New strain of Pediococcus pentosaceus alleviates ethanol-induced liver injury by modulating the gut microbiota and short-chain fatty acid metabolism
Xian-Wan Jiang, Ya-Ting Li, Jian-Zhong Ye, Long-Xian Lv, Li-Ya Yang, Xiao-Yuan Bian, Wen-Rui Wu, Jing-Jing Wu, Ding Shi, Qing Wang, Dai-Qiong Fang, Kai-Cen Wang, Qiang-Qiang Wang, Yan-Meng Lu, Jiao-Jiao Xie, Lan-Juan Li
Abstract
Key Words: Pediococcus pentosaceus; Alcoholic liver disease; Gut microbiota; Probiotic; Short-chain fatty acid
INTRODUCTION
Alcoholic liver disease (ALD), which includes a spectrum of conditions, remains one of the most common causes of liver cirrhosis and liver failure worldwide, and it is responsible for 1.2% and 0.7% of all global deaths in men and women, respectively[1]. Most heavy drinkers exhibit liver steatosis, but only 10%–35% of them progress to alcoholic hepatitis, and even fewer ultimately develop cirrhosis, indicating that multiple factors, such as sex, genetics, obesity, drinking patterns and comorbidity, are involved in the progression of ALD[2-4]. Furthermore, recent studies have also suggested a close association between intestinal dysbiosis and the progression of ethanol-induced liver injury[4].
Intestinal dysbiosis has been shown to be associated with the pathogenesis of ALD, which includes changes in the microbiota composition and bacterial overgrowth. Several studies have reported alterations in the microbiota composition in patients with ALD, with a decreasing proportion ofBacteroidetesand increasing proportions ofFirmicutesandActinobacteria; a lower abundance of lactic acid-producing bacteria, such asLactobacillusandPediococcus, was also closely associated with ALD[5-7]. In addition, intestinal bacterial overgrowth is very common in patients with ALD and in animal models involving ethanol feeding[8,9]. Ethanol-induced intestinal bacterial overgrowth contributes to bacterial translocation that is associated with the expression of the antimicrobial peptides Reg3β and Reg3γ, which inhibit gram-positive bacteria and protect against bacterial overgrowth, and Reg3β- and Reg3γ-deficient mice exhibit mucosal bacterial overgrowth and increased translocation of bacteria to the liver[10]. Fecal microbiota transplantation from patients with ALD to germ-free mice increased the susceptibility to ethanol-induced liver injury and revealed a causal relationship between intestinal dysbiosis and the progression of ALD[4]. Moreover, alcohol and the metabolite acetaldehyde disrupt the gut mucosal barrier and increase gut permeability, promoting the translocation of pathological bacteria and microbiotaassociated products such as lipopolysaccharide from the gut to the liver, which promotes immune cell activation by Toll-like receptors (TLRs) and the release of cytokines and chemokines to induce liver inflammation and fibrosis[11,12]. Thus, manipulation of the microbiota with prebiotics, probiotics and fecal microbiota transplantation to restore gut homeostasis represent new therapeutic strategies for ALD[13].
Pediococcus pentosaceus(P. pentosaceus) belongs to theLactobacillaceaefamily and is widely used as a probiotic. Several strains ofP. pentosaceushave been proven to exert anti-inflammatory effects on fatty liver and intestinal inflammation. Our laboratory has focused on the isolation of potential probiotics from the gut microbiota of healthy volunteers.P. pentosaceusCGMCC 7049 is a newly isolated strain of bacteria that has been shown to be acid-tolerant and resistant to bile salts, with a high tolerance to 5% ethanol[14]. As shown in our previous studies, the administration ofP. pentosaceuseffectively inhibits pathogenic bacteria, such asStaphylococcus aureusandClostridium difficile[14,15]. The relative abundance ofPediococcusis also significantly reduced in patients with ALD and animal models involving ethanol feeding[9,16]. Based on findings from previous studies, we screened the ethanol-resistant strain ofP. pentosaceusCGMCC 7049 to further evaluate its potential protective effect on the chronic plus binge NIAAA animal model of ALD[17,18]. The short-chain fatty acid (SCFA) concentrations and composition of the gut microbiota were analyzed to explore the potential mechanisms.
