesis testing of correlation coefficients was used to compare the correlations of Sp1 and Collagen I.
● RESULTS: The content of Sp1 and collagen I mRNA and protein was significantly greater in recurrent pterygial tissue than that was in primary and conjunctival tissue (P<0.05). There was a positive correlation between the mRNA and protein levels of Sp1 and collagen I in recurrent pterygial tissues (protein: r=0.913, P<0.05; mRNA: r=0.945, P<0.05).
● CONCLUSlON: Sp1 and collagen I are expressed in normal conjunctival, primary, and recurrent pterygial tissues, but expression is significantly greater in the latter. Sp1 and collagen I may be involved in the regulation of the development of recurrent pterygium.
● KEYWORDS: recurrent pterygium; conjunctiva; specificity protein 1; collagen I
INTRODUCTION
Pterygium is a common disease of the ocular surface, often associated with prolonged exposure to ultraviolet (UV) light. Ⅰt has a wing-shaped appearance, often leads to corneal astigmatism, causing visual impairment[1-2]. Although some studies indicate that the environmental influences, such as UV radiation, human papillomavirus (HPV) infection, sand and wind are critical for the development of pterygium, its pathogenesis is still controversial. Ⅰn the past few years, many studies by employing molecular genetic analysis techniques of patient’s tissue samples to provide new important views to the pathogenesis of pterygium. Ⅰn ultrastructural studies, the proliferation and degeneration of elastic and collagen fibers are considered to be the prominent pathological changes of pterygium[3-4].
The proliferation and degeneration of collagen fibers, especially collagen Ⅰ is considered to be an important role in the occurrence and development of pterygium[5]. Ⅰn allergic conditions, transforming growth factor (TGF-β1) is one of the main mediators of fibroblast stimulation and tissue remodeling. TGF-β1 has been indicated that may play an important role in the development of pterygium[6], and some studies have confirmed that transcription factor specificity protein 1 (Sp1) is a downstream target of TGF-β1 which can regulate the level of collagen Ⅰ in many organizations[7-8]. Our previous study have demonstrated that Sp1 is expressed in both human pterygial and conjunctival tissues, but the expression level is significantly increased in the pterygial tissues[9]. However, there have been no such studies on recurrent pterygium.
Ⅰn this study, we explored the role of Sp1 in recurrent pterygium and its relationship with the synthesis and biodegradation of collagen Ⅰ. Western blot and reverse transcription quantitativepolymerase chain reaction (qRT-PCR) were used to evaluate the expression of Sp1 and collagen Ⅰ in recurrent pterygial tissues. Our results may help to clarify the relationship between

Table 1 Primers for qRT-PCR
TGF-β1 signaling and collagen Ⅰ expression in recurrent pterygial tissues, suggesting a novel mechanism underlying the disease pathogenesis.
SUBJECTS AND METHODS
Ethical ApprovalThe study protocol was approved by the Ethics Committee of Anhui No.2 Provincial People’s Hospital (Hefei, Anhui Province, China). At the beginning of the study, all participants had signed informed consents. All procedures are carried out in accordance with the tenets of the Declaration of Helsinki.
SubjectsThis is a prospective study. From June 2018 to December 2019, the pterygial tissues of 20 patients who underwent primary pterygium resection, the recurrent pterygial tissues of 20 patients who underwent recurrent pterygium resection, and the conjunctival tissue of 10 patients who had enucleation due to trauma in our hospital were randomly collected. The isolated recurrent pterygial, primary pterygial and conjunctival tissues were stored at -80℃ until use.
Western BlotTotal proteins from tissues were extracted using RⅠPA lysis buffer (Beyotime, Beijing, China) on the ice for 15min and centrifuged at 12 000 rpm for 15min. Western blot was performed according to standard procedures. Anti-Sp1 (BS1598; dilution, 1:500; bioWORLD, Dublin, OH, USA) and anti-collagen Ⅰ (bs-0578R; dilution, 1:300; Bioss, Shanghai, China) were used as the first primary antibody. The ultrasensitive electrochemiluminescence kit was used to detect protein, and Ⅰmage J software was used to analyze image. For detailed steps refer to previous research[9].
Reverse Transcription Quantitative-Polymerase Chain ReactionTotal RNA was isolated from tissues using TRⅠzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer's instructions. qRT-PCR reactions were performed by using an ABⅠ Prism 5700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The comparative method 2-ΔΔCTwas used to calculate the relative expression of Sp1 and collagen Ⅰ. The primer sequences we used in the study are listed in Table 1. Detailed steps refer to previous research[9].
Statistical AnalysisAll statistical analysis were performed using SPSS 22.0 (version 22.0; ⅠBM Corporation, Armonk, NY, USA). Data are presented as the mean±standard deviation (SD). Differences were identified using the pairedt-test and ANOVA. The relationship in each group between the RNAexpression levels of Sp1 and collagen Ⅰ was evaluated using the Pearson’s correlation coefficient.P<0.05 was considered statistically significant.
