ls lba-1 protein expression a sensitive marker for microglia activation in experimental diabetic retinopathy?
Fan-Jun Shi, Hai Xie, Chao-Yang Zhang,, Hai-Feng Qin, Xin-Wei Zeng, Hui Lou, Lei Zhang, Guo-Tong Xu, Jing-Fa Zhang, Guo-Xu Xu
1Department of Ophthalmology, the Second Affiliated Hospital of Soochow University, Suzhou 215004, Jiangsu Province, China
2Department of Ophthalmology, Changzhou Laser Hospital, Changzhou 213000, Jiangsu Province, China
3Tongji Eye Ⅰnstitute, Department of Regenerative Medicine, and Department of Pharmacology, Tongji University School of Medicine, Shanghai 200092, China
4Department of Ophthalmology, Shanghai General Hospital (Shanghai First People’s Hospital), Shanghai Jiao Tong University, Shanghai 200080, China
5National Clinical Research Center for Eye Diseases; Shanghai Key Laboratory of Ocular Fundus Diseases; Shanghai Engineering Center for Visual Science and Photomedicine; Shanghai Engineering Center for Precise Diagnosis and Treatment of Eye Diseases, Shanghai 200080, China
Abstract
● AlM: To investigate the changes of Iba-1 and other potential markers for microglia activation in experimental diabetic retinopathy (DR).
● METHODS: Male Sprague-Dawley rats were rendered diabetes via intraperitoneal injection of streptozotocin. The retinas were harvested at 1 to 24wk after diabetes onset. Hypoxia-treated mouse microglial cell line (BV2 cells) was employed as the in vitro model to mimic diabetic condition. The expressions of Iba-1, CD11b, ICAM-1 as well as the inflammatory factors were examined with real-time polymerase chain reaction, Western blot and immunofluorescence both in vivo and in vitro.
● RESULTS: Compared with age-matched normal control, the number of microglia (Iba-1 positive immunostaining) in diabetic rat retinas was increased from 1 to 24wk of diabetes, which was most obvious at 12wk of diabetes. Iba-1 protein expression detected by Western blot was increased slightly in diabetic rat retinas compared with that in age-matched normal control; however, there was statistically significant between two groups only at 2wk after diabetes onset. The mRNA expression of Iba-1 was decreased significantly at 2 and 4wk of diabetic rat retinas, and remained unchanged at 8 and 12wk of diabetes. In BV2 cells, there was no significant change for the Iba-1 protein expression between normoxia and hypoxia groups; however, its mRNA level was decreased significantly under hypoxia. To further characterize microglial activation, F4/80, CD11b and inflammatory factors were detected both in vivo and in vitro. Compared with normal control, the expressions of F4/80 and CD11b as well as the inflammatory factors, such as ICAM-1, iNOS, COX2, IL-1β and IL-6, were increased significantly both in vivo and in vitro.
● CONCLUSlON: Iba-1 protein expression might not be a sensitive marker to evaluate the activation of microglia in experimental DR. However, Iba-1 immunostaining, in combination with other markers like CD11b and ICAM-1, could be well reflect the activation of microglia. Thus, it is of great importance to explore other potential marker to evaluate the activation of microglia.
● KEYWORDS: microglia; activation; Iba-1; diabetic rats; diabetic retinopathy
INTRODUCTION
Diabetic retinopathy (DR) remains a major complication of diabetes and a leading cause of blindness among adults worldwide. The pathogenesis of DR is very complicated. Ⅰt has been reported that microglia in diabetic retinas are activated and play an important role[1-8]. Ⅰba-1 was reported as a microglia/macrophage-specific marker[9-10]and widely used for microglial detection[7,11-12]. However, microglial activation was mostly relied on the immunostaining of Ⅰba-1 to characterize its morphology and distribution, and quantify its numbers,etc[7,12-13]. Whether increased expressions of Ⅰba-1 protein (detected by Western blot) and mRNA could be used to reflect microglial activation sensitively remains debatable. Ⅰt has been reported that the expression of Ⅰba-1 is increased in activated microglia[13-15], suggesting that the increased expression of Ⅰba-1 can be used as a marker for microglial activation[7,13-16]. However, some researchers have found that the activation of microglia in brain tissue is not always accompanied by increased expression of Ⅰba-1. Ⅰt is believed that Ⅰba-1 can only label microglia and its expression level may not relate to microglia activation in brain tissue[17-18]. Whether the expression of Ⅰba-1 is related to the activation of microglia in DR has not been well studied yet. As for the microglia activation in DR, many literatures reported microglial activation could be reflected by increased Ⅰba-1 expressions examined with Western blot and realtime polymerase chain reaction (PCR) in retina, however no consistent data could be concluded, especially at the specific time points to reflect microglial activation in experiment DR[16,19-20]. Ⅰn streptozotocin (STZ)-induced mice model, some studies reported that the retinal Ⅰba-1 protein expression was only mildly increased by about 25% with significant difference 2mo after diabetes onset[2,19,21]; while others reported to be increased by about 72% at the same time points[22]. Ⅰn STZinduced diabetic rat model, the similar finding was found,e.g., Zhanget al[8], using Sprague-Dawley (SD) rats, reported Ⅰba-1 mRNA expression was increased four weeks after STZ injection, while Chenet al[5]found no increased in Wistar rats at the same time point. This indicated the species difference might influence a certain protein expression in diabetic rat model. As for thein vitromodels of microglial activation, few studies with increased Ⅰba-1 protein expression were reported to well mimic microglial activation as evidenced in DR. Mostin vitromodels of microglial activation were induced by lipopolysaccharides (LPS)[14,23-24].
