Diagnostic efficacy of the Japan Narrow-band-imaging Expert Team and Pit pattern classifications for colorectal lesions: A meta-analysis
Yu Zhang, Hui-Yan Chen, Xiao-Lu Zhou, Wen-Sheng Pan, Xin-Xin Zhou, Hang-Hai Pan
Abstract
Key Words: Colorectal neoplasms; Colonoscopy; Chromoendoscopy; Japan Narrow-bandimaging Expert Team; Pit pattern; Meta-analysis
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignant tumors in the world[1]. Accurate identification and treatment of early CRC have signicantly reduced its incidence and mortality[2]. However, early CRC is generally asymptomatic, and coloscopy allows the direct visual inspection of the intestinal tract and same-session detection, biopsy, and subsequent removal of lesions. Endoscopic evaluation of colorectal lesions is important to guide the selection of an appropriate treatment. The ideal endoscopic management of colorectal lesions involves two steps. First, any mucosal lesions need to be detected. Second, the lesions need to be characterized based on histological characteristics assessedviaendoscopic evaluation; which forms the basis of the endoscopic judgment on whether the lesions need to be resected. In general, there is a consensus that low-grade dysplasias (LGDs), high-grade dysplasias (HGDs), and superficial submucosal invasive (SM-s) carcinomas are considered appropriate for endoscopic resection, while observation is recommended for hyperplastic polyps (HPs), in contrast, surgery is recommended for deep submucosal invasive (SM-d) carcinomas[3].
Kudoet al[4]first proposed the “Pit patterns” (the opening shape of a colorectal crypt) based on the classification of colorectal lesionsviamagnifying endoscopy, and indigo carmine dye contrast[4]. Pit pattern diagnosis (Type I-V) is clinically significant as it can differentiate between neoplasia and non-neoplasia, characterize the degree of histological atypia in a tumor, and reveal the invasion depth of early carcinoma.
Usually, standard magnification requires the use of chromoagents (e.g., indigo carmine, crystal violet, or methylene blue) to clarify the pit structures in these diagnostic procedures. However, with the emergence of electronic staining endoscopic equipment, chemical staining endoscopy has gradually been replaced. In addition, simpler and more convenient procedures are desirable for magnifying procedures. It has been suggested that narrow-band-imaging (NBI) technology is as effective as chromoendoscopy in differentiating the gross type of colorectal lesions[5]. The Colon Tumor NBI Interest Group proposed the NBI International Colorectal Endoscopic (NICE) classification in 2010, which is based on non-magnifying NBI observations[6]. However, the NICE classification cannot distinguish between benign adenoma and superficial mucosal carcinoma, therefore, it plays a limited role in guiding endoscopic treatment strategies. As more endoscopic devices are being equipped with a magnifying function, NBI combined with magnifying endoscopy is increasingly being used, which further improves the diagnostic efficiency, and plays an important role in estimating the invasion depth of the lesion. In order to better guide the endoscopic treatment strategy, in 2014, the Japan NBI Expert Team (JNET) proposed the JNET classification as a universal NBI magnifying endoscopic classification[7]. The JNET classification focuses on vessel and surface patterns to diagnose colorectal lesions as Types 1, 2A and B, 3.
Recently, several studies have proposed that the JNET classification of colorectal lesionsviaNBI magnifying endoscopy is a useful and objective tool for differentiating the gross type of colorectal lesions. However, differences in the diagnostic performance of the JNET classification have not been reported according to each gross type. Although several investigators now accept the application of the JNET classification for colorectal lesions, to what extent we can trust the results of the JNET classification, and whether the Pit pattern classification can be replaced by the JNET classification are aspects that remain unclear.
Few studies have compared the diagnostic efficacy of the JNET and Pit pattern classifications for each gross type of lesion, and determined the correlation between endoscopic features and pathological findings, and, to our knowledge, there are no meta-analyses on this topic. Accordingly, we performed a systematic review and metaanalysis to analyze the data on existing magnifying endoscopy trials using the JNET classification and the Pit pattern classification for characterization of colorectal lesions, and, to obtain a statistically convincing conclusion on the diagnostic accuracy, and practicability of these two comparable methods.
MATERIALS AND METHODS
Literature search
We performed a systematic literature search of articles in PubMed, Web of Science, Embase and the Cochrane Library (January 1990 to May 2020) containing quantitative data, and manually searched the reference lists of retrieved articles. The following search terms were used: “Japan Narrow-Band Imaging Expert Team”, “Japan NBI Expert Team”, JNET, “Pit pattern”, “Kudo’s classification”, “Colorectal Neoplasm” “Neoplasm, Colorectal”, “Colorectal Carcinoma”, “Carcinoma, Colorectal”, “Carcinomas, Colorectal”, “Colorectal Carcinomas”, “Colorectal Cancer”, “Cancer, Colorectal”, “Cancers, Colorectal”, “Colorectal Cancers”, “Colorectal Tumors”, “Colorectal Tumor”, “Tumor, Colorectal”, “Tumors, Colorectal”, “Neoplasms, Colorectal”, “Colon polyps”, “Colorectal polyps”, and “Colorectal lesions”. The queries used are displayed in the supplementary materials. All similar possible word variations were also searched. The attained records were retrieved and managed with EndNote X 9.0 (Bld 10136, Thomson Reuters, New York, NY, United States).
Study selection
Studies were included when all of the following conditions were met: (1) Studies in which all participants received the JNET or Pit pattern classification for colorectal lesions diagnosedviaendoscopy; (2) Histological diagnosis was chosen as the gold standard; and (3) Studies in which sufficient data were reported to calculate true positive (TP), false positive (FP), false negative (FN), and true negative (TN) results. In addition, we included only the latest-published article when the same population was reported in more than one article. However, conference papers and duplicate published studies that fulfilled the above two criteria were excluded. This metaanalysis was performed in compliance with the preferred reporting items for systematic reviews and meta-analyses statement (PRISMA)[8].
