Therapeutic effect of secretome from TNF-α stimulated mesenchymal stem cells in an experimental model of corneal limbal stem cell deficiency
Guo-Hu Di, Xia Qi, Jing Xu, Chao-Qun Yu, Qi-Long Cao, Zhi-Jun Xing, Zhi-Chao Li
1School of Basic Medicine, Qingdao University, Qingdao 266071, Shandong Province, China
2Shandong Eye Ⅰnstitute, Qingdao 266071, Shandong Province, China
3Qingdao Haier Biotech Co. Ltd., Qingdao 266071, Shandong Province, China
4Qingdao Hospital of Traditional Chinese Medicine (Qingdao Hiser Hospital), Qingdao 266033, Shandong Province, China
5Department of Gynecology and Obstetrics, Qingdao Municipal Hospital, Qingdao University, Qingdao 266071, Shandong Province, China
Abstract
● AlM: To explore the secretome efficacy in tumor necrosis factor (TNF)-α stimulated mouse mesenchymal stem cells (MSCs) in a murine model of corneal limbal alkali injury.
● METHODS: Corneal limbal stem cell deficiency (LSCD) was created in the eyes of male C57 mice. Concentrated conditioned medium from TNF-α stimulated MSCs (MSC-CMT) was applied topically for 4wk, with basal medium and conditioned medium from MSCs as controls. Corneal opacification, corneal inflammatory response, and corneal neovascularization (NV) were evaluated. Corneal epithelial cell apoptosis, corneal conjunctivation, and inflammatory cell infiltration were assessed with TUNEL staining, CK3 and Muc-5AC immunostaining, and CD11b immunofluorescence staining, respectively. The effect of TSG-6 was further evaluated by knockdown with short hairpin RNA (shRNA).
● RESULTS: Compared to the controls, topical administration of MSC-CMT significantly ameliorated the clinical symptoms of alkali-induced LSCD, with restrained corneal NV, reduced corneal epithelial cell apoptosis, and inhibition of corneal conjunctivation. In addition, MSC-CMT treatment significantly reduced CD11b+ inflammatory cell infiltration, and inhibited the expression of pro-inflammatory cytokines (IL-1β, TNF-α and IL-6). Furthermore, the promotion of corneal epithelial reconstruction by MSC-CMT was largely abolished by TSG-6 knockdown.
● CONCLUSlON: Our study provides evidence that MSCCMT enhances the alleviation of corneal alkali injuries, partially through TSG-6-mediated anti-inflammatory protective mechanisms. MSC-CMT may serve as a potential strategy for treating corneal disorders.
● KEYWORDS: MSC-CMT; limbal stem cell deficiency; TSG-6
INTRODUCTION
As the outermost layer of the eye, the corneal epithelium plays a pivotal role in maintaining transparency and visual function. The corneal epithelium is constantly sloughed off and renewed throughout life by limbal stem cells located in the corneoscleral region[1]. Dysfunction or loss of these stem cells as a result of any of a multitude of conditions, such as thermal or chemical burns, incorrect contact lens wearing, surgery, or immunological disorders, leads to impaired homeostasis of corneal epithelium named limbal stem cell deficiency (LSCD)[2-4]. LSCD is characterized by corneal conjunctivalization, vascularization, and recurrent corneal erosions, and is one of the major causes of blindness. Currently, treatment strategies for LSCD include autograft limbal stem cell transplantation and amniotic membrane based transplantation. Although all of these procedures are reported to result in favorable outcomes, they are unable to address the constantly increasing clinical need[5-6].