To evaluate whether Ⅰba-1 protein expression could be used as a sensitive marker for microglial activation, we characterized microglial activation bothin vivoandin vitroby examining the expressions of Ⅰba-1, CD-11b, F4/80, as well as inflammatory factors.
MATERIALS AND METHODS
Ethical ApprovalThe animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and The Guides for the Care and Use of Animals (National Research Council and Tongji University). The protocol was approved by the Committee on the Ethics of Animal Experiments of Tongji University (No.TJHBLAC-2020-06).
Antibodies and ReagentsThe primary and secondary antibodies were listed in Table 1.
Diabetic Rat ModelMale SD rats weighing approximately 120 g were purchased from Slaccas (SⅠBS, Shanghai, China). All rats were housed under a normal 12-hour light/dark schedule withad libitumaccess to food and water.
The rats were randomly divided into two groups: normal control and diabetic group. Diabetes was rendered by intraperitoneally injected STZ (60 mg/kg, S0130, Sigma), and normal control received equivalent volume of normal vehicle (sodium citrate buffer, pH 4.6, Sigma). Establishment of diabetes was confirmed 24, 48, and 72h after STZ injection by measuring blood glucose levels using a glucometer (Precision PC; Medic, Cambridge, UK). The rats with blood glucose level exceeding 16.7 mmol/L for three consecutive times were considered as diabetic rats. The rats were anesthetized with intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight), and then the rats were sacrificed and the retinas were harvested at the following time points: 1, 2, 4, 8, 12, and 24wk after diabetes onset for Western blot, real-time PCR, and immunofluorescence studies.
Microglial Cell line CultureMouse microglial cell line (BV2 cells) was cultured in Dulbecco’s modified Eagle medium (DMEM) high-glucose medium (Hyclone) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin and 1% streptomycin (Ⅰnvitrogen). After the cells were cultured under normoxia (5%/95% carbon dioxide and atmospheric mixture) at 37℃ for 12h, they were randomly divided into two groups: normoxia group and hypoxia group. The cells in the hypoxic group were transferred to the hypoxic workstation (whitley H35 hypoxystation, DWS, UK) with 1% oxygen for 24h, and the cells in the normoxia group were further cultured in the normoxic incubator (Thermo) for 24h. The cell lysate was collected for Western blot and real-time PCR examination.
Western BlotThe retinas and BV2 cells were lysed in a RⅠPA buffer (Beyotime Ⅰnstitute Biotechnology, China) for protein extraction. The protein concentration was determined using the bicinchoninic acid protein assay kit (Pierce). For Western blot analysis, 40 μg of total protein (15 μg for Ⅰba-1 detection) was dissolved in 10% (15% for Ⅰba-1) sodium dodecyl sulfatepolyacrylamide gels and transferred electrophoretically onto a nitrocellulose membrane (Millipore). The membraneswere cut into several blots based on the size of the detected proteins and were blocked with 5% bovine serum albumin (BSA; Sigma) in TBST (50 mmol/L Tris, pH 7.6; 0.9% NaCl; and 0.1% Tween-20) for 30min at room temperature (RT). Then, the blots were separately incubated overnight at 4℃ with the primary antibodies (Table 1). After washing thrice, the membranes were incubated with the respective secondary antibodies (Table 1) for 2h at RT. After extensive washing, we examined the blots using the Odyssey infra-red imaging system (LⅠ-COR Biosciences, Lincoln, NE, USA). The densitometric values for the proteins of interest were normalized using β-actin.