Data extraction
Data from the included studies were extracted and cross-checked by 2 authors independently (Zhang Y and Pan HH). If there was a discrepancy in their opinions, it was discussed with other authors to achieve a consistent result. The extracted data included the name of the first author, year of publication, demographics of the population, type of endoscope, number of included patients, number of colorectal lesions examined, design of the study, and the type of classification for colorectal lesions. The number of TP, FP, TN, and FN results for the JNET, and Pit pattern classifications were the main statistics extracted from the studies. We computed sensitivity [TP/(TP + FN)] and specificity [TN/(TN + FP)] for each technique separately.
Quality assessment
The quality of the included studies was independently assessed by 2 authors independently (Zhou XX and Chen HY) using the quality assessment of diagnostic accuracy studies II (QUADAS-II) tool[9].
Statistical analysis
The original data from each study (TP, FP, TN and FN) were integrated through metaanalysis, and the pooled sensitivity, specificity, positive likelihood ratio, and diagnostic odds ratio (DOR) of both the JNET and Pit pattern classifications were calculated by the DerSimonian Laird random effects model[10]. The heterogeneity of pooled sensitivity and specificity was calculated using theI2statistic, and a high degree of heterogeneity was set atI2> 50%[11]. Mose's constant linear model was used to perform the summary receiver operating characteristic curve[12]. Cochrane’s Q test was used to evaluate the accuracy of the JNET and Pit pattern classifications in the diagnosis of colorectal lesions. When heterogeneity was present, the Spearman correlation coefficient, and thePvalue or heterogeneity ratio caused by the threshold effect were calculated. Meta-regression was conducted to explore the existing source of heterogeneity. Publication bias was assessed using the Deeks’ Funnel Plot Asymmetry Test. Thet-test was used to compare the statistical significance of the area under the curve (AUC) and pooled sensitivity, with the significance set atP< 0.05. The statistical software used for the diagnostic accuracy test was Stat 15.1. Revman5.3 was used to evaluate the quality of the included studies.
RESULTS
Study selection
A total of 1146 articles were initially searched (351 in Web of Science, 241 in PubMed, 44 in Cochrane Library, and 510 in Embase), and 31 studies[5,13-42]with a total of 14674 patients were ultimately included in this meta-analysis. 1114 studies were excluded of which 306 studies were duplicate references, 442 studies were excluded based on title and abstract, and 70 studies were excluded after full-text review. A detailed PRISMA flow diagram is shown in Figure 1.
Description of studies and qualitative analysis
All studies used the JNET or Pit pattern classifications as the diagnostic criteria for colorectal lesions examinedviaendoscopy. The JNET classified colorectal lesions into four categories: Type 1 is a hyperplastic polyp (HP)/sessile serrated lesion (SSL), Type 2A is a LGD/adenoma, Type 2B is a HGD/M-SM-s and Type 3 is a SM-d[5]. The Pit pattern classification has five corresponding categories: Type I is normal mucosa, Type II is hyperplasia/SSL, Types IIIL/IV are LGD/adenomas, Types IIIS/VI-L,and VI-H/VNare associated with HGD/M-SM-s lesions, and SM-d lesions, respectively[13]. The detailed interpretation criteria are shown in Table 1. Our study was restricted to only cross-sectional outcomes such as sensitivity and specificity, and the screening tests were compared to the reference standard (histopathological diagnosis). General information on the included studies is presented in Table 2. Of the 31 studies[5,13-42], 11 were retrospective[5,13-22], and 20 were prospective[23-42]. Seven studies[14-20]used the JNET classification alone, 21 studies[22-42]used the Pit pattern classification alone, and only 3 studies[5,13,21]used both the JNET and Pit pattern classifications in the same population. Thirteen studies[5,13-16,18-21,24,28,35,41]used narrow-band imaging magnifying endoscopy (NBI-ME), 13 studies used magnifying chromoendoscopy[22,25-27,29,30,32-35,37,38,42], and the other studies used BLI-magnification[17,23](n= 2), NBI[31,39](n= 2), andchromoendoscopy[36,40](n= 2). The QUADAS-II quality assessment for each study is presented in Figure 2.

Table 1 Detailed tentative criteria for interpretation of the Japan Narrow-band-imaging Expert Team and Pit pattern classifications compared with histologic diagnosis
Diagnostic efficacy
In total, 19227 colorectal lesions in 14674 patients were identified in the 31 included studies. Table 3 summarizes the pooled sensitivity, specificity, DOR, and AUC in each category of the JNET and Pit pattern classifications corresponding to the pathological results.
Ten studies[5,13-21]involving 13479 colorectal lesions reported the diagnostic efficacy of the JNET classification. The pooled values for each category were as follows. Type 1 (non-neoplastic): Sensitivity, 0.73 (95%CI: 0.55-0.85); specificity, 0.99 (95%CI: 0.97-1.00) (Figure 3A); DOR, 245 (95%CI: 64-936); and AUC, 0.97 (95%CI: 0.95-0.98) (Figure 4A). Type 2A: sensitivity, 0.88 (95%CI: 0.78-0.94); specificity, 0.72 (95%CI: 0.64-0.79) (Figure 8A); DOR, 19 (95%CI: 11-33); and AUC, 0.84 (95%CI: 0.81-0.87) (Figure 4B). Type 2B: sensitivity, 0.56 (95%CI: 0.47-0.64); specificity, 0.91 (95%CI: 0.79-0.96) (Figure 5A); DOR, 13 (95%CI: 7-24); and AUC, 0.72 (95%CI: 0.68-0.76) (Figure 4C); Type 3: sensitivity, 0.51 (95%CI: 0.42-0.61); specificity, 1.00 (95%CI: 1.00-1.00) (Figure 6A); DOR, 801 (95%CI: 267-2398); and AUC, 0.90 (95%CI: 0.87-0.93) (Figure 4D).