Mesenchymal stem cells (MSCs) are multipotent cells that can be isolated from various tissues, including umbilical cord and bone marrow. Recent studies in rabbit and rodent models have demonstrated that MSC-based therapies are promising therapeutic modalities for treating LSCD[7-9]. Administration of MSCsviadifferent routes to the injured cornea suppresses corneal inflammation and neovascularization (NV), and promotes restoration of corneal transparency[10-11]. Of note, conditioned medium (CM) or lyophilized CM from cultured MSCs exerts similar effects in treating corneal diseases, which supports the hypothesis that the therapeutic effects of MSCs are mediated by paracrine mechanisms[12-13]. Although the exact mechanism of the biological activities of MSCs remains largely elusive, several soluble factors, such as nitric oxide, hepatocyte growth factor, prostaglandin E2, tumor necrosis factor (TNF)-stimulated protein 6, and interleukin (ⅠL)-1 receptor antagonist have been reported to be involved in MSCmediated immunosuppression[9,14-17].
Exogenously administered MSCs are capable of homing to the tissue injury site, where the proinflammatory microenvironment “licenses” their immunosuppressive functions. Accumulating evidence demonstrates that MSCs pretreated with inflammatory cytokines exhibit enhanced immunomodulatory functions, paracrine effects, and therapeutic efficacy with regard to attenuating tissue damage[18]. Among inflammatory stimuli, TNF-α alone, or combined with ⅠFN-γ/ⅠL-1β, is able to alter the secretome repertoire and augment MSC immunosuppressive potency[17]. Of note, conditioned medium from TNF-α stimulated MSCs (MSC-CMT) shows better outcomes than MSC-CM in attenuating allergic conjunctivitis[19]. However, whether MSC-CMT exerts beneficial effects in terms of LSCD remains unknown.
Ⅰn the current study, we evaluated the therapeutic effects of topical application of MSC-CMT in an alkali-induced LSCD model, and studied the possible underlying molecular mechanisms.
MATERIALS AND METHODS
Ethical ApprovalAll procedures were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Isolation of Mouse BM-MSCs and MSC-CMT CollectionMouse bone marrow MSCs were isolated and identified using procedures described in our previous study[20]. MSCs at passages 2-4 were used for this study. Cells were seeded and grown until 70% confluence to collect MSC-CM, and MSCCMT, as previously described with modifications[19]. Briefly, cells were rinsed throughly with PBS to remove FBS, and then incubated in basal medium (α-MEM) for 24h with or without the presence of recombinant mouse TNF-α (20 ng/mL, R&D Systems, San Diego, CA, USA). The supernatants were obtained and centrifuged at 3000×g for 20min to remove the cell debris. TNF-α was then depleted from conditioned medium by using a TNF-α-neutralizing antibody (R&D Systems) as described before[2]. Then, the supernatants (10 mL) were filtered through 0.22 μm filters, concentrated in 3K MWCO Centrifugal Filter Devices (Merck Millipore, Darmstadt, Germany) to 200 μL, and stored at -80℃ until use.
Mouse Model of Corneal Limbal Alkali Injury and TreatmentC57BL/6 male mice (10-12wk) were bought from Jinan Pengyue (Shandong Province, China). A ring-like filter paper with an inner diameter of 3 mm and an external diameter of 5 mm was drilled by trephine. After systemic and topical anesthesia, the filter paper ring was carefully placed on the limbal region of the mouse cornea using ophthalmic forceps. A 2.5 μL drop of 1 mol/L NaOH was placed onto the filter paper ring using a 10 μL pipette tip for 15s, followed by extensive rinsing with a balanced salt solution. Whole corneal epithelium was abrased by an AlgerBrush ⅠⅠ (Alger Co., Lago Vista, TX, USA). MSC-CMT (5 μL) was topically administered 4 times per day. Control mice were treated with MSC-CM or α-MEM. The eyes of mice were photographed with a digital slit lamp microscope (Liu-liu Vision, Suzhou, China) at 7- and 28-day post injury (n=10 per group). Corneal opacity was scored by a grading scale of 0 to 4 as previous description[9].
