Immune response activation following hyperthermic intraperitoneal chemotherapy for peritoneal metastases: A pilot study
Giammaria Fiorentini, Donatella Sarti, Alberto Patriti, Emilio Eugeni, Francesco Guerra, Francesco Masedu,Andrew Reay Mackay, Stefano Guadagni
Giammaria Fiorentini, Donatella Sarti, Department of Onco-Hematology, Azienda Ospedaliera“Ospedali Riuniti Marche Nord”, Pesaro 61122, Italy
Alberto Patriti, Emilio Eugeni, Francesco Guerra, Department of General Surgery, Azienda Ospedaliera “Ospedali Riuniti Marche Nord”, Pesaro 61122, Italy
Francesco Masedu, Andrew Reay Mackay, Stefano Guadagni, Department of Applied Clinical Sciences and Biotechnology, University of L’Aquila, L’Aquila 67100, Italy
Abstract BACKGROUND Hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastases(PM) is considered to be feasible, safe and to improve survival.AIM To investigate whether an immune response is activated following HIPEC for PM.METHODS Six patients were enrolled in this study. Peripheral blood samples were obtained from each patient prior to (day 0) and post-procedure (day 30), and used to evaluate the number of CD3+ total, CD3+/CD4+ T-Helper, CD3+/CD8+ cytotoxic T,CD3+/CD56+ natural killer and CD19+ B lymphocyte numbers, and CD4+: CD8+ T lymphocyte ratios.RESULTS The total numbers of CD3+, CD3+/CD4+ T-Helper, CD3+/CD8+ cytotoxic T,CD3+/CD56+ natural killer and CD19+ B lymphocytes, and CD4+: CD8+lymphocyte ratios were increased in all but one patient 30 d following the cytoreductive surgery-HIPEC procedure, and these increases were significant (P≤ 0.05) for CD3+/CD4+ T Helper and CD3+/CD8+ cytotoxic T lymphocyte numbers.CONCLUSION This report provides the first evidence that HIPEC exhibits immunomodulating activity in PM patients, resulting in generalized activation of the adaptive immune response. Moreover, the majority of lymphocyte populations increased following HIPEC and continued to be elevated several weeks following the procedure, consistent with a potential authentic immunomodulating effect rather than a normal inflammatory response, to be fully characterised in future studies.
Key words: Immune response; Hyperthermic intraperitoneal chemotherapy; Peritoneal metastases; Regulatory T lymphocytes; Pseudomyxoma peritonei; Colorectal cancer
INTRODUCTION
Cytoreductive surgery (CRS) was originally introduced for debulking peritoneal metastases (PM) in ovarian cancer patients and improved survival[1]. Despite some difficulty in being accepted by the medical oncology community, CRS was subsequently adopted for the treatment of pseudomyxoma peritonei (PMP), with beneficial results, confirming a role for CRS in the treatment of peritoneal disease[1].CRS methodology was subsequently improved by Sugarbakeret al[2], who introduced six distinct peritonectomy procedures for complete cytoreduction. Intraperitoneal (IP)chemotherapy was subsequently used as an adjuvant therapy to CRS. In several clinical trials, hyperthermic intraperitoneal chemotherapy (HIPEC), in particular,resulted in survival improvements[3]. Today, CRS combined with HIPEC is considered the standard treatment for PMP and peritoneal mesothelioma[4]and, according to current Network NCCN Guidelines[5,6], is also considered for the management of colorectal PM.
HIPEC, pressurized intraperitoneal aerosol chemotherapy, hepatic artery infusion,trans-arterial chemo-embolization and thermal ablation are all under investigation as approaches for locoregional treatment[7,8]. However, only thermal tumor ablation has been reported to induce a potential immunomodulatory effect, illustrated by the intense inflammatory cell response that is associated with thermally ablated tumors[9,10], and characterized by ablated tissue transitional zone infiltration by immune and inflammatory cell populations, including: Dendritic cells, neutrophils,macrophages, B and T lymphocytes and natural killer (NK) cells[10], with a systemic increase in immune cells also detected[8].
The effect of HIPEC is similar to that of radiofrequency ablation, with both procedures inducing hyperemic stress-induced tumor necrosis, suggesting that HIPEC may also exhibit immunomodulatory activity. Therefore, the aim of this study was to investigate whether HIPEC for PM induces an immune response.