Twenty-one studies[5,13,21-42]involving 6150 colorectal lesions reported the diagnostic value of the Pit pattern classification. The pooled values for each category were as follows. Types I + II (non-neoplastic): sensitivity, 0.86 (95%CI: 0.81-0.90); specificity, 0.94 (95%CI: 0.90-0.96) (Figure 7); DOR, 88 (95%CI: 48-156); and AUC, 0.95 (95%CI: 0.93-0.97) (Figure 9A). Types IIIL+ IV: sensitivity, 0.80 (95%CI: 0.67-0.89); specificity, 0.80 (95%CI: 0.74-0.86) (Figure 8B); DOR, 17 (95%CI: 8-34); and AUC, 0.87 (95%CI: 0.83-0.89) (Figure 9B). Types IIIS+ VI-L: sensitivity, 0.45 (95%CI: 0.23-0.69); specificity, 0.88 (95%CI: 0.75-0.94) (Figure 5B); DOR, 6 (95%CI: 1-26); and AUC, 0.79 (95%CI: 0.75-0.82) (Figure 9C). Types VN+ VI-H: sensitivity, 0.73 (95%CI: 0.55-0.85); specificity, 0.99 (95%CI: 0.98-1.00) (Figure 6B); DOR, 449 (95%CI: 93-2182); and AUC, 0.98 (95%CI: 0.97-0.99) (Figure 9D).
The Student’st-test was used to compare the sensitivities, specificities, and AUCs of each corresponding category of these two classifications. A statistically significant difference was found in sensitivity between JNET Type 3, and Pit pattern Types VN+ VL-H(P< 0.05), however, no significant difference was found in specificity, and AUC. The results showed that no significant differences was found between the remaining categories among sensitivities, specificities, and AUCs: JNET Type 1 (non-neoplastic)vsPit pattern Types I + II (non-neoplastic), JNET Type 2AvsPit pattern Types IIIL+ IV, and JNET Type 2BvsPit pattern Types IIIS+ VI-L.
Heterogeneity analysis
Significant heterogeneity existed among the included studies. Estimation of Spearman’s correlation coefficient (coef) and theP-value for each category of the JNET and Pit pattern classifications are shown in Table 4. Significant differences were noted for JNET Type 1 (non-neoplastic) (coef. = 0.14,P= 0.02), JNET Type 3 (coef. = -0.17,P= 0.03), and Pit pattern Types I + II (non-neoplastic) (coef. = -0.12,P= 0.02), while for the remaining subtypes of these two classifications, there were no significant differences. These results indicated the threshold effect existed in JNET Types 1, 3, and Pit pattern Types I + II. Additionally, for the non-threshold effect, we performed meta-regression analysis, including the population in the study (Asian