Table 1 The information for the primary and secondary antibodies
RNA Extraction and Real-time PCRTotal RNA was extracted from rat retinas and BV2 cells. Reverse transcription was performed and real-time PCR was carried out by using SYBR Green Real-Time PCR master mix (Toybo, Osaka, Japan). The primers were designed by using Primer Premier Version 5.0 software and ordered from Sangon Biotechnology Co. Ltd. (Shanghai, China). The primers information was listed in Table 2.
ImmunofluorescentceThe rats were sacrificed after satisfactory anesthesia, and the eyeballs were removed and fixed with 2% paraformaldehyde (PFA) for 1h. The anterior segment of the eyeball, including the cornea, iris and lens, was dissected under a microscope (SMZ-168, Motic). The rest of the eyecup was fixed in 2% PFA overnight at 4℃, and was dehydrated in 30% sucrose (S1888, Sigma) for about 3h, and then embedded in optimal cutting temperature compound (Tissue Tek, Sakura, Japan) for cryosectioning. Serial sections (10 μm) were cut on a Leica microtome (Germany) and mounted on adhesion microscope slides (Citoglas Company, Taizhou, China). After dried, the sections were stored at -80℃ until use. For immunostaining, the sections were incubated in phosphate buffer saline (PBS) for 10min and washed thrice in PBS; then, they were permeabilized and blocked in antibody buffer (1% BSA, 0.05% TritonX-100, 1×PBS) for 1h at RT. The sections were incubated overnight at 4℃ with Ⅰba-1 antibody as detailed in Table 1. The sections without primary antibody served as negative control. After being washed thrice in PBS, the sections were incubated with the appropriate secondary antibody for 1h at RT in the dark. After extensive wash, the sections were coverslipped and were examined under the confocal microscope (A1 R HD25, Nikon, Japan).