MATERIALS AND METHODS
Culture of P. pentosaceus
P. pentosaceusCGMCC 7049 was cultured in MRS broth in an anaerobic chamber. Anaerobic workstations (Electrotek, England) filled with 10% H2, 10% CO2, and 80% N2were used to maintain anaerobic conditions. The cultured bacteria were centrifuged and washed twice with sterile phosphate buffered saline (PBS).P. pentosaceuswas resuspended in PBS and adjusted to a dose of 2 × 109colony forming units (CFUs) for animal experiments.
Animals and experimental design
Eight- to ten-week-old C57BL/6 mice were fed a liquid diet for one week to adapt to alcohol feeding. The mice were then randomly divided into three groups: the mice in the control group received a pair-fed control diet and oral gavage with sterile PBS (n= 8), mice in the EtOH group received a ten-day Lieber-DeCarli diet containing 5% ethanol and oral gavage with PBS (n= 10), and the mice in theP. pentosaceusgroup received a 5% ethanol Lieber-DeCarli diet but were treated withP. pentosaceus(n= 10). The mice in theP. pentosaceusgroup were administered 2 × 109CFUs ofP. pentosaceussuspended in PBS, and an equal volume of PBS was administered by oral gavage to the control group and EtOH group once daily. Mice in the EtOH group andP. pentosaceusgroup received oral gavage with one dose of ethanol (5 g/kg) on day 11, and the control group received the same volume of isocaloric maltose dextrin solution as the control reagent instead of ethanol by oral gavage; the mice were sacrificed nine hours later. Blood and tissue samples (liver and gut) were harvested to evaluate gut barrier function and liver injury-related parameters. Fresh cecal contents were collected after the mice were sacrificed on day 11 and immediately stored at -80°C until 16S rRNA gene sequencing and SCFA analyses.
Biochemical assays
A dry chemistry analyzer was used to measure the concentrations of aspartate transaminase (AST) and alanine aminotransferase (ALT). The liver tissue samples were homogenized, the supernatant was collected, and hepatic triglyceride (TG) concentrations were measured with a triglyceride assay kit (Applygen Technologies Inc., China). Endotoxin was quantified indirectly with lipopolysaccharide-binding protein (LBP) due to the inaccurate results of direct measurements, and serum LBP concentrations were determined using an LBP ELISA Kit (Guduo, Shanghai, China) according to the manufacturer’s protocols.
Serum cytokine analysis
Serum cytokine concentrations were measured using a 23-Plex Panel kit with the MAGPIX system according to the manufacturer’s protocols. The following cytokines and chemokines were detected using this multiplex assay: Interleukin (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 (p70), IL-13, IL-17A, IL-18, interferon-γ, tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-3α, C-C chemokine ligand 5, vascular endothelial growth factor, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor, and keratinocyte-derived protein chemokine (KC).
Liver and gut histology
The liver and gut tissues were fixed with 4% paraformaldehyde overnight and then embedded in paraffin. Hematoxylin and eosin (HE) staining of liver and intestinal sections was performed, and the sections were then analyzed with a digital pathology system. For the analysis of hepatic lipid accumulation, liver samples were frozen in optimal cutting temperature compound and cryosectioned. After air-drying, the sections were fixed and stained with oil red O in propylene glycol and counterstained with hematoxylin.
Immunohistochemical and immunofluorescence staining
Immunohistochemical staining was performed on paraffin sections to detect neutrophil (myeloperoxidase [MPO], Abcam, 1:200 dilution) infiltration. Briefly, liver sections were deparaffinized with xylene and dehydrated with a gradient of ethanol solutions. Rabbit MPO antibodies and secondary anti-rabbit antibodies were applied. Images were obtained with the NanoZoomer Digital Pathology system. Immunofluorescence staining was performed in colon sections to detect a tight junction protein (ZO-1). The cells were incubated with a ZO-1 antibody overnight, followed by an incubation with secondary goat anti-rabbit antibodies and DAPI staining for 45 min. Images were captured with a 340 confocal microscope (Zeiss, Oberkochen, Germany).
RNA isolation and real-time PCR analysis
RNA was purified from the liver and gut with the RNeasy Plus kit. An Applied Biosystems system was used for quantitative PCR (qPCR). The mRNA expression of the following genes was assessed using qPCR: Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), TNF-α, IL-6, IL-10, MCP-1, ZO-1, TLR2, TLR4, Reg3β, Reg3γ, MUC-1, MUC-2, and MUC-4.