or non-Asian), design of thestudy (retrospective or prospective), patient sample size (≥ 100 or < 100), QUADAS-2 score (≥ 7 or < 7), publication year (before, or after 2014), as well as the type of endoscopy as covariates. The sources of potential heterogeneity in the sensitivity and specificity were detected by univariate regression analysis, and the results are shown in Table 5. Deeks’ Funnel Plot Asymmetry Test revealed no publication bias in each category of these two classifications (Table 6).
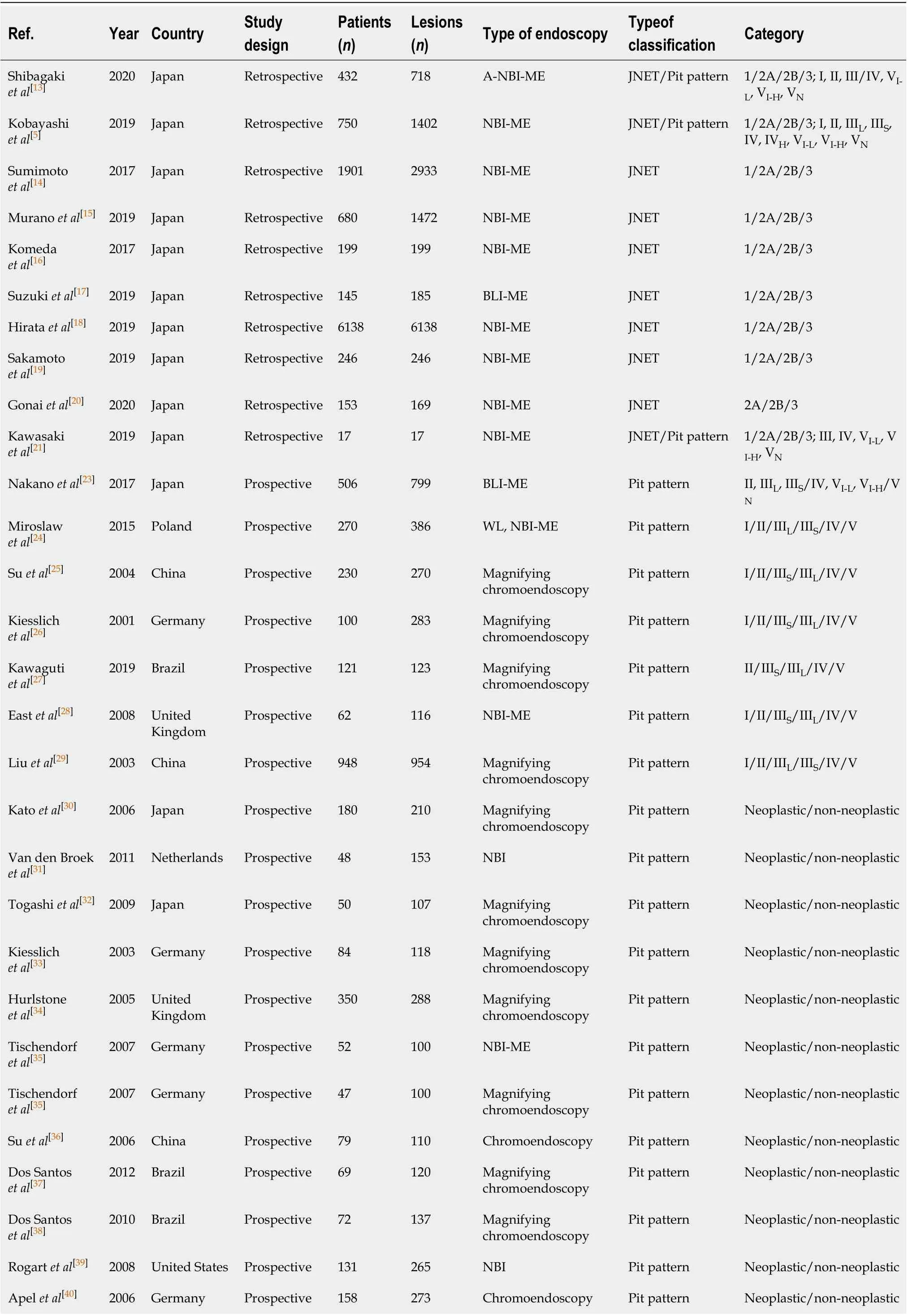
Table 2 General characteristics of the included studies

Van den Broek et al[41]2008 Netherlands Prospective 50 98 NBI-ME Pit pattern Neoplastic/non-neoplastic Chiu et al[42]2007 China Prospective 133 180 Magnifying chromoendoscopy Pit pattern Neoplastic/non-neoplastic Liu et al[22]2008 China Retrospective 223 451 Magnifying chromoendoscopy Pit pattern Neoplastic/non-neoplastic JNET: Japan Narrow-band-imaging Expert Team; NBI: Narrow band imaging; NBI-ME: NBI magnifying endoscopy; WL: White light; BLI: Blue laser imaging; A-NBIME: NBIME with acetic acid enhancement.

Table 3 Summary of the results of each category for the Japan Narrow-band-imaging Expert Team classification and the Pit pattern classification corresponding to histological diagnosis in the included studies
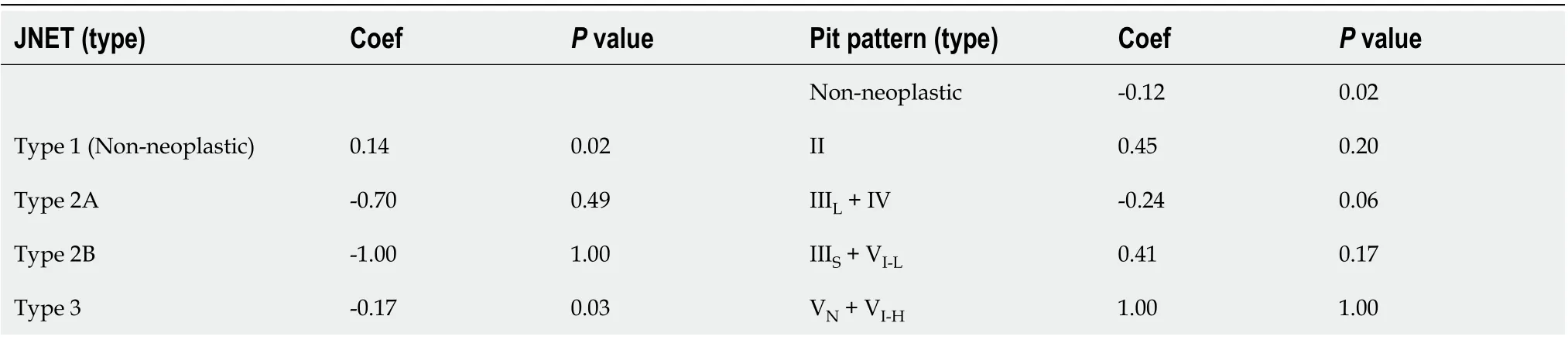
Table 4 Summary of the results of Spearman’s correlation coefficient of each category for the Japan Narrow-band-imaging Expert Team classification and the Pit pattern classification corresponding to histological diagnosis in the included studies
DISCUSSION
To best of our knowledge, no meta-analysis has been reported that systematically compared the diagnostic efficacy of detailed histologic characteristics and interobserver diagnostic agreements between the JNET and Pit pattern classifications. The present meta-analysis compared and evaluated the diagnostic outcomes of each category of these two classifications corresponding to the histological diagnosis. Our results revealed that the diagnostic performance of the JNET classification isequivalent to the Pit pattern classification in each corresponding category. We also proposed a treatment strategy for colorectal lesions using the JNET classification with its confidence level.