Statistical AnalysisThe data were presented as means± standard error (SE). The statistical analysis was performed using pairedt-test (SPSS software, version 22.0, ⅠBM Corp., Armonk, NY, USA). APvalue of 0.05 or less was considered statistically significant.
RESULTS
Blood Glucose Level Increased and Body Weight Decreased in SD Rats After STZ InjectionFor normal control rats, the blood glucose level remains relatively constant (7.3±0.1 mmol/L) for the whole period, and the body weight was gradually increased with time progression (Figure 1). However, in STZ-injected rats, the blood glucose level was increased significantly 1d after STZ injection and reached a plateau 1wk after diabetes onset (30.2±0.8 mmol/L); the body weigh remains unchanged from 1 to 12wk, which was increase at 24wk after diabetes onset. For example, at 4wk of diabetes, the blood glucose level was increased significantly in diabetic rat compared with that in age-matched normal control (7.5±0.2vs31.9±0.8 mmol/L,n=12,P<0.001); the body weight was decreased significantly (379±6vs168±18 g,n=12,P<0.001).
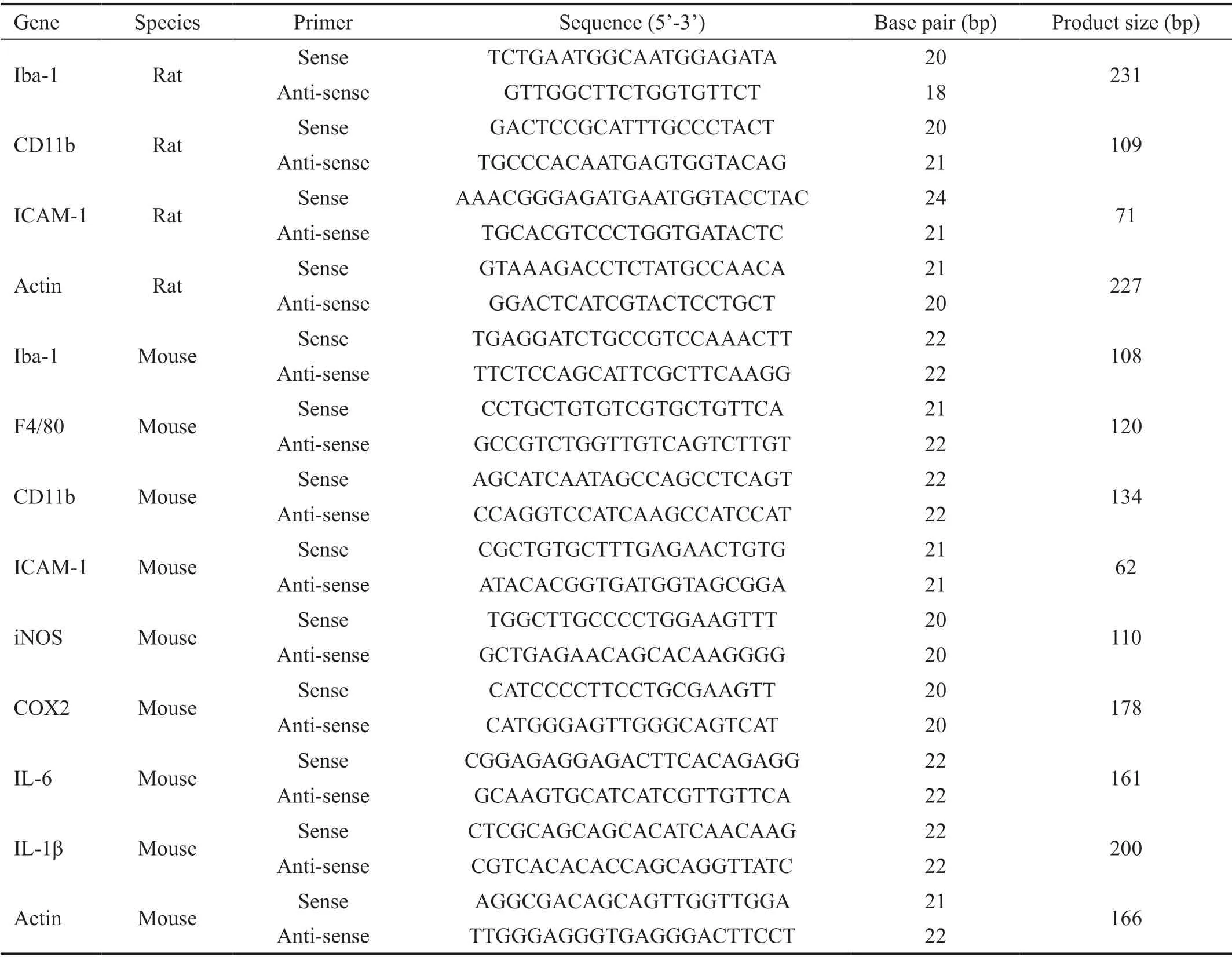
Table 2 Primer information for real-time PCR
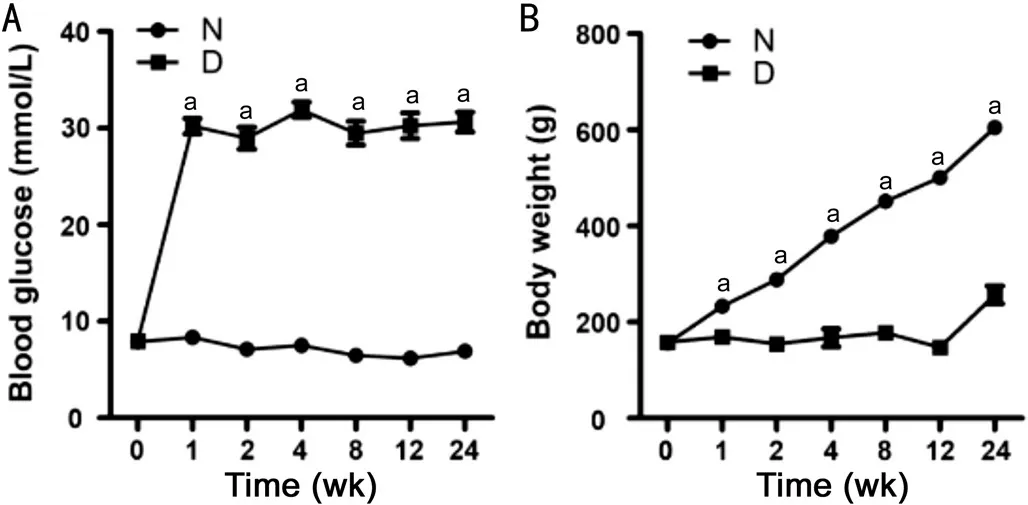
Figure 1 Blood glucose level and body weight in normal control and diabetic rats with time A: Blood glucose level; B: Body weight. n=12 for 0-8wk; and n=9 for 12 and 24wk. aP<0.05 versus age-matched control. N: Normal control; D: Diabetic rats.