SCFA analysis
Gas chromatography–mass spectrometry (GC-MS) was performed to measure the levels of SCFAs, including acetic acid, propionic acid, butyric acid, isobutyric acid, 2-methylbutyric acid and valeric acid, in the cecal contents. SCFA concentrations in cecal contents are reported in milligrams per gram (mg/g) of feces.
16S rRNA gene sequencing analysis
Total bacterial DNA was obtained with a QIAamp Fast DNA Stool Mini Kit (Qiagen, CA, United States). DNA extraction was verified by standard agarose gel electrophoresis and quantified with a Nanodrop 2000 spectrophotometer. Library preparations and sequencing were performed at BGI (Shenzhen, China). PCR was performed to prepare amplicons from the V3-V4 region using the 341F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) primers. Libraries were finally submitted for sequencing on the Illumina MiSeq platform. Paired-end raw reads from the Illumina platform were overlapped and merged using FLASH with standard parameters. Quality control of the merged reads was performed using the QIIME platform. Finally, the reads were taxonomically assigned by mapping them to the Greengenes reference database based on 97% sequence similarity using closed-reference operational taxonomic unit (OTU) mapping in QIIME. Calculations of strain composition, rarefied alpha diversity indices and beta diversity indices were performed using QIIME. The similarity of microbiota communities among groups was determined by performing a principal coordinate analysis (PCoA). The differentially abundant biomarkers among groups were further investigated with linear discriminant analysis effect size (LEfSe) analysis. The raw sequencing data were deposited in the GenBank Sequence Read Archive (Accession number: PRJNA555778).
Statistical analysis
The results are presented as means ± SE. The normality of the data was evaluated with the Kolmogorov–Smirnov test. One-way ANOVA followed by Tukey’s multiple comparison tests were used to evaluate the statistical significance of differences among groups. Spearman’s rank correlation coefficients were calculated to establish the correlations between gut bacterial genera and injury-related indices.P< 0.05 was considered statistically significant. Statistical analyses were performed and graphs were constructed using GraphPad Prism version 7.0 (GraphPad Software Inc., La Jolla, CA, United States) and SPSS 20.0 (SPSS, Inc., Chicago, IL, United States).
RESULTS
P. pentosaceus alleviated ethanol-induced liver inflammation and steatosis
We established the chronic plus binge NIAAA animal model of experimental ALD to evaluate the therapeutic properties ofP. pentosaceus. Ethanol-fed mice exhibited a significant induction of liver steatosis (Figure 1A), which was confirmed by the measurement of hepatic TG concentrations and oil red O staining of hepatic neutral lipids (Figure 1B and D). In addition,P. pentosaceussupplementation attenuated liver steatosis, with lower hepatic TG levels and less fat accumulation in hepatocytes in the histological images. Moreover, compared to the pair-fed control mice, ethanol-fed mice exhibited signs of liver injury and inflammation, as indicated by increases in serum ALT and AST levels (Figure 1D) and neutrophil infiltration (Figure 1C). However,P. pentosaceusadministration protected against liver injury by reducing ALT and AST levels and neutrophil infiltration, which was also confirmed by the quantification of MPO-positive cells after immunohistochemical staining for MPO in the liver (Figure 1C and D).
P. pentosaceus alleviated alcoholic steatohepatitis by reducing endotoxin levels and regulating the cytokine profile
The serum LBP and cytokine levels were subsequently assessed. The serum level of LBP was increased in ethanol-fed mice, but the mice treated withP. pentosaceusshowed lower levels of LBP than mice in the EtOH group (Figure 2). Additionally, the expression of TLR4 mRNA was also upregulated in the liver of ethanol-fed mice compared with control mice, while TLR4 expression was downregulated in theP. pentosaceus-treated group (Figure 2). The serum cytokine and chemokine levels were also measured, and the levels of proinflammatory cytokines, including IL-5, IL-6, TNFα, G-CSF, KC, MIP-1α and MCP-1, were markedly increased in ethanol-fed mice. As expected,P. pentosaceustreatment significantly reduced the levels of the proinflammatory cytokines IL-5, TNF-α, G-CSF, KC, MIP-1α and MCP-1 (Figure 2).