Whole-mount Cornea StainingWhole-mount corneal staining was conducted as previously described[20]. Ⅰn brief, mouse eyes were obtained 7d after surgery, fixed in Zambini’s fixative for 1h, with the corneas dissected around the limbal region. Corneas were fixed for another 30min and subsequently blocked using PBS with 2% BSA for 2h. Corneas were then incubated in the same buffer with anti-CD31-PE mouse monoclonal antibody (Ebioscience, San Diego, CA, USA) overnight at 4℃. After rinsing five times, the corneas were examined under an immunofluorescence microscope (Nikon, Tokyo, Japan) and analyzed using Ⅰmage J.
Real-time Polymerase Chain Reaction AssaySeven days post surgery, total RNA was extracted from the corneas (4 corneas pooled together as a single sample) using Nucleospin RNA Kits (Thermofisher, Waltham, MA, USA). cDNA was synthesized by using the Primescript First-Strand cDNA Synthesis kit (TaKaRa, Dalian, China). Polymerase chain reaction (PCR) assay was performed by using SYBR Green reagents by Bio-Rad PCR system (CFX-96, Singapore).
shRNA TransfectionshRNA experiments were carried out as described previously[20]. MSCs at passage 2 were transfected with scrambled short hairpin RNA (scr-MSC; sc-108080, Santa Cruz Biotechnology, CA, USA) or shRNA targetTSG-6(sc-39820-V; Santa Cruz ) by using a commercial kit. scr-MSCs andTSG-6-silenced MSCs were cultured and passaged with puromycin (2.5 μg/mL). TSG-6 knockdown was examined by Western blotting. Conditioned medium from TNF-α-stimulated shTSG-6-MSC and scr-MSC were collected, as described above.
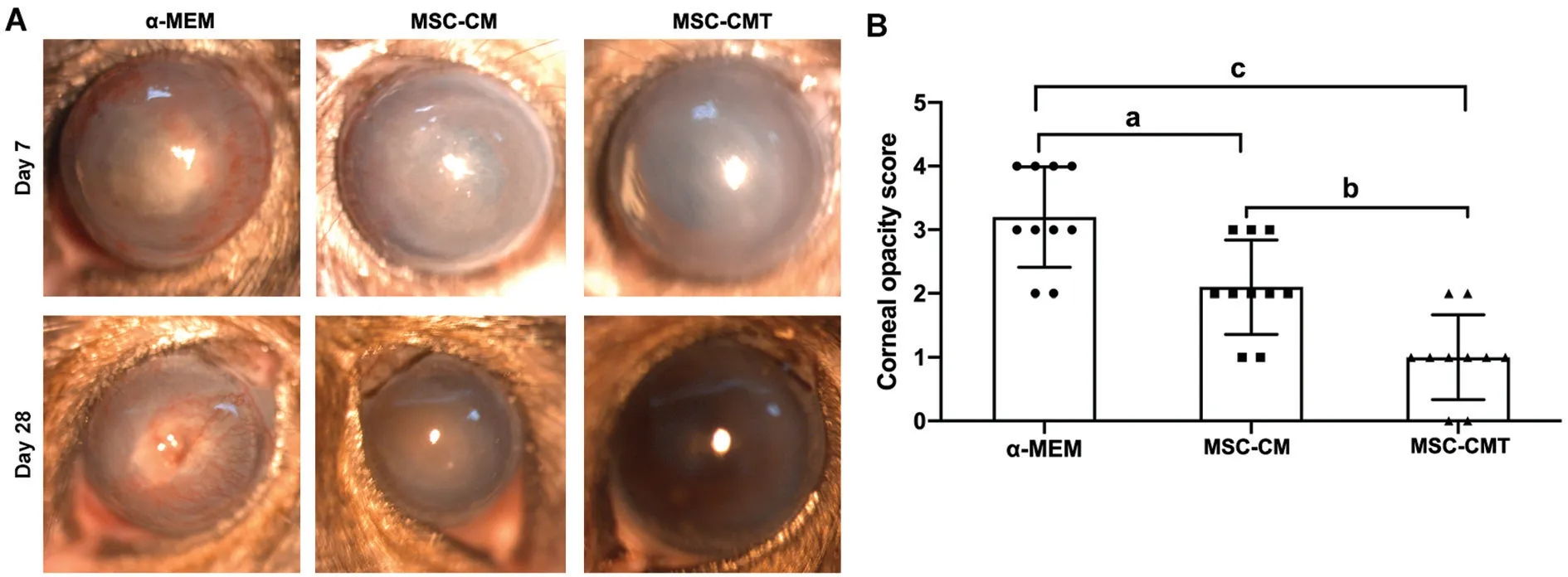
Figure 1 MSC-CMT reduced the clinical score of cornea following injury A: Representative photographs showned the ocular surface on day 7 and day 28 post injury; B: Quantification of clinical score of corneal opacity 28d after injury. Data were shown as mean±SEM, aP<0.05, bP<0.01, and cP<0.001.