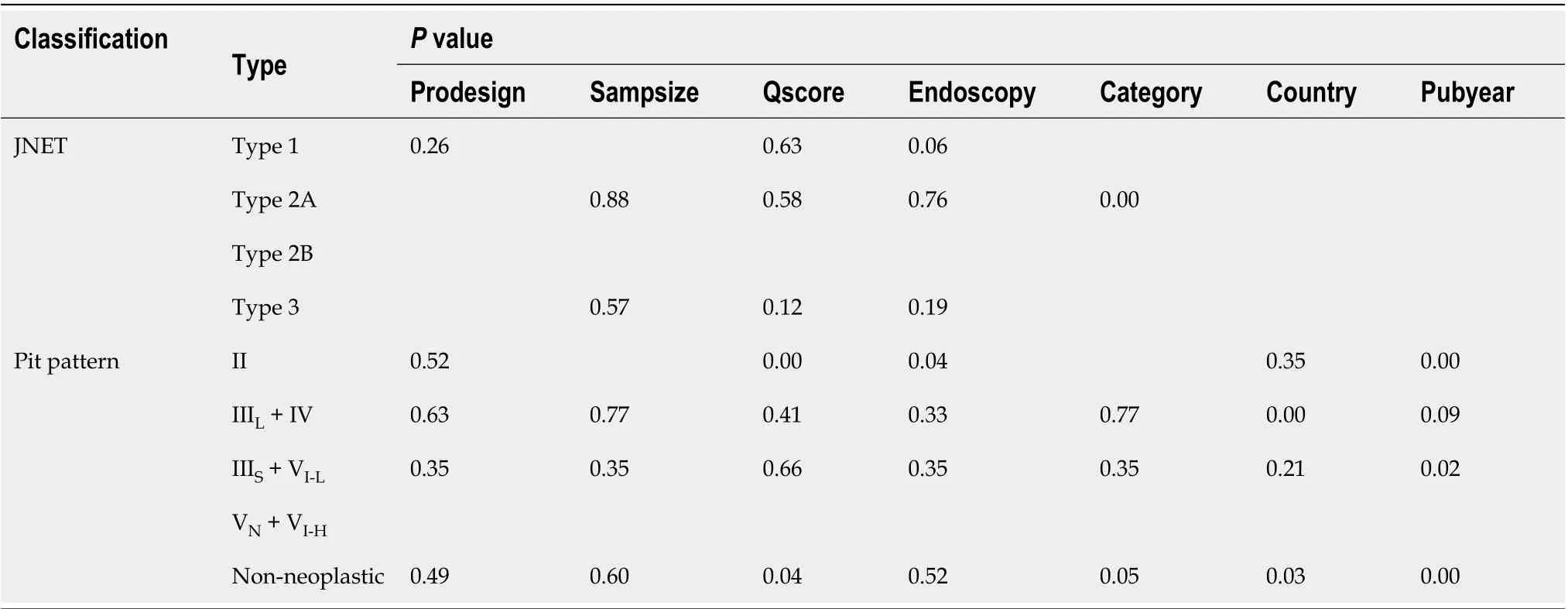
Table 5 Summary of the results of meta-regression analysis of each category for the Japan Narrow-band-imaging Expert Team classification and the Pit pattern classification corresponding to histological diagnosis in the included studies

Table 6 Summary of the results of Deek’s test for publication bias of each category for the Japan Narrow-band-imaging Expert Team classification and the Pit pattern classification corresponding to histological diagnosis in the included studies
Endoscopic diagnosisviamagnification colonoscopy has been reported to provide high diagnostic accuracy and improve the prognosis of colorectal lesions. The Pit pattern classification is the most frequently used criteria for the accurate diagnosis of colorectal neoplasms. To date, several trials[5,13-21]have evaluated the efficacy of the JNET classification, which provides useful criteria for optical-histologic diagnoses of colorectal lesions. Whereas, JNET Types 1 and 2A correspond to Pit pattern Types I + II, and Type IIIL+ IV, respectively; JNET Type 2B and 3 correspond to Pit pattern Types IIIS+ VI-Land VI-H+ VN, respectively. However, there are some differences between the JNET classification and Pit pattern classification. The Pit pattern classification is only based on the surface structure of lesions, while the JNET classification is based on the surface structure combined with the microvascular structure of lesions. In addition, the JNET classification is more concise in terms of guiding appropriate treatment strategies. According to our proposed treatment strategy, most JNET Type 1 lesions are HPs, which generally do not require resection and could be followed up by endoscopy. However, whether using the JNET classification or Pit pattern classification, it is difficult to distinguish between HP and SSL with endoscopy. Endoscopic resection is also recommended if the colorectal lesion is large or tends to enlarge obviously with endoscopy follow-up. Type 2A lesions could be resected by polypectomy or endoscopic mucosal resection (EMR). Type 2B lesions should be resected en bloc by endoscopic submucosal dissection (ESD) to obtain a precise histologic diagnosis concerning the invasion depth and determine endoscopic curability. If the lesion is relatively small, EMR is also recommended. Lastly, surgical resection is recommended for Type 3 lesions. However, few studies[5,13-21]have been published that have used the JNET classification for treatment of colorectal lesions in practice.