P. pentosaceus improved intestinal integrity and reduced bacterial overgrowth
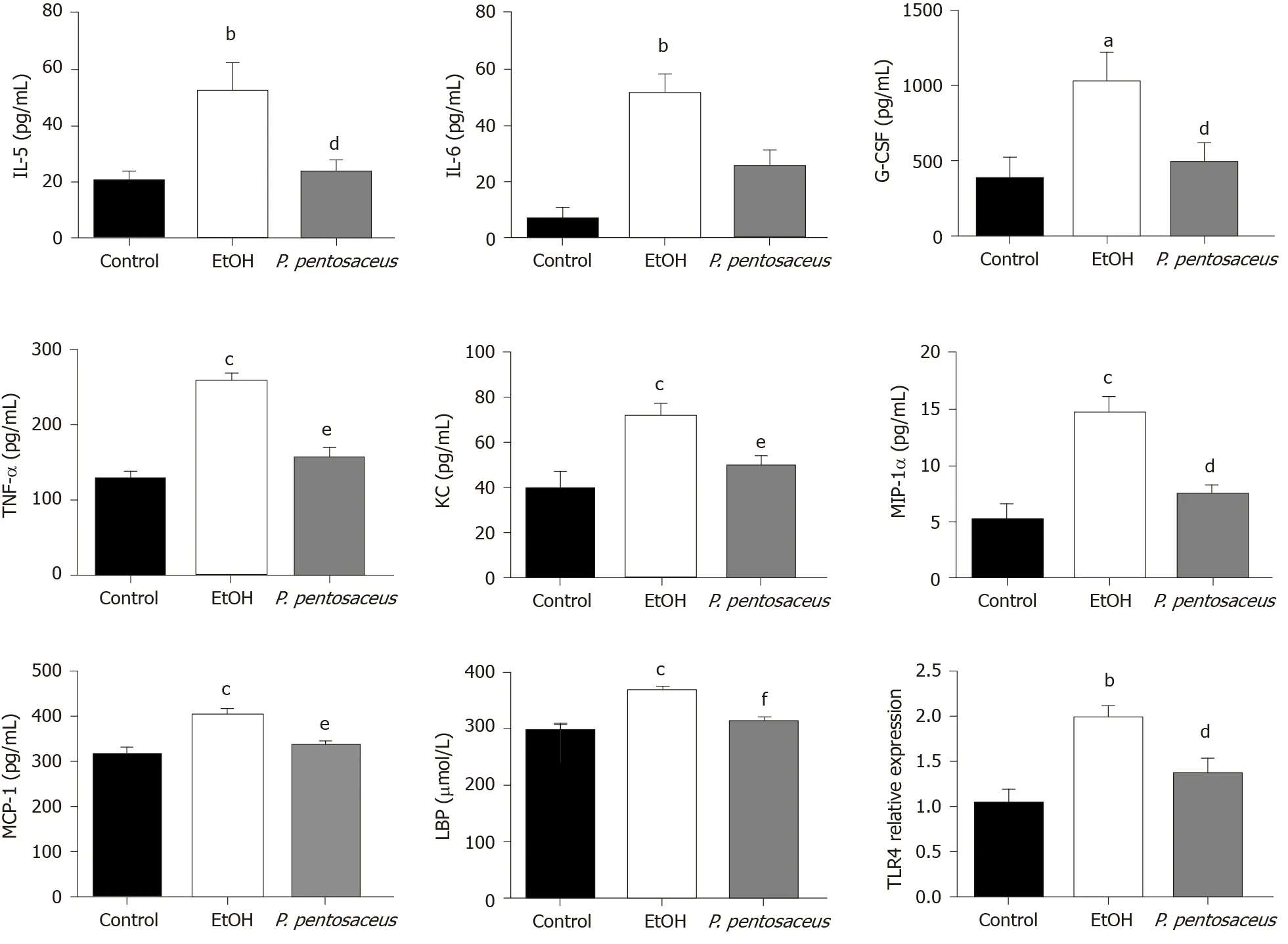
Figure 2 Effects of the Pediococcus pentosaceus treatment on lipopolysaccharide-binding protein and proinflammatory cytokine levels during ethanol-induced liver injury. The serum levels of interleukin (IL)-5, IL-6, granulocyte colony-stimulating factor, tumor necrosis factor-α, keratinocytederived protein chemokine, macrophage inflammatory protein-1α, monocyte chemoattractant protein-1 and lipopolysaccharide-binding protein and expression of the Toll-like receptor 4 mRNA in the liver. All data are presented as means ± SE. aP < 0.05, bP < 0.01, cP < 0.001 vs the Control group. dP < 0.05, eP < 0.01, fP < 0.001 vs the EtOH group. P. pentosaceus: Pediococcus pentosaceus; IL: Interleukin; G-CSF: Granulocyte colony-stimulating factor; TNF-α: Tumor necrosis factor-α; KC: Keratinocyte-derived protein chemokine; MIP-1α: Macrophage inflammatory protein-1α; MCP-1: Monocyte chemoattractant protein-1; LBP: Lipopolysaccharidebinding protein; TLR4: Toll-like receptor 4.