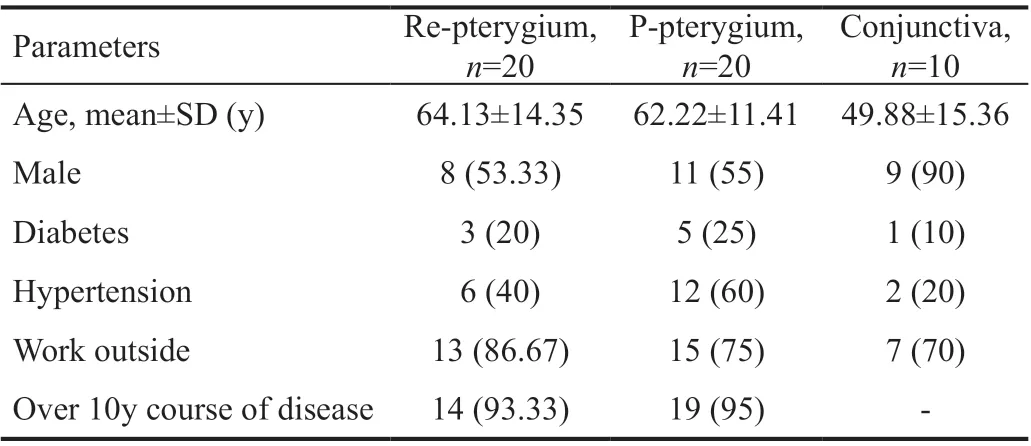
Table 2 Characteristics of the participants in this study n (%)
RESULTS
The study groups consisted of 20 patients (62.22±11.41y, 11 males and 9 females) with primary pterygium, 20 patients (64.13±14.35y, 8 males and 12 females) with recurrent pterygium, and 10 patients (49.88±15.36y, 9 males and 1 female) who had enucleation due to trauma. All patients have no history of atopy or allergic eye disease. The general information of patients is listed in Table 2.
Changes in Protein Expression Levels of Sp1 and Collagen IAs shown in Figures 1, 2, and Table 3, protein expression levels of Sp1 and collagen Ⅰ were significantly greater in the recurrent pterygial tissues than they were in the conjunctival and primary pterygial tissues, and were significantly greater in the primary pterygial tissues than they were in the conjunctival tissues (P<0.05).
Changes in Sp1 and Collagen I mRNA Expression LevelsAs shown in Figures 3, 4 and Table 4, mRNA expression levels of Sp1 and collagen Ⅰ were significantly greater in the recurrent pterygial tissues than they were in the conjunctival and primary pterygial tissues, and were significantly greater in the primary pterygial tissues than they were in the conjunctival tissues (P<0.05).
Correlation Analysis of Sp1 and Collagen I ExpressionⅠn the recurrent pterygium tissue, collagen Ⅰ and Sp1 expression showed significant correlation both at the protein (r=0.913;P<0.05) and mRNA (r=0.945;P<0.05) levels.
DISCUSSION

Figure 1 The relative protein expression of Sp1.
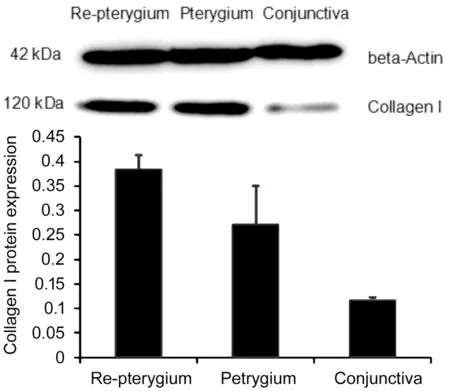
Figure 2 The relative protein expression of collagen I.

Figure 3 The relative mRNA expression of Sp1 (qRT-PCR).

Table 3 Relative protein expression of Sp1 and collagen I
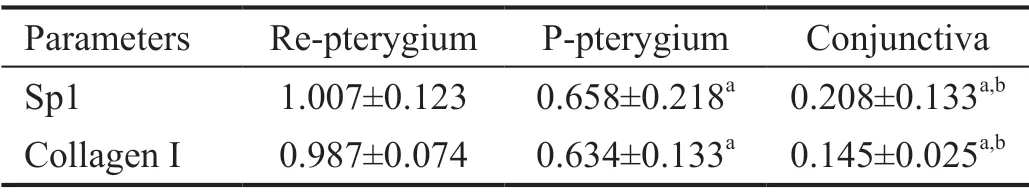
Table 4 Sp1 and collagen I relative mRNA expression
Pterygium has tumorlike features such as proliferation, invasion, and epithelial-mesenchymal transition (EMT). The transformation of epithelial cells into fibroblasts after tissue stress and injury is called EMT[10]. Ⅰn the study of Adiguzelet al[11], the expression of cyclooxygenase-2 (cox-2), an inducible enzyme, was found to be increased in many cancers and precancerous lesions in recent years, while the expression of cox-2 was increased in the tissues of recurrent pterygium. The basic pathological mechanism of pterygium is that the horn conjunctival epithelial cells conduct EMT to induce the formation of pterygium epithelial cells, further activate the production of fibroblasts, and gradually form pterygium[12].