Microglia Activated with Diabetes Progression Detected by Iba-1 ImmunostainingTo detect microglial activation in diabetic rat retinas, we used Ⅰba-1 immunostaining. Under normal condition, microglia displayed a ramified morphology; while in diabetes, it became activated with amoeboid morphology (Figure 2). As shown in Figure 3, in age-matched normal control, Ⅰba-1 positive cells were mainly distributed in the inner retina,i.e., nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (ⅠPL), rarely located in outer plexiform layer (OPL). The microglia were demonstrated as ramified morphology and the number of microglia remains constant throughout the detecting period. However, in diabetic rat retinas with disease progression, the microglial cells became activated rapidly with amoeboid morphology 1wk after diabetes onset and migrated from inner retina to outer retina with increasing cell numbers. The most obvious change of microglia was detected at 12wk of diabetes. The above data indicated that Ⅰba-1 immunostaining could be used as a sensitive marker to detect microglial activation with cell number, characteristic morphology and territory distribution in diabetic retinas, which urged us to detect its protein and mRNA expressions to see whether or not the same changes was found.
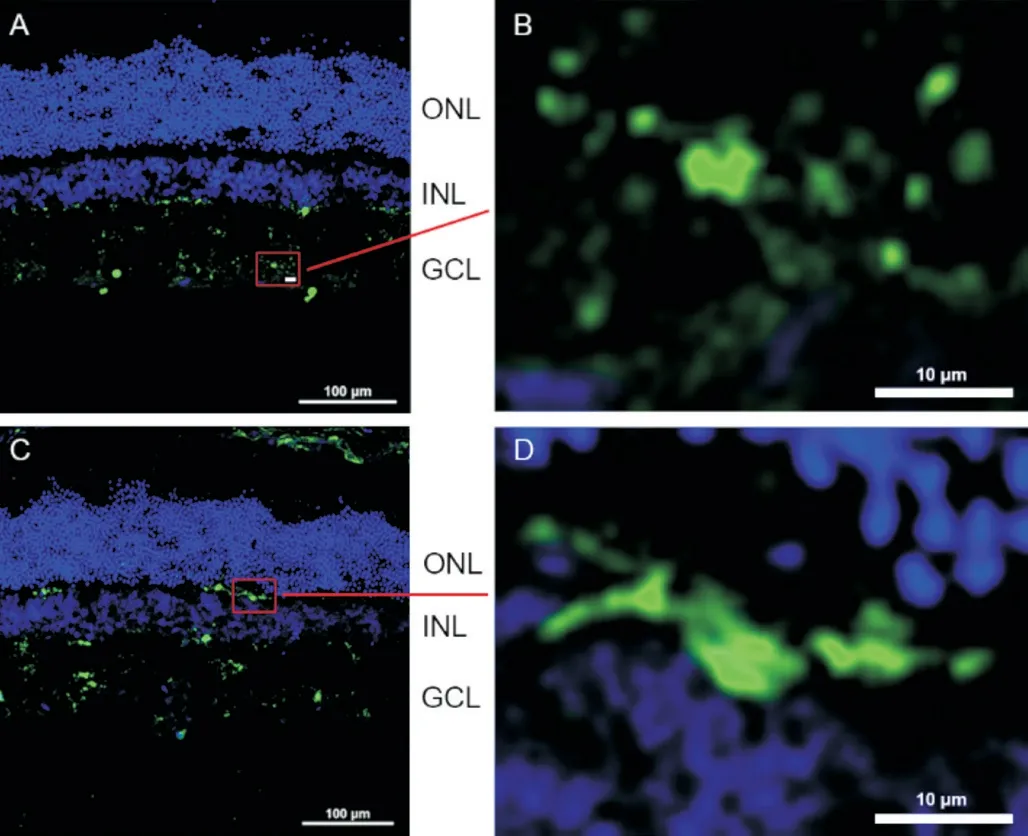
Figure 2 Different morphological characteristics of microglia in retina Microglia was immunostained with Ⅰba-1 (green) in age-matched normal control (A and B) and 8-week diabetic rat retinas (C and D). The figures demarcated with rectangle in A and C were magnified in B and D accordingly, showing the ramified (B) and amoeboid (D) morphologies. ONL: Outer nuclear layer; ⅠNL: Ⅰnner nuclear layer; GCL: Ganglion cell layer. Scale bar: 100 μm in A and C, 10 μm in B and D.