According to HE staining of colon tissues, alcohol exposure significantly increased tissue inflammation in the absence of a loss of villi or intestinal crypts.P. pentosaceussupplementation significantly ameliorated the histological abnormalities associated with ethanol-induced intestinal epithelial injury (Figure 3A). Tight junction proteins are essential to maintain intestinal barrier function, and thus we next evaluated the expression of ZO-1 in the colon. Alcohol feeding significantly reduced the expression of ZO-1 mRNA, whereas the expression of ZO-1 mRNA was upregulated followingP. pentosaceussupplementation. The results of immunofluorescence staining were also consistent with the gene expression results, as the fluorescence intensity of ZO-1 decreased during alcohol feeding andP. pentosaceussupplementation stabilized the expression of this tight junction protein and improved intestinal integrity (Figure 3A and B). Moreover, we also investigated the expression of mucin mRNAs that protect against pathogen penetration of the mucus layer after alcohol administration. Although the expression of intestinal mucin mRNAs, including MUC-1 and MUC-2, was not significantly different between the EtOH group and the control group, significantly higher mucin mRNA expression (MUC-1, MUC-2 and MUC-4) was observed in mice treated withP. pentosaceus(Figure 3B). In addition, we further evaluated the overall bacterial load and the expression of the antibacterial peptides Reg3β and Reg3γ. The total bacterial load was significantly higher in ethanol-fed animals than in the control animals, butP. pentosaceussupplementation significantly ameliorated intestinal bacterial overgrowth during alcohol feeding (Figure 3B). The expression of Reg3β mRNA was significantly inhibited in alcohol-fed mice compared with control mice. Conversely, much higher levels of Reg3β mRNA were observed in mice that received theP. pentosaceussupplement (Figure 3B).
P. pentosaceus ameliorated ethanol-induced intestinal dysbiosis
Bacterial DNA was extracted and the gut microbiota composition was analyzed using 16S rRNA sequencing. The resulting paired reads were assembled and filtered to generate 1077504 valid tags (44896 tags per sample) for subsequent analysis. The nonchimeric sequences were then clustered into 398 OTUs based on 97.0% sequence similarity.
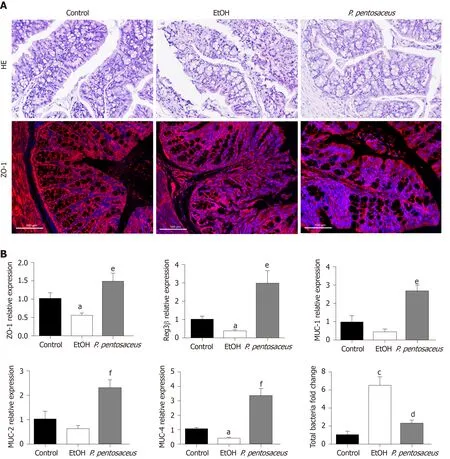
Figure 3 Histopathological examination of the colon and the expression of intestinal barrier markers. A: Representative histological images of the colon stained with hematoxylin and eosin and images of immunofluorescence staining for ZO-1. Scale bar: 100 μm; B: Relative mRNA levels of ZO-1, mucin (MUC)-1, MUC-2, MUC-4, Reg3β and the total bacterial 16S rRNA. All data are presented as means ± SE. aP < 0.05, cP < 0.001 vs the Control group. dP < 0.05, eP < 0.01, fP < 0.001 vs the EtOH group. P. pentosaceus: Pediococcus pentosaceus; HE: Hematoxylin and eosin; MUC: Mucin.