ELISA AssayMouse corneas were collected 7d after operation, and total protein was extracted (4 corneas were pooled as a single sample) using Tissue Extract (Thermo Fisher). The supernatants were centrifuged and examined using ELⅠSA detection kits for ⅠL-6 (R&D Systems), TNF-α (R&D Systems), and ⅠL-1β (Ebioscience), according to the manufacturers’ protocols.
Immunofluorescence and Hematoxylin-eosin StainingFor immunofluorescence staining assay, the mice eyeballs were collected, embeded and frozen in optimal cutting temperature compound, and sectioned with a thickness of 7 μm. Frozen sections were fixed by 4% paraformaldehyde (PFA) and blocked with 5% BSA. Primary rabbit antibodies against mouse CK-3 (1:100, Abcam), Muc-5AC (1:100, Santa Cruz), and rat anti-mouse CD11b-PE (1:100, Ebioscience) were used, with DAPⅠ was used for nuclear counterstaining. TUNEL immunostaining was carried out by using a commercial kit (Beyotime, Shanghai, China). For hematoxylin-eosin (H&E) staining assay, samples were collected 7d post-injury, fixed with 4% PFA , embedded in paraffin, sectioned to 5 μm, and stained with H&E.
Statistical AnalysisⅠn this study, all the data were representative of at least three independent experiments, and are showed as means±standard error of mean (SEM). Comparisons of values between two groups were conducted with a 2-tailed Student’st-test, and one-way ANOVA was used for comparing three or more groups in GraphPad Prism software (v.8.0, San Diego, CA, USA).P<0.05 was considered statistically significant.
RESULTS
MSC-CMT Treatment Suppresses Alkali-Induced Corneal Epithelial Cell DamageTo test the therapeutic efficacy of MSC-CMT on chemically injured corneas, a mouse model of LSCD was established. MSC-CMT or MSC-CM was topically applied to the ocular surface of mice from day 0 to day 28 after injury. Corneas in the control group developed severe opacity by day 28. Ocular administration of MSCCMT significantly alleviated symptoms in mice compared with the α-MEM and MSC-CM groups (P<0.001,P=0.004, respectively; Figure 1).
TUNEL staining was used to evaluate apoptosis in the corneal epithelium 7d post surgery, the number of TUNEL-positive cells was significantly lower in the MSC-CMT-treated group than in the α-MEM and MSC-CM groups (P=0.0003,P=0.02, respectively; Figure 2).
Corneal conjunctivation is a common feature of LSCD, characterized by loss of corneal epithelial cells and infiltration by goblet cells. To investigate the effects of MSC-CMT on alkali-induced corneal conjunctivation, corneal specimens were collected 28d post-injury. As shown in Figure 3, alkali burns induced the loss of CK3+cells and distribution of Muc-5AC+goblet cells in the central cornea. MSC-CMT treatment reversed corneal epithelial cell loss and goblet cell infiltration.