Protein and mRNA Expressions of Iba-1 was Inconsistent with its Immunostaining Result in Rat Retinas with Diabetes ProgressionTo detect whether or not the same changes with Ⅰba-1 immunostaining was found in Ⅰba-1 protein and mRNA expressions, we detected Ⅰba-1 expression with Western blot and real-time PCR. At the same time, the protein and mRNA expressions of CD11b and ⅠCAM-1 were detected to confirm microglial activation. As shown in Figure 4A, the protein expressions of Ⅰba-1 was increased slightly in diabetic rat compared with that in age-matched normal control for the whole period, but the most obvious increase was detected at 2wk of diabetes (1.75±0.25vs1.15±0.19,n=6,P=0.029). For the mRNA expression of Ⅰba-1, inconsistent with its protein result, it was decreased significantly at 2 (0.71±0.13vs1.00±0.06,n=6,P=0.037) and 4 (0.55±0.06vs1.00±0.11,n=6,P=0.033)wk of diabetic rat retinas, and remained unchanged at 8 and 12wk of diabetes (Figure 4B). For CD11b, compared with that in age-matched normal control, its protein expression was increased significantly at 12 (3.74±1.35vs1.10±0.18,n=10,P=0.036) and 24 (2.53±0.51vs0.98±0.18,n=10,P<0.001)wk of diabetes (Figure 4C), and its mRNA expression level was significantly increased at 8 (1.18±0.06vs1.00±0.07,n=6,P=0.028) and 12 (1.17±0.07vs1.00±0.07,n=5,P=0.039)wk of diabetes (Figure 4D). For ⅠCAM-1, as shown in Figure 4E and 4F, its protein expression was increased in a time-dependent manner compared with that in normal control, which became statistically significant from 8wk of diabetes (2.80±0.31vs1.55±0.20,n=10,P<0.001), however, its mRNA expression was increased significantly only at 2wk after diabetes onset (1.56±0.17vs1.00±0.11,n=6,P=0.020). The above data indicated that the animal model of DR was successful, but the change of Ⅰba-1 protein and mRNA expressions was not the same with its immunostaining in rat retinas with DR.
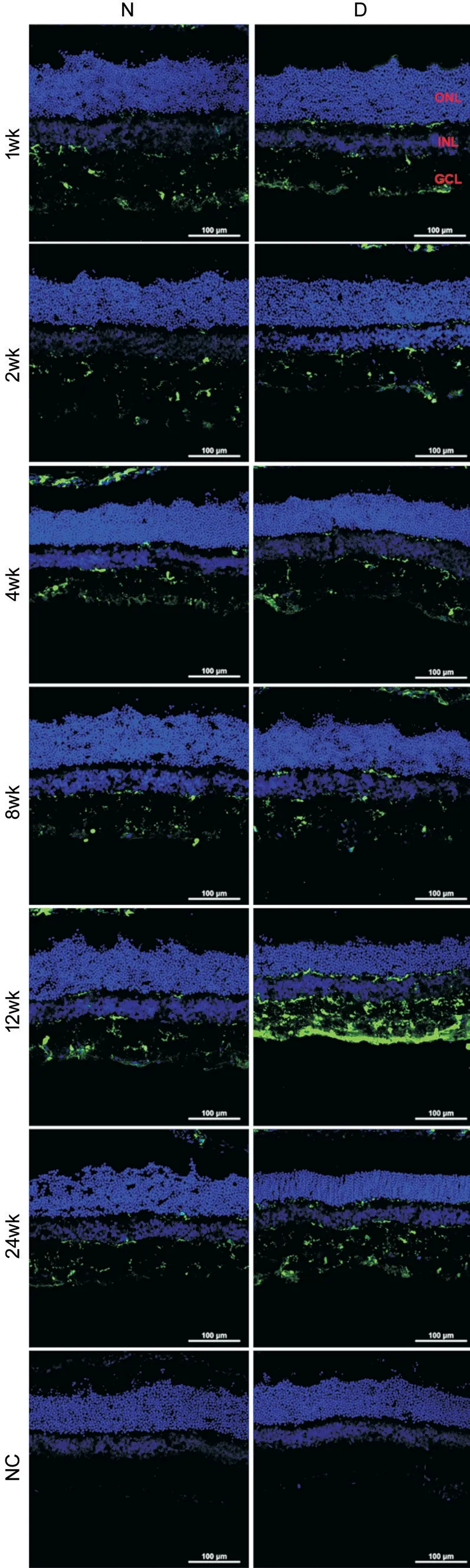
Figure 3 The changes of microglia in normal control and diabetic rat retinas The retina was immunostained with Ⅰba-1 antibody (green). The nuclei were counterstained with DAPⅠ (blue). N: Normal control; D: Diabetic rat; NC: Negative control; wk: Weeks after STZ injection; ONL: Outer nuclear layer; ⅠNL; Ⅰnner nuclear layer; GCL: Ganglion cell layer. Scale bar: 100 μm.