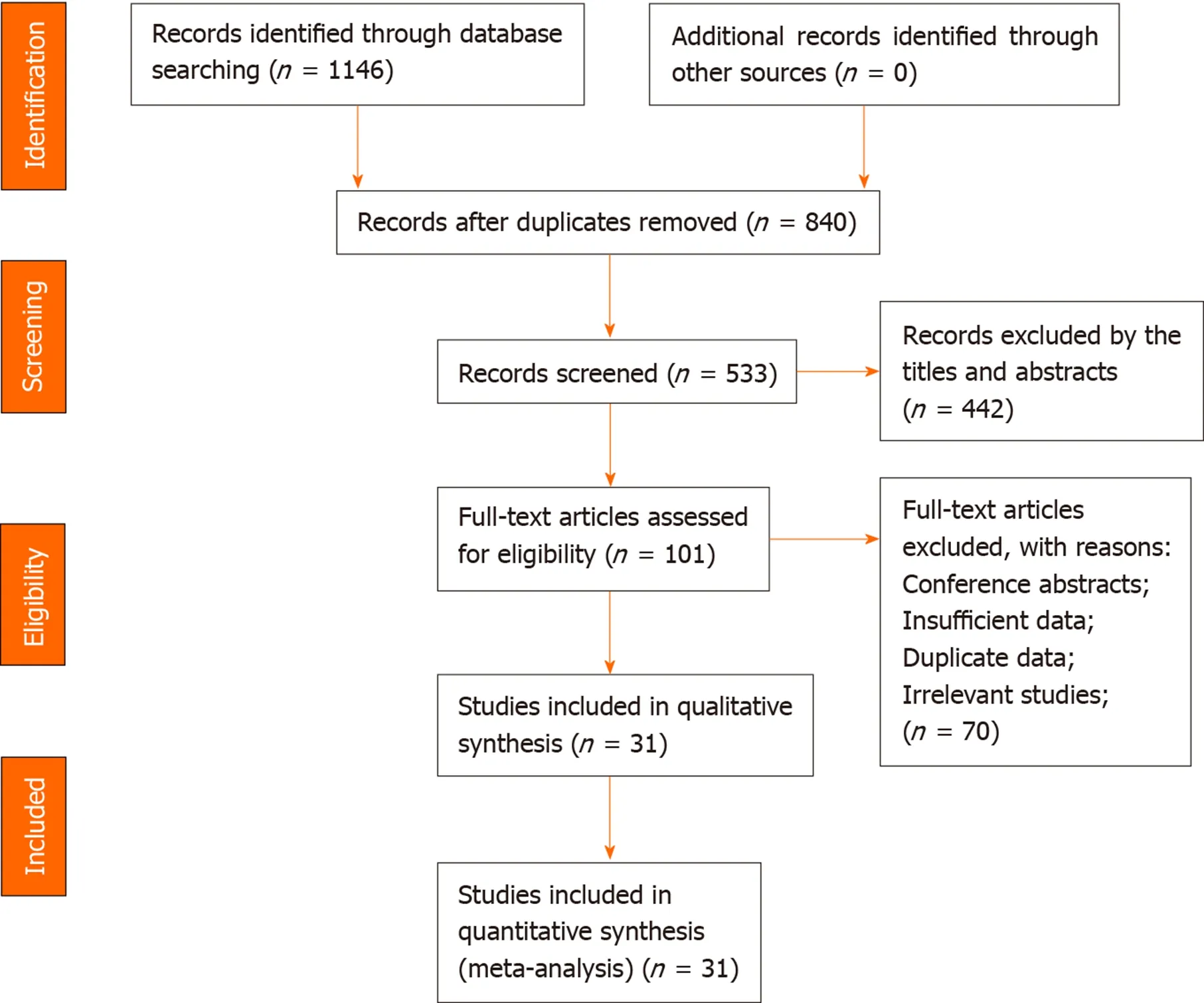
Figure 1 Study identification, inclusion and exclusion for meta-analysis.
The JNET Type 1 is considered to have as high diagnostic efficacy as the Pit pattern Type II, moreover, no significant difference was found between these categories in this meta-analysis, which implies that the magnification technology could accurately distinguish non-neoplastic from neoplastic lesions and guide clinicians to formulate the appropriate treatment.
In this study, JNET Type 2B had a relatively low pooled sensitivity and AUC of 0.56 and 0.72, respectively. Interestingly, similar results were also obtained for Pit pattern Types IIIS+ VI-L, with a sensitivity of 0.45 and AUC of 0.79. Through data analysis, it was found that, in these two categories, the evaluation regarding the dysplasia of some lesions was too low, or the depth of invasion was too shallow. Firstly, one reason might be that a large lesion often contained several pathological features, for example, a lesion contained two or more histological features of HGD, SM-s carcinoma, and SMd carcinoma at the same time. Most of the surface structures showed the features of HGD, or SM-s carcinoma, while, only a focal, or the deep part of these lesions showed the features of SM-d carcinoma; therefore, the lesions were identified as Type 2B. Secondly, due to the large size, or the special location of the lesions, the endoscopist might be unable to observe the full picture of these lesions, which might be one reason for the low sensitivity. In addition, due to the spontaneous or contact bleeding of some large lesions, the blood might be attached to the surface of the lesions and then affect the judgment of pathological Types. Therefore, for large lesions, special location lesions and pedicled lesions, endoscopists are required to observe carefully, to obtain the whole picture of the lesions, and make a comprehensive judgement, which might be helpful in improving sensitivity.
Generally, SM-d carcinomas correspond to JNET Type 3. Our meta-analysis showed that JNET Type 3 has a lower sensitivity than that of Pit pattern Type VN+ VL-H. Some polypoid advanced lesions could have a slightly less irregular NBI appearance than Type 3 because the surface microvillous structure persisted, which might be classified into JNET Type 2B. Thus, it has been proposed that Type 2B can be divided into two subtypes, Type 2B-low, and Type 2B-high[14]. The classification subtypes help to optimize the choice of treatment strategies, which also indicates that the JNET classification may need to be updated and optimized by experts to further improve the sensitivity of diagnosis. In addition, two studies[5,14]suggested that the endoscopist needed to perform an additional Pit pattern diagnosis using chromoendoscopy to differentiate Type 2B from Type 3, which might help to improve sensitivity; however, this requires further validation. In terms of specificity, compared with the Pit pattern classification, the overall JNET types were slightly higher, which was attributed to the evaluation of vessel structure by NBI-ME.
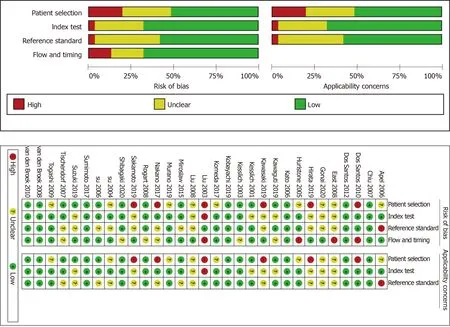
Figure 2 Quality assessment of the included studies.