MATERIALS AND METHODS
Study population and data collection
In this observational study, data were collected from patients with PM prospectively submitted for CRS and HIPEC, from October 2018 to August 2019, in accordance with Research Ethics Committee regulations. The inclusion criteria were: Age > 18 and < 75 years, diagnosis of PC with T4 stage (including cases with bowel perforation),mucinous subtype, or positive cytology of peritoneal lavage and written informed consent. Exclusion criteria were: Pre-operative chemotherapy or oncology medical treatment, concomitant tumor and autoimmune or rheumatologic disorders.
Cytoreductive surgery and HIPEC
CRS and HIPEC were performed according to learning curve and training program recommendations[11]. HIPEC, performed immediately after R0 primary tumor debulking (intraoperative administration), involved 60-min infusion/lavage with Oxaliplatin 400 mg/m2and Mitomycin C 15 mg/m2, with drugs added to perfusion solutions at temperatures ≥ 42°C.
Immunological parameters
Peripheral blood (PB) samples were collected in ethylenediaminetetraacetic acid, prior to (day 0) and following (day 30) CRS-HIPEC procedures, and immunophenotypic analysis was performed within 24 h (Figure 1). Single-cell suspensions were labelled with fluorochrome-conjugated anti-human CD3, CD4, CD8, CD19 and CD56 monoclonal antibodies or matched isotypes, and CD3+total, CD3+/CD4+T-Helper,CD3+/CD8+cytotoxic T, CD3+/CD56+NK and CD19+B lymphocytes, were quantified by fluorescence-activated cell sorting, an antibody-based cell sorting method for heterogeneous mixtures based upon the specific light scattering and fluorescent characteristics of each antibody-labelled cell-type[12]. Additional immunological parameters included the CD4+: CD8+T lymphocyte ratio. Immunological populations were also analyzed in stained samples, after exclusion of debris and doublets, as previously described[13].
Outcome measures
The primary outcome was to assess adaptive immune response activation and decreased immunosuppression, and the secondary outcome was to monitor recurrence-free survival, by computed tomography scan or magnetic resonance imaging.
Statistical analysis
The characteristics of recruited immune/inflammatory cell populations are described qualitatively and by quantitative variables. Unilateral paired Student’st-tests were used to demonstrate intra-patient and intra-cohort treatment response variability,trend, and statistical significance associated with aPvalue of ≤ 0.05.
RESULTS
Overall characteristics of the patients
The 6 patients in this study comprised 3 males (50%) and 3 females (50%), with a median age of 55 years (range 48-71 years). Two patients (33%) presented with PM from colorectal cancer and the other 4 (77%) with PMP (Table 1).
Computed tomography scans, performed 3, 6 and 12 mo following CRS-HIPEC,demonstrated complete responses in all 6 patients, associated with a median progression-free survival of 12 mo. No complications were observed and the mean hospitalization-time, following CRS-HIPEC, was 10 ± 2 d. One patient however, was hospitalized for 58 d with severe oxaliplatin toxicity, characterized by grade 4 hematopoietic toxicity, according to National Cancer Institute, Common Terminology Criteria for Adverse Events (CTCAE v4.03), and grade 3 hematomas in the abdominal wall and pelvis. The other patients did not exhibit any HIPEC drug-associated adverse events.
Evaluation of system lymphocyte populations in patient PB samples (Tables 2 and 3), revealed increases in systemic CD3+total, CD3+/CD4+T Helper, CD3+/CD8+cytotoxic T, CD3-/CD56+NK, CD19+B lymphocyte numbers and increased CD4+:CD8+T lymphocyte ratios, statistically significant for CD3+/CD4+T Helper and CD3+/CD8+Cytotoxic T lymphocyte populations (unilateral paired Student’sttest,P< 0.05) (Figure 2). These increases were observed in all but one patient, who exhibited reduced systemic lymphocyte counts post-HIPEC, consistent with severe oxaliplatin toxicity.
DISCUSSION
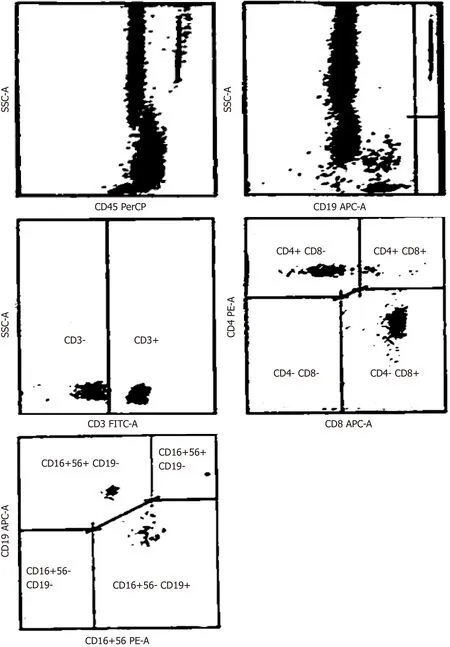
Figure 1 Representative example of fluorescence-activated cell sorting immunophenotypic analysis.