The microbial alpha diversity was evaluated with the Chao, Shannon and Simpson indices. The Chao richness index and Shannon and Simpson diversity indices were significantly reduced after alcohol consumption, but the changes in microbial diversity were partially reversed byP. pentosaceustreatment (Figure 4B and Supplementary Figure 1). Furthermore, we constructed a PCoA plot with unweighted UniFrac distances to assess microbial beta diversity among the three groups (Figure 4A). The microbial communities of the three groups were distinctly separated, and the microbial communities in the alcohol-fed group were clustered and significantly different from the microbial communities in the control group (ANOSIM,P= 0.002,r= 0.71). Likewise, the microbial composition also differed between theP. pentosaceusgroup and the EtOH group (ANOSIM,P= 0.003,r= 0.23). Based on these results,P. pentosaceussupplementation altered the bacterial community and increased the microbial diversity.
We further investigated the changes in the microbial composition at the phylum and genus levels. At the phylum level, alcohol feeding resulted in an increase in the abundance ofActinobacteriaandFirmicutes, but lower proportions ofBacteroidetescompared to the control group (Figure 4C). However,P. pentosaceussupplementation restored the abundance ofBacteroidetesandVerrucomicrobiaand prevented the expansion ofFirmicutes(Figure 4C). At the genus level, significant reductions in
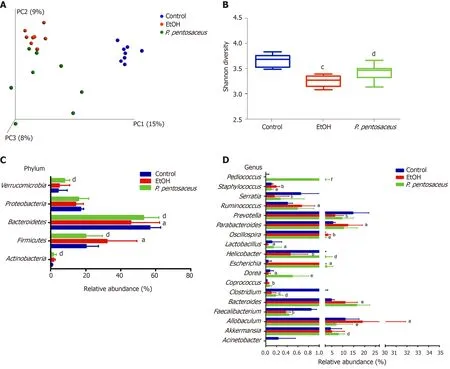
Figure 4 Alterations in the bacterial diversity and microbial composition. A: Principal co-ordinates analysis plot of the microbiomes of different groups; each symbol represents one sample; B: Shannon diversity indices among the three groups; C: The relative abundance of taxa at the phylum level; D: The relative abundance of taxa at the genus level. All data are presented as means ± SE. aP < 0.05, bP < 0.01, cP < 0.001 vs the Control group. dP < 0.05, eP < 0.01, fP < 0.001 vs the EtOH group. PC: principal co-ordinates; P. pentosaceus: Pediococcus pentosaceus.
Prevotella, Helicobacter, Lactobacillus, Faecalibacterium, andClostridiumand increases inEscherichia, Staphylococcus, andParabacteroideswere observed in mice after alcohol consumption (Figure 4D). In contrast,P. pentosaceusadministration restored the relative abundance ofLactobacillus, Pediococcus, ClostridiumandAkkermansiaand decreased the abundance ofStaphylococcus, OscillospiraandAllobaculum(Figure 4D). We performed a LEfSe analysis to investigate the microbial markers between the groups. Similarly,Prevotella, Helicobacter, Lactobacillus, Faecalibacterium, Clostridiumand
Serratiawere more frequently represented in the control group, whereasEscherichia,Staphylococcus,Parabacteroides,OscillospiraandAllobaculumwere the predominant microbiota in the EtOH group (Figure 5A). Remarkably, the familyLactobacillaceaeand the generaLactobacillusandPediococcuswere depleted after alcohol feeding and were significantly overrepresented afterP. pentosaceussupplementation (Figure 5B).
P. pentosaceus supplementation increased SCFA levels
GC-MS was used to measure the concentrations of SCFAs, including acetic acid, propionic acid, butyric acid, isobutyric acid, 2-methylbutyric acid and valeric acid, in mouse feces. Ethanol feeding resulted in marked reductions in propionic acid, butyric acid, valeric acid, and 2-methylbutyric acid concentrations (Figure 6). Conversely,P. pentosaceusadministration increased the production of propionic acid and butyric acid compared to the EtOH group (Figure 6). A decreasing trend in the levels of acetic acid and isobutyric acid was observed in mice after ethanol consumption, but this trend was not statistically significant among the three groups (Figure 6). Thus, alcohol feeding significantly decreased the production of SCFAs, butP. pentosaceussupplementation partially reversed this trend.