Figure 3 Forest plots of pooled sensitivity and specificity. A: Japan Narrow-band-imaging Expert Team type 1; B: Pit pattern II.
There are several limitations to this meta-analysis. First, the high degree of statistical heterogeneity with a highI2value could not be avoided. The quality of endoscopic images, type of endoscopy, size of the population, year of publication, and experience of the endoscopists (expert or non-expert) possibly affected the heterogeneity of the included studies. For the Pit pattern classification, chemical staining magnifications were used in most of the included studies, but several studies used electronic staining magnification, and a few studies also used non-magnifying technology, which might have affected the results. Second, previous studies of the JNET classification were all retrospective single-center studies and the included populations were all Japanese. This indicates a potential need for large-scale prospective multi-center validation studies of the JNET classification in the future. Additionally, it is better to compare the JNET classification with the Pit pattern classification in the same endoscopic and histopathologic center.
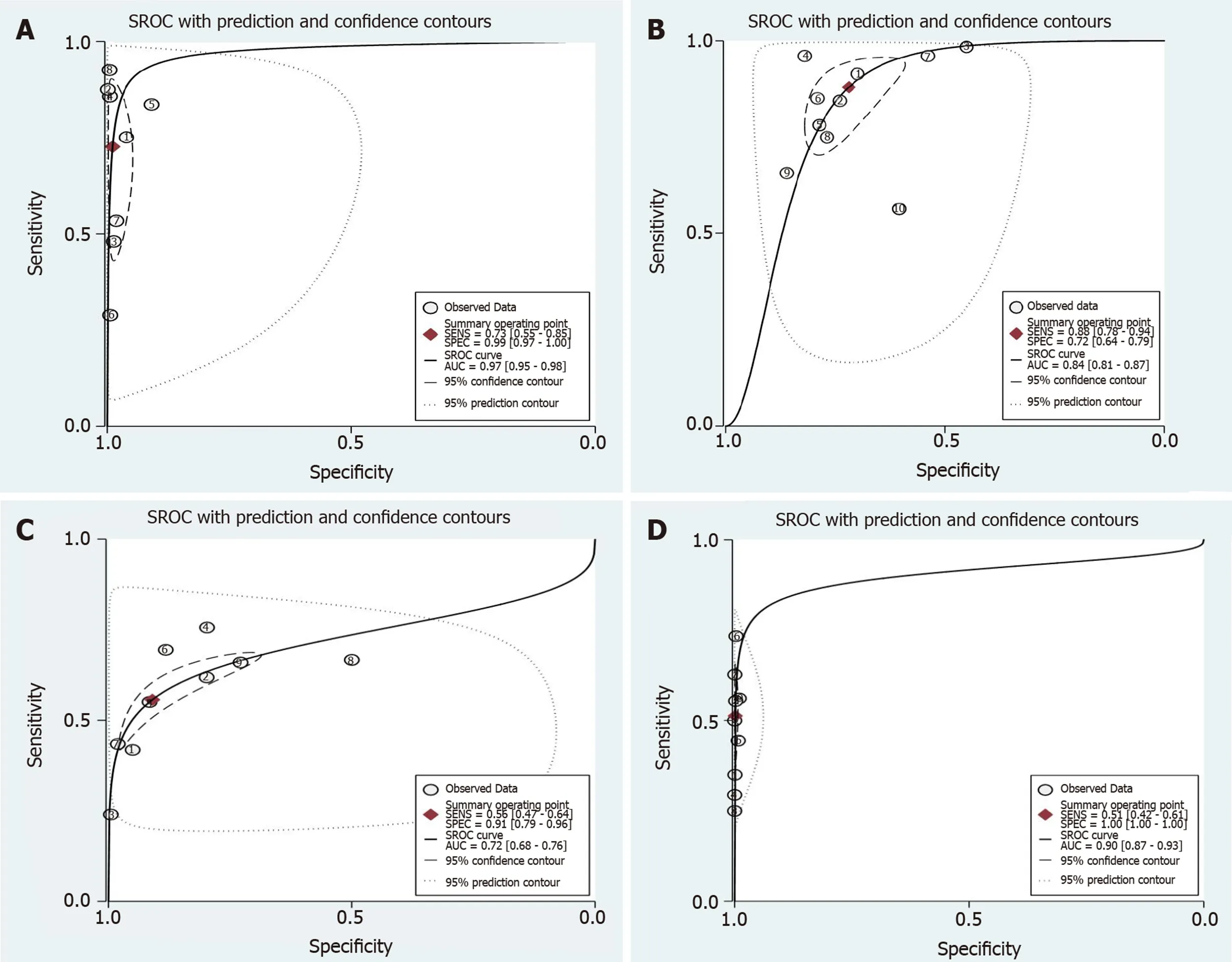
Figure 4 Summary receiver operating characteristic of the Japan Narrow-band-imaging Expert Team classification. To diagnose colorectal lesions with the corresponding 95% confidence region. A: Type 1; B: Type 2A; C: Type 2B and Type 3. SROC: Summary receiver operating characteristic.

Figure 5 Forest plots of pooled sensitivity and specificity. A: Japan Narrow-band-imaging Expert Team type 2B; B: Pit pattern IIIS + VI-L.
CONCLUSION

Figure 6 Forest plots of pooled sensitivity and specificity. A: Japan Narrow-band-imaging Expert Team type 3; B: Pit pattern VN + VI-H.

Figure 7 Forest plots of pooled sensitivity and specificity of non-neoplastic lesions by Pit pattern.