The peritoneum is the second most common site of colon cancer recurrence[14].However, early imaging detection of peritoneal metastases is difficult and adjuvant systemic treatment does not reduce peritoneal dissemination. Therefore, current international clinical practice guidelines suggest HIPEC for treatment of patients at high risk of PM, as this procedure has been shown to reduce peritoneal recurrences in the long-term and to prolong overall survival[6,14-18].
Hyperthermia induces tissue necrosis immediately adjacent to the thermal source and induces apoptosis in more peripheral or transitional zone tissues, surrounded by tissues unaffected by thermal ablation[10]. Tumor cell necrosis and apoptosis result in the release of tumor-debris, tumor antigens and damage-associated molecular patterns, all of which could theoretically activate the immune system. For this reason,we evaluated systemic immune cell profiles in PM patients treated with CRS-HIPEC,as a potential index of immune response activation by this procedure. The results obtained provide the first evidence that HIPEC induces immune system activation in PM patients, characterized by induction of a generalized adaptive immune response and decrease in immunosuppression. Furthermore, this CRS-HIPEC-induced immunological effect was long-lived and lasted for several weeks, consistent with authentic immunomodulation, rather than a normal inflammatory response.
In our opinion, therefore, HIPEC is not only a useful chemotherapeutic procedure but also stimulates the immune system. In fact, 60-min of HIPEC appears to promotenot only efficacious cytotoxicity but also sufficient release of tumor debris, antigens and damage-associated molecules to activate the immune system and promote cytotoxic T lymphocyte maturation.

Table 1 Sample characteristics
The limitation of this pilot study, however, is that it only provides preliminary evidence that this methodological approach is appropriate for evaluating immunological changes following CRS and HIPEC, and is, therefore, a forerunner to a future confirmatory large cohort study. Furthermore, this study was not designed to evaluate treatment safety, efficacy or effectiveness[19], justifying the small sample size.The data should also be interpreted with some care, since HIPEC is performed immediately following tumor debulking, implicating surgical trauma as an alternative source of physiological and immunological alteration. Indeed, the normal physiological response to tissue injury involves a complex integration of inflammatory, immunological, neuroendocrine and metabolic mechanisms, and incision, dissection, organ manipulation and vascular alterations all induce acute inflammation for the purpose of host defense and tissue repair[10], with negative immunosuppressive feedback activated to avert an exaggerated immunological/inflammatory response[20]. Notwithstanding this, the difference in the characteristics of acute inflammation due to surgical stress-induced and HIPECinduced long-lived increases in systemic CD3+, CD3+/CD4+T Helper, CD3+/CD8+cytotoxic T, CD3-/CD56+NK, CD19+B lymphocytes, and CD4+: CD8+T lymphocyte ratio, statistically significant for CD3+/CD4+T Helper and CD3+/CD8+cytotoxic T lymphocyte populations, supports the hypothesis that HIPEC activates the immune system, with differences consistent with generalized activation of an adaptive immune response.
In conclusion, this pilot study provides the first evidence that HIPEC activates the immune response in PM patients, supporting an additional immunomodulatory function for this procedure. Of course, results from this small patient cohort pilot study must await confirmation in a larger patient cohort, which could also benefit from comparing the CRS-HIPEC-induced immune response activation to that induced by HIPEC combined with minimally-invasive surgical procedures.