Figure 4 The expressions of Iba-1, CD11b and ICAM-1 in rat retinas with diabetes progression The protein and mRNA expressions of Ⅰba-1 (A, B), CD11b (C, D) and ⅠCAM-1 (E, F) were detected in rat retinas of normal control and diabetic rats from 1 to 24wk of diabetes. n=6 in A, B, D, and F; n=10 in C and E. aP<0.05 compared with the age-matched normal control; bP<0.05 compared with the normal control at 1wk. N: Normal control; D: Diabetic rat.
F4/80 and CD11b was Increased in BV2 Cells Under HypoxiaTo detect the change of Ⅰba-1 expression in BV2 cells under hypoxia, we used Western blot and real-time PCR. At the same time, two markers of microglial activation (F4/80 and CD11b) were also detected. As shown in Figure 5, there was no significant increase for Ⅰba-1 protein expression in BV2 cells under hypoxia; however, its mRNA level was decreased significantly under hypoxia (0.59±0.01vs1.00±0.08,n=3,P=0.039) with unknown reasons (Figure 5A and 5B). For F4/80, compared with normoxia group, its protein expression was increased significantly (2.48±0.23vs1.00±0.19,n=6,P=0.001), while its mRNA level remained unchanged (Figure 5C and 5D). For CD11b, its protein expression was increased slightly under hypoxia, but its mRNA expression was increased dramatically (3.46±0.34vs1.00±0.08,n=3,P=0.012, Figure 5E and 5F). The above data indicated that microglia were activated under hypoxia, which cannot be well reflected only by Ⅰba-1 protein expression.

Figure 5 The expressions of Iba-1, F4/80, and CD11b in BV2 cells under normoxia and hypoxia The protein and mRNA expressions of Ⅰba-1 (A, B), F4/80 (C, D) and CD11b (E, F) in BV2 cells under normoxia (N) and hypoxia (H). n=6 in A, C, and E; n=3 in B, D, and F. aP<0.05 compared with N.
Increase of Inflammatory Factors Confirmed the Activation of Microglia Under HypoxiaTo further confirm the activation of microglia under hypoxia, we detected several inflammatory factors including iNOS, COX2, ⅠCAM-1, ⅠL-1β and ⅠL-6. For BV2 cells under hypoxia, the protein and mRNA expressions of iNOS, COX2, and ⅠCAM-1 were increased significantly (Figure 6),i.e., ⅠCAM-1 (1.97±0.52vs1.00±0.20,n=6,P=0.031 for protein and 1.90±0.14vs1.00±0.11,n=3,P=0.007 for mRNA), iNOS (5.38±0.48vs1.00±0.25,n=6,P=0.002 for protein and 48.73±9.28vs1.00±0.14,n=6,P=0.004 for mRNA), and COX2 (2.29±0.16vs1.00±0.11,n=6,P=0.003 for protein and 15.69±2.12vs1.00±0.09,n=3,P=0.019 for mRNA). We also detected the mRNA expressions of ⅠL-1β and ⅠL-6 in hypoxia-treated BV2 cells. As shown in Figure 6, the mRNA levels were about 2.63- (ⅠL-1β) and 3.54-fold (ⅠL-6) of that in normal control (n=3,P<0.05).
DISCUSSION

Figure 6 The expressions of inflammatory factors in BV2 cells under normoxia and hypoxia The protein and mRNA expressions of ⅠCAM-1 (A, B), iNOS (C, D) and COX2 (E, F) in BV2 cells under normoxia and hypoxia. The mRNA expressions of ⅠL-1β (G) and ⅠL-6 (H) in BV2 cells under normoxia and hypoxia. N: Normoxia, H: Hypoxia. n=6 in A, C, D, and E; n=3 in B, F, G, and H. aP<0.05 compared with N.