In conclusion, this meta-analysis has shown that the diagnostic efficacy of the JNET classification is equivalent to that of the Pit pattern classification as both classifications are divided into four major categories according to similar histopathology. The sensitivity of JNET Type 2B can be further improved by differentiating subtypes and combining it with the Pit pattern classification. Due to its simpler and clearer application, it is easier to guide the choice of treatment strategy, which suggests that we can promote the application of the JNET classification for colorectal lesions in the clinic. However, future prospective multi-center studies with a uniform endoscopic and histopathology protocol are required to validate our results.

Figure 8 Forest plots of pooled sensitivity and specificity. A: Japan Narrow-band-imaging Expert Team type 2A; B: Pit pattern IIIL + IV.
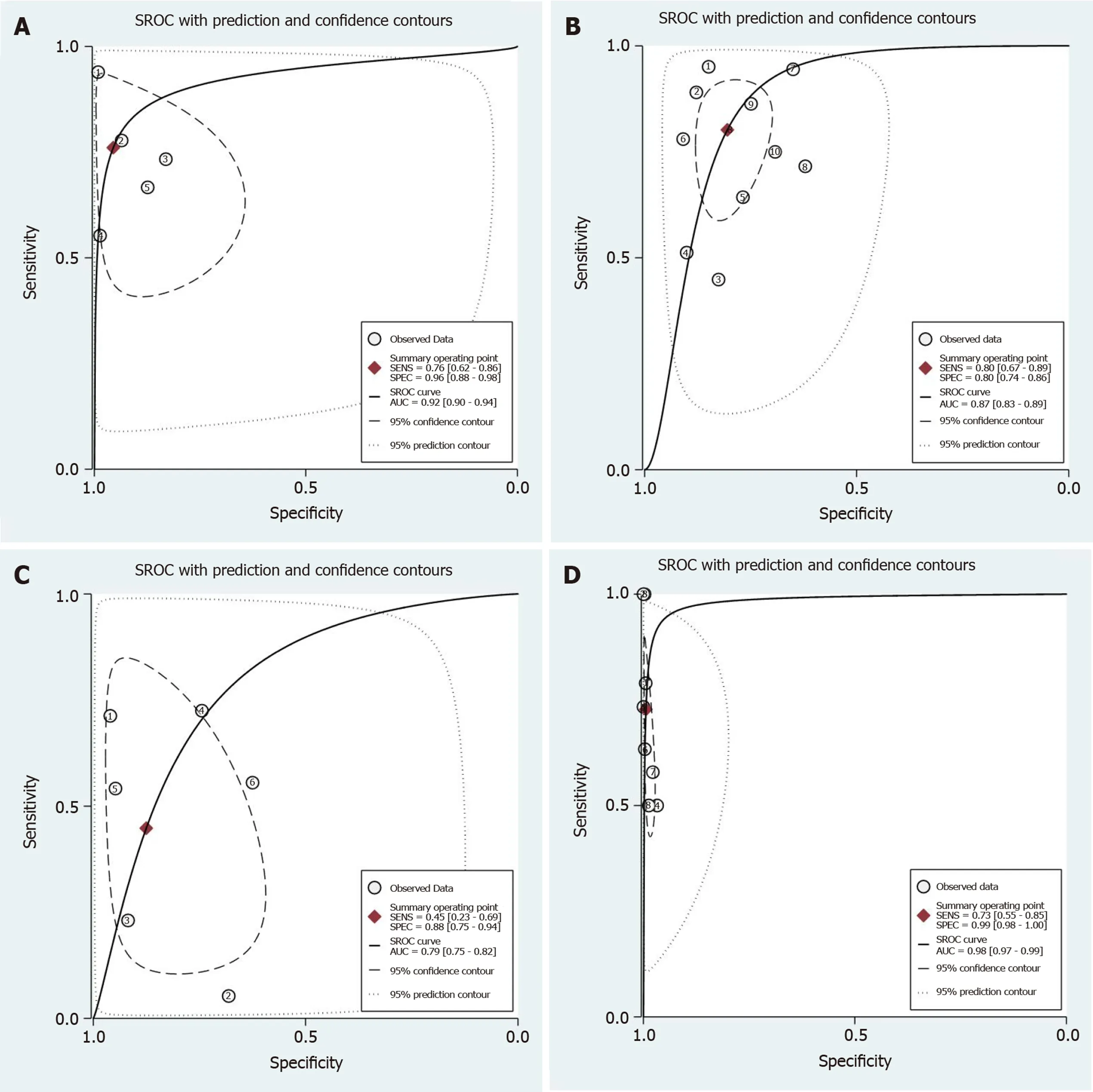
Figure 9 Summary receiver operating characteristic of Pit pattern classification. To diagnose colorectal lesions with the corresponding 95% confidence region. A: II; B: IIIL + IV; C: IIIS + VI-L and VN + VI-H. SROC: Summary receiver operating characteristic.
ARTICLE HIGHLIGHTS

 World Journal of Gastroenterology2020年40期
World Journal of Gastroenterology2020年40期
- World Journal of Gastroenterology的其它文章
- Cirrhotic portal hypertension: From pathophysiology to novel therapeutics
- New strain of Pediococcus pentosaceus alleviates ethanol-induced liver injury by modulating the gut microbiota and short-chain fatty acid metabolism
- Prediction of clinically actionable genetic alterations from colorectal cancer histopathology images using deep learning
- Compromised therapeutic value of pediatric liver transplantation in ethylmalonic encephalopathy: A case report
- Predicting cholecystocholedochal fistulas in patients with Mirizzi syndrome undergoing endoscopic retrograde cholangiopancreatography
- Novel endoscopic papillectomy for reducing postoperative adverse events (with videos)