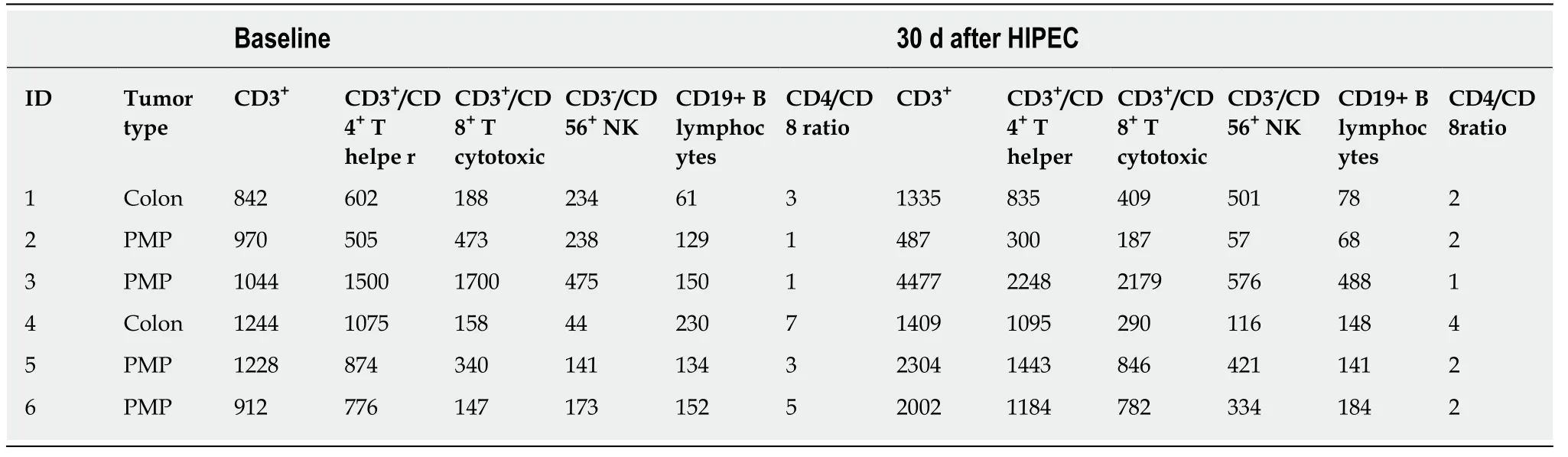
Table 2 Immunological analysis: absolute values, cells/L
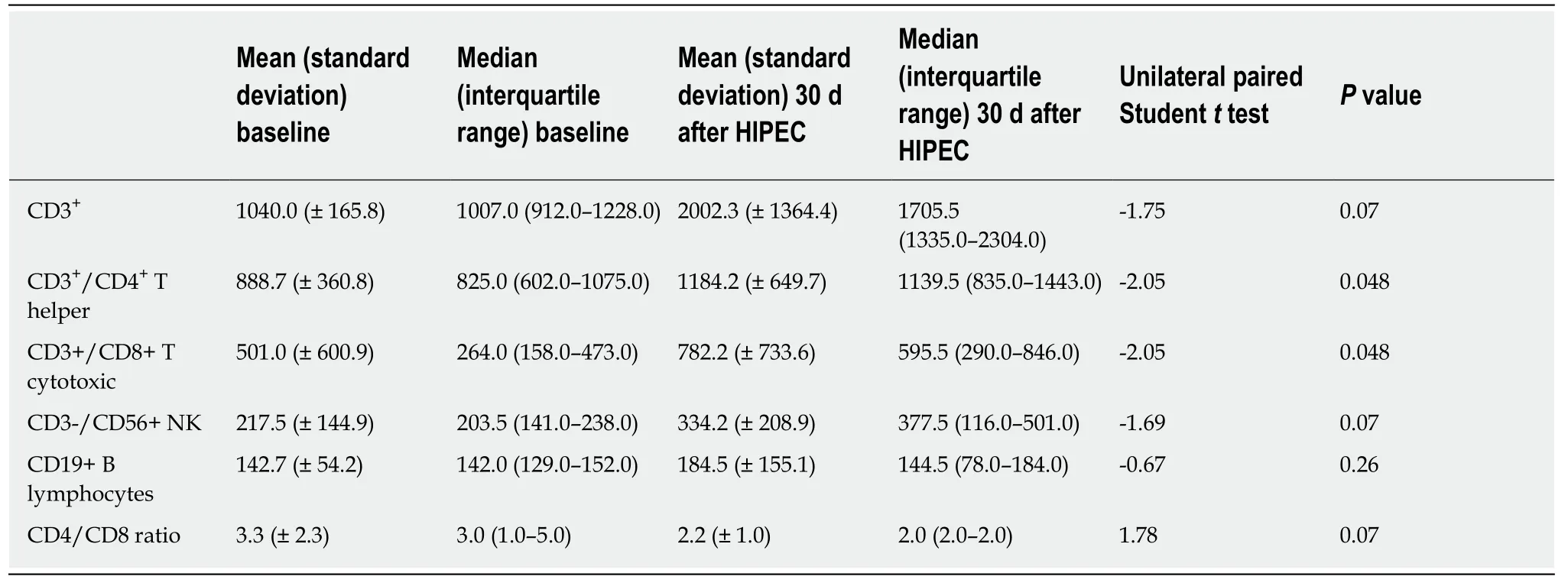
Table 3 Immunological response: statistical analysis
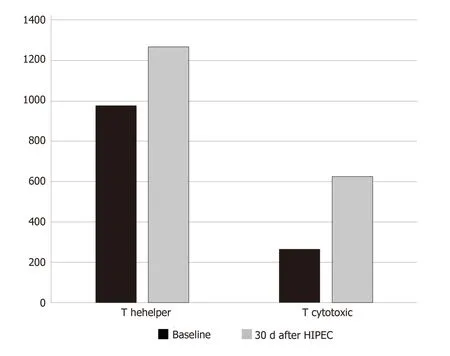
Figure 2 Median values (absolute value cells/mL) of T helper and T cytotoxic levels at baseline and 30 d after hyperthermic intraperitoneal chemotherapy.HIPEC: Hyperthermic intraperitoneal chemotherapy.