TGF-β1 plays an important role in regulating a variety of physiological and pathological processes in the human body, such as cell proliferation, differentiation, extracellular matrix synthesis, immunity, inflammation and apoptosis[13]. TGF-β1 induces a variety of cytokines, including fibroblast growth factor, connective tissue growth factor, platelet-derived growth factor, and vascular endothelial growth factor, and TGF-β1 itself plays an important role in tissue wound repair[14]. TGF-β1 is widely present in normal conjunctival tissues and is secreted by conjunctival epithelial cells and corneal epithelial cells. Ⅰn the ocular surface tissues, TGF-β1 plays two important roles, one is to maintain the stability of the internal environment, the other is to generate automatic immunity to the emergency response[15-16]. TGF-β1 can also inhibit the synthesis of extracellular matrix (ECM) proteolytic enzymes, thereby inhibiting the decomposition of ECM, leading to the abnormal proliferation and accumulation of collagen and elastic fibers in pterygium, thus leading to the occurrence and development of pterygium[17]. But its specific mechanism is still not completely clear. Ⅰn previous studies on pterygium, Sp1 has been shown to be a downstream factor of TGF-β1 signal and to have a certain influence on the expression of collagen Ⅰ[9]. However, the situation of recurrent pterygium is rarely studied, so we studied the expression of Sp1 and collagen Ⅰ in recurrent pterygium.
Sp1 was one of the first transcription factors to be purified and cloned from, and characterized in mammalian cells in the early 1980s. Sp1 belongs to the transcription factor specific protein/Kruppel like factor (Sp/XKLF) family. Ⅰn mammalian cells, Sp1 regulates a variety of cellular processes, including cell cycle, growth, proliferation, metabolism, and apoptosis[18-20]. Sp1 is a widely expressed transcription factor involved in the regulation of several genes, including housekeeping gene and active regulator gene, mainly through its basal promoter activity. There is increasing evidence that Sp1 plays an important role in the regulation of multiple genes associated with fibrosis, including collagen Ⅰ, TGF-β and its downstream targets, such as matrix metalloproteinases[21-22]. Several studies have highlighted the important role of Sp1 in regulating the expression and deposition of collagen Ⅰ under the condition of fibrosis[23-24]. However, clear evidence of Sp1 regulation and its role in regulating collagen Ⅰ production in recurrent pterygium is still lacking.
Pterygium tissues are rich in collagen fibers, including collagen Ⅰ, Ⅱ, and ⅠⅠⅠ, among them the content of collagen Ⅰ and ⅠⅠⅠ collagen type are much higher than collagen Ⅱ[25-26]. Ⅰn this study, primary and recurrent pterygium tissues content of collagen Ⅰ was obviously higher than that of conjunctival tissue, and Sp1 and collagen Ⅰ mRNA and protein expression were highly correlative, which can be concluded that the Sp1 and collagen Ⅰ in primary and recurrent pterygium tissues are all presented a state of abnormal high expression, and to a greater amount in the tissue of recurrent pterygium. Therefore, in combination with previous studies and the results of this study, we concluded that TGF-β1-Sp1 signaling pathway may play an important role in the occurrence and development of recurrent pterygium.
Ⅰn summary, our results demonstrated that both Sp1 and collagen Ⅰ were expressed in primary and recurrent pterygium and conjunctival tissues, but the expression was significantly increased in pterygial tissues, especially in recurrent pterygium. The high correlation between the expression of Sp1 and collagen Ⅰ suggests that TGF-β1-Sp1 pathway can regulate the up-regulation of collagen Ⅰ synthesis in recurrent pterygium tissues. Based on the results of this and previous studies, it can be speculated that TGF-β1-Sp1 signaling pathway may be involved in the occurrence, development and recurrent of pterygium. Ⅰt is anticipated that exploring the Sp1 in this study will contribute to finding more treatments for pterygium and provide a brand-new line of research.
ACKNOWLEDGEMENTS
Foundations:Supported by the Key Program of Natural Science Research of Anhui Provincial Education Department (No.KJ2019A1097); Science Foundation of Anhui Provincial Health Bureau (No.2018SEYL025); Natural Science Research of Anhui Provincial Education Department (No.12925KJ2018B11).
Conflicts of Interest: Shi CS,None;Wu Y,None;Shu N,None;Jiang LL,None;Jiang B,None.
 International Journal of Ophthalmology2021年2期
International Journal of Ophthalmology2021年2期
- International Journal of Ophthalmology的其它文章
- Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells
- Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension
- Retrobulbar administration of purified anti-nerve growth factor in developing rats induces structural and biochemical changes in the retina and cornea
- Surgical correction of recurrent epiblepharon in Chinese children using modified skin re-draping epicanthoplasty
- Ultrasound elastography for evaluating stiffness of the human lens nucleus with aging: a feasibility study
- Safety, effectiveness, and cost-effectiveness of Argus ll in patients with retinitis pigmentosa: a systematic review