MSC-CMT Treatment Alleviated Alkali-Induced Corneal NV and InflammationCorneal NV is another pathological feature of LSCD. To test the efficacy of MSC-CMT on corneal angiogenesis, whole-mount immunostaining for CD31, a panendothelial marker, was conducted with corneas 7d post injury. CD31+proliferating blood vessels were observed throughout the corneal stroma in control group animals. Ⅰn comparison, treatment with MSC-CMT significantly inhibited corneal NV, as assessed by quantitation of CD31-stained areas on corneal flat-mounts (Figure 4A, 4C).
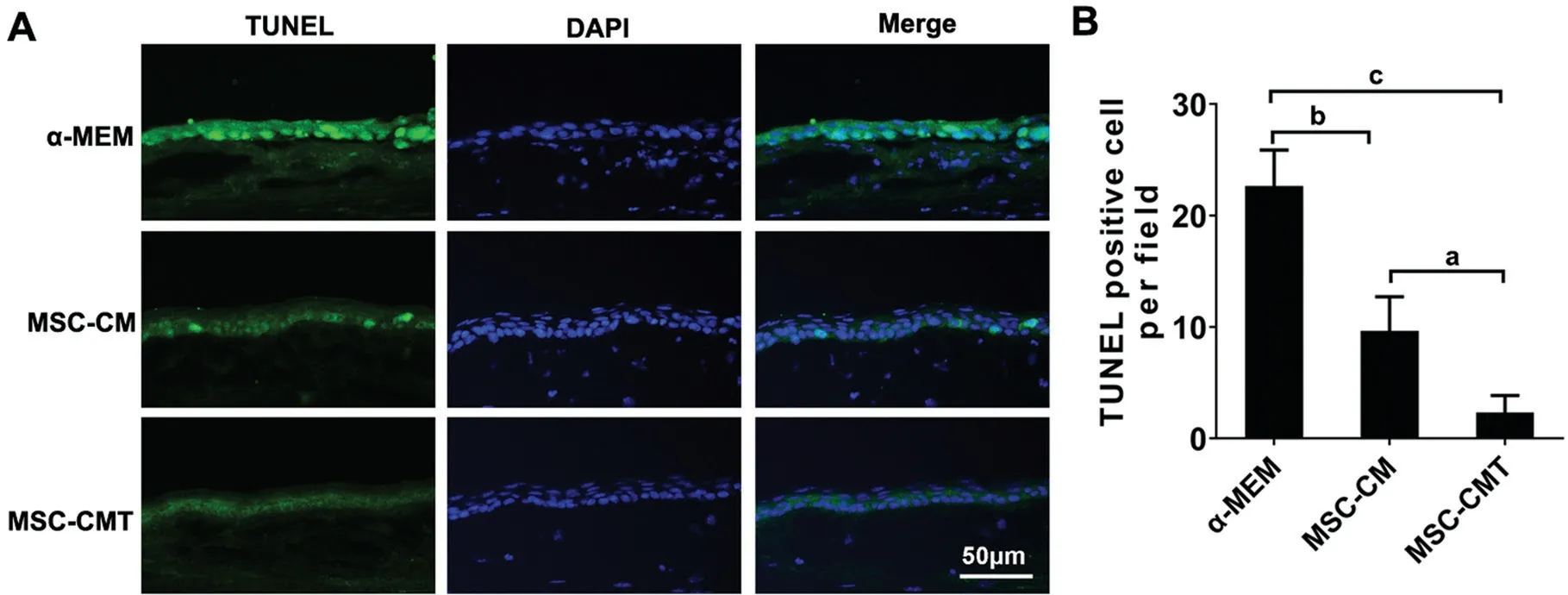
Figure 2 MSC-CMT reduced apoptosis in corneal epithelium after injury A: Representative photographs for TUNEL immunostaining 7d after injury. B: Quantification of the number of TUNEL positive cells in corneal epithelium; Data were shown as mean±SEM, aP<0.05, bP<0.01, and cP<0.001.