ARTICLE HIGHLIGHTS
Research background
According to current Network NCCN Guidelines, cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is considered the standard treatment for pseudomyxoma peritonei and peritoneal mesothelioma. In several clinical trials, HIPEC, in particular, resulted in survival improvements.
Research motivation
Thermal tumor ablation has been reported to induce a potential immunomodulatory effect,illustrated by the intense inflammatory cell response, including: Dendritic cells, neutrophils,macrophages, B and T lymphocytes and natural killer cells. The induction of this immune response might be important in increasing tumor response and improving survival.
Research objectives
The aim of this study was to investigate whether HIPEC for peritoneal metastases induces an immune response, which was determined by analyzing the immune population before and 30 d after HIPEC.
Research methods
Peripheral blood samples were collected prior to (day 0) and following (day 30) CRS-HIPEC procedures, and immunophenotypic analysis was performed within 24 h. The levels of immunological populations were quantified by fluorescence-activated cell sorting, an antibodybased cell sorting method for heterogeneous mixtures based upon the specific light scattering and fluorescent characteristics of each antibody-labelled cell-type as reported in the literature.Immunological populations were also analyzed in stained samples, after exclusion of debris and doublets, as previously described.
Research results
The evaluation of system lymphocyte populations revealed an increase in systemic CD3+total,CD3+/CD4+T Helper, CD3+/CD8+cytotoxic T, CD3-/CD56+natural killer, CD19+B lymphocyte levels and CD4+/CD8+T lymphocyte CD3+/CD8+Cytotoxic T lymphocyte ratios. Statistical significance was observed for CD3+/CD4+T Helper and CD3+/CD8+Cytotoxic T lymphocyte populations (unilateral paired Student’sttest,P< 0.05). These increases were observed in all but one patient, who exhibited reduced systemic lymphocyte counts post-HIPEC, consistent with severe oxaliplatin toxicity.
Research conclusions
The results obtained provide new evidence that HIPEC induces immune system activation in pseudomyxoma peritonei patients, characterized by induction of a generalized adaptive immune response and decrease in immunosuppression. Another new finding was that the CRS-HIPECinduced immunological effect was long-lived and lasted for several weeks, consistently with authentic immunomodulation, rather than a normal inflammatory response.
Research perspectives
This pilot study provides the first evidence that HIPEC activates the immune response,supporting an additional immunomodulatory function for this procedure. Further studies are required to confirm these results in a large cohort study and to evaluate treatment safety, efficacy or effectiveness.
 World Journal of Clinical Oncology2020年6期
World Journal of Clinical Oncology2020年6期
- World Journal of Clinical Oncology的其它文章
- Angioimmunoblastic T-cell lymphoma accompanied by pure red cell aplasia: A case report
- National Comprehensive Cancer Network guidelines compliance of a sarcoma service: A retrospective review
- Preoperative markers for the prediction of high-risk features in endometrial cancer
- Role of microRNA dysregulation in childhood acute leukemias:Diagnostics, monitoring and therapeutics: A comprehensive review
- Lingual lymph nodes: Anatomy, clinical considerations, and oncological significance
- Active surveillance in low risk papillary thyroid carcinoma