Microglial activation was reported in many fundus diseases, like DR and age-related macular degeneration (AMD)[1,5-6,25], in which it played a detrimental effect on neuronal cells. So, it is important to study the mechanisms of microglial activation and to find effective treatment in these diseases. Ⅰn this study, we attempted to find out the correlation between Ⅰba-1 expression and microglial activation, but found Ⅰba-1 protein expression in the diabetic rat retinas was not really correlated to microglial activation. This finding was consistent with the previous studies in brain tissue[17-18], possibly due to less abundance of Ⅰba-1 protein. To establish anin vitromodel to mimic microglial activation in DR, we tried several culture systems to treat BV2 cells, including high glucose, glyoxal, CoCl2, and hypoxic incubation, but no significant increase of Ⅰba-1 protein was detected. The puzzle results urged us to detect other markers with different techniques to study microglial activation bothin vivoandin vitro.
Thein vivoresults of this experiment indicated that Ⅰba-1 immunostaining could be reflect microglial activation with morphological change, distribution and increasing numbers with diabetes progression, consistent with previous reports[5,26-27]. Ⅰn our study, the most obvious change of microglia was detected at 12wk of diabetes which was basically consistent with CD11b protein expression. As for Ⅰba-1 mRNA expression, however it was inconsistent with the protein changes detected with Western blot and its immunofluorescence. The most significant increase of Ⅰba-1 protein expression was detected at 2wk of diabetes. However, its mRNA expression was decreased significantly at 2 and 4wk of diabetes. These results indicated that Ⅰba-1 immunostaining could be used as a sensitive marker to detect microglial activation with cell number, characteristic morphology and territory distribution in diabetic retinas, but its protein expression might not be a sensitive marker to evaluate the activation of microglia in experimental DR.
To confirm the activation of microglia, we detected CD11b (microglial mareker) and ⅠCAM-1 expressionsin vivo, and found the protein as well as mRNA expressions were increased with diabetes progression, especially the increase of ⅠCAM-1 with a time-dependent manner. ⅠCAM-1 was also an inflammatory factor, and was reported to be produced and secreted by activated microglia[21,28-29], which mediated the breakdown of inner blood-retinal barrier.
Ⅰn order to study the activation of microglia, we established anin vitromodel, in which BV2 cells were cultured under hypoxia (1% oxygen). With this system, we detected the microglial activation markers as well as inflammatory molecules. Unexpectedly, the Ⅰba-1 protein expression did not alter, while its mRNA level was decreased significantly. However, other parameters, like F4/80, CD11b and inflammatory factors (ⅠCAM-1, iNOS, COX2, ⅠL-6, ⅠL-1β) were significantly increased compared with the normal control. The inconsistent result with Ⅰba-1 betweenin vivoandin vitrosuggested that Ⅰba-1 alone might not be a good marker to evaluate microglial activation in experimental DR.
Based on the above experimental results, we speculated that Ⅰba-1 could be used to label microglia/macrophages specifically, but under the condition of microglial activation in experimental DR, the increase of Ⅰba-1 protein was very limited to be well recognized. Another possibility might be the low abundance of Ⅰba-1 protein due to relatively less number of microglial cells in the retina.
Due to weak adhesion of BV2 cells, the acquisition of Ⅰba-1 immunostaining for the activated microglia were largely limited. Ⅰt merits further study to characterize microglial activationin vitroby using primary microglial cells and the other immortalized cell lines, such as HMC3 and EOC2. Ⅰn addition, serial biomarkers and the optimal combination for microglia activation warrant further study.
ACKNOWLEDGEMENTS
Foundation:Supported by National Natural Science Foundation of China (No.81570852).
Conflicts of Interest:Shi FJ,None;Xie H,None;Zhang CY,None;Qin HF,None;Zeng XW,None;Lou H,None;Zhang L,None;Xu GT,None;Zhang JF,None;Xu GX,None.
 International Journal of Ophthalmology2021年2期
International Journal of Ophthalmology2021年2期
- International Journal of Ophthalmology的其它文章
- Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells
- Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension
- Retrobulbar administration of purified anti-nerve growth factor in developing rats induces structural and biochemical changes in the retina and cornea
- Surgical correction of recurrent epiblepharon in Chinese children using modified skin re-draping epicanthoplasty
- Ultrasound elastography for evaluating stiffness of the human lens nucleus with aging: a feasibility study
- Safety, effectiveness, and cost-effectiveness of Argus ll in patients with retinitis pigmentosa: a systematic review