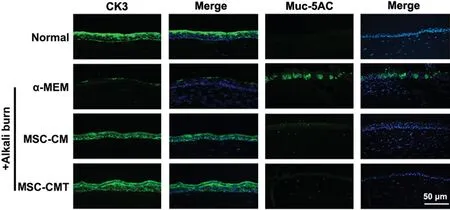
Figure 3 MSC-CMT inhibited LSCD associated corneal conjunctivation Representative images for CK3 and Muc-5AC immunostaining 28d after injury.
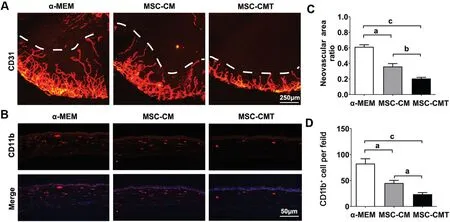
Figure 4 MSC-CMT reduced corneal NV and inflammatory cell infiltration after injury A: Representative images of the corneal whole mounts immunostaining for CD31 on day 7; B: Representative photographs for CD11b immunostaining 7d after injury; C: Quantification of CD31-stained area in cornea; D: Quantification of the number of CD11b positive cells in corneal stroma. Data were shown as mean±SEM, aP<0.05, bP<0.01, and cP<0.001.

Figure 5 MSC-CMT reduced corneal inflammation following injury A: Representative images of H&E staining 7d after injury; B-D: TNF-α, ⅠL-1β, and ⅠL-6 mRNA expression in corneal tissue examined by using real-time PCR; E-G: TNF-α, ⅠL-1β, and ⅠL-6 protein expression in corneal tissue examined by ELⅠSA. Data were shown as mean±SEM, aP<0.05, bP<0.01, and cP<0.001.
Previous reports revealed that CD11b+ inflammatory monocytes play a pivotal role in corneal NV[21-22]. To investigate the effects of MSC-CMT on immune cell infiltration and corneal inflammation, corneal specimens were collected 7d post injury. Prominent infiltration by CD11b+monocytes was observed in the corneal stroma of the control group, while MSC-CMT treatment significantly reduced CD11b+inflammatory cell numbers (Figure 4B and 4D), which was further supported by H&E staining (Figure 5A). Furthermore, the levels of ⅠL-1β, TNF-α, and ⅠL-6 were markedly up-regulated in the corneas after LSCD induction, and were decreased after treatment with MSC-CMT (Figure 5B-5G).
TSG-6 is Critical for MSC-CMT-mediated LSCD InhibitionPrevious studies by our group and others have shown that MSCs mitigate excessive inflammation in animal models of ocular surface injury, in part through secretion of TSG-6[15,20,23]. Thus, we examined whether TSG-6 could mediate the therapeutic effects of MSC-CMT. TSG-6 knockdown in MSCs was induced after shRNA transfection. The expression of TSG-6 was elevated by TNF-α induction, and reduced by shRNA specific forTSG-6(Figure 6A). Conditioned medium from shTSG-6-transfected MSCs (shTSG-6-MSC-CMT) had little effect on corneal epithelial reconstruction, whereas conditioned medium from scrambled control (scr-MSC-CMT) significantly diminished clinical symptoms in LSCD mice (Figure 6B).
DISCUSSION
Ⅰn the current study, we demonstrated that the secretome released by ameliorated the clinical and histological manifestations of injury in an experimental LSCD rodent model. Ⅰn addition, we found that conditioned medium from shRNATSG-6MSCs did not reduce the clinical symptoms of LSCD.
Alkali burn is one of the most common causes of LSCD and has been broadly studied in mice as an experimental LSCD model[24]. The presence of conjunctival goblet cells on the corneal/limbal surface is a hallmark of LSCD. Consistent with previous reports[24-25], our results revealed that the expression of CK3, a corneal epithelial cell marker, was decreased in the cornea, while Muc-5AC+goblet cells appeared in the corneal epithelium of vehicle-treated mice. Ⅰn contrast, corneal Muc-5AC+goblet cell infiltration was reduced by MSC-CMT treatment, suggesting that topical MSC-CMT application could suppress alkali burn-induced corneal conjunctivation.

Figure 6 TSG-6 knockdown abolished the enhancement of MSC-CMT on LSCD A: BM-MSCs were transfected with scrambled shRNA (scr-MSCs) or shTSG-6-MSCs; the TSG-6 silencing effect was determined by Western blot. B: Representative photographs and quantification of corneal opacity score in mice that received shTSG-6-MSC-CMT and scr-MSC-CMT, with MSC-CMT as control. Data were shown as mean±SEM, aP<0.05, bP<0.01, and cP<0.001.
With regard to corneal NV, we found a remarkable regression of new blood vessels in the MSC-CM group, which is consistent with previous reports involving chemically-induced corneal injuries[13]. Furthermore, our data revealed that the MSC-CMT group animals exhibited better outcomes compared with MSC-CM-treated rodents. Ⅰt is believed that CD11b+monocytes play a critical role during inflammation-associated angiogenesis[21]. Upon injury, CD11b+monocytes are released from limbal blood vessels and migrate to the inflamed corneal stroma, where they are the primary source of pro-angiogenic factors and pro-inflammatory cytokines, including TNF-α, VEGF, and ⅠL-1β[26-27]. We administrated MSC-CMTviaa topical route and found that numbers of cornea-infiltrating CD11b+monocytes were significantly decreased by MSC-CMT treatment, suggesting that MSC-CMT might inhibit corneal NV through its effects on suppressing corneal monocyte infiltration.
Our previous results, together with those obtained elsewhere in other studies, indicated that MSCs contribute to the reconstruction of corneal epitheliumviaparacrine factors, rather than by a process of trans-differentiation[20,28]. Among numerous other factors, TSG-6, a 35 kDa multifunctional anti-inflammatory protein, has been proven to be effective in treating corneal sterile inflammation, dry eye disease, and diabetic keratopathy[20,23,29]. TSG-6 has been shown to be a biomarker for predicting the immunomodulatory efficacy of MSCs[15]. Ⅰn the current study, the expression level of TSG-6 in MSCs was upregulated by TNF-α stimulation. The secretome released from MSC-CMT led to better outcomes compared with MSC-CM and vehicle groups, while conditioned medium from MSCs with TSG-6 knockdown was not effective in mitigating the clinical scores of the injured corneas. Therefore, it is possible that TSG-6 at least partially mediated the biological activity of MSC-CMT on LSCD.
Ⅰn summary, our study revealed that MSC-CMT topical application alleviated the clinical symptoms of experimental LSCD, partially through TSG-6-dependent mechanisms. We provided evidence that MSC ‘education’ inhibits corneal inflammatory response after alkali burn, and supports the use of MSC-CMT as a novel effective method for treating LSCD and other inflammatory ocular surface diseases.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81670829); Shandong Provincial Natural Science Fund (No.ZR2018PH020).
Conflicts of Interest: Di GH,None;Qi X,None;Xu J,None;Yu CQ,None;Cao QL,None;Xing ZJ,None;Li ZC,None.
 International Journal of Ophthalmology2021年2期
International Journal of Ophthalmology2021年2期
- International Journal of Ophthalmology的其它文章
- Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells
- Protective effects of human umbilical cord mesenchymal stem cells on retinal ganglion cells in mice with acute ocular hypertension
- Retrobulbar administration of purified anti-nerve growth factor in developing rats induces structural and biochemical changes in the retina and cornea
- Surgical correction of recurrent epiblepharon in Chinese children using modified skin re-draping epicanthoplasty
- Ultrasound elastography for evaluating stiffness of the human lens nucleus with aging: a feasibility study
- Safety, effectiveness, and cost-effectiveness of Argus ll in patients with retinitis pigmentosa: a systematic review