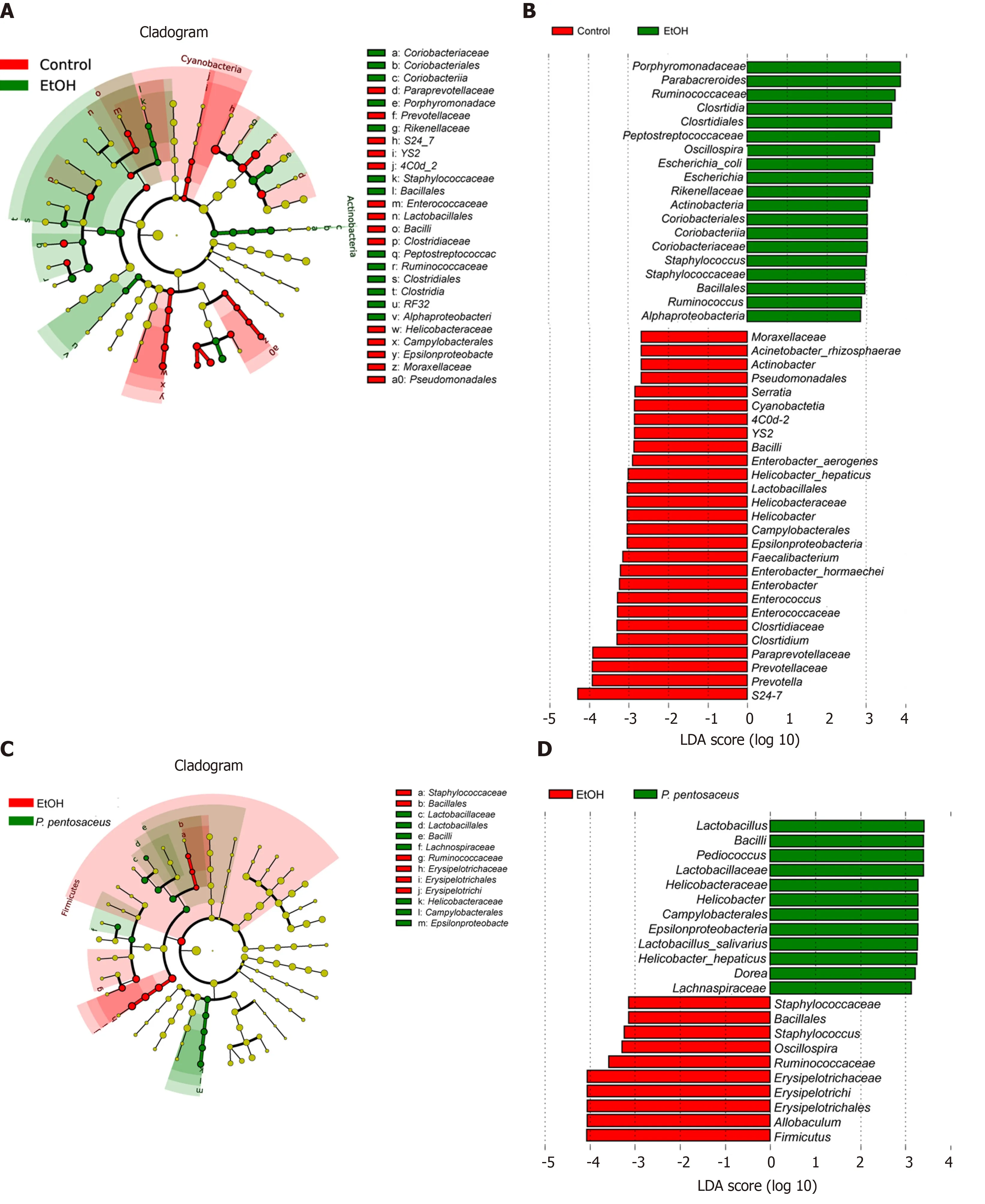
Figure 5 The effects of Pediococcus pentosaceus supplementation on the microbial community according to the linear discriminant analysis effect size analysis. Cladogram showing the most differentially abundant taxa among groups. The circle sizes in the cladogram are proportional to the bacterial abundance. Taxa enriched in microbiota from different groups were identified using linear discriminant analysis effect size. P. pentosaceus: Pediococcus pentosaceus; LDA: Linear discriminant analysis.
Correlation analysis of representative microbial genera, gut barrier markers and liver injury-related indices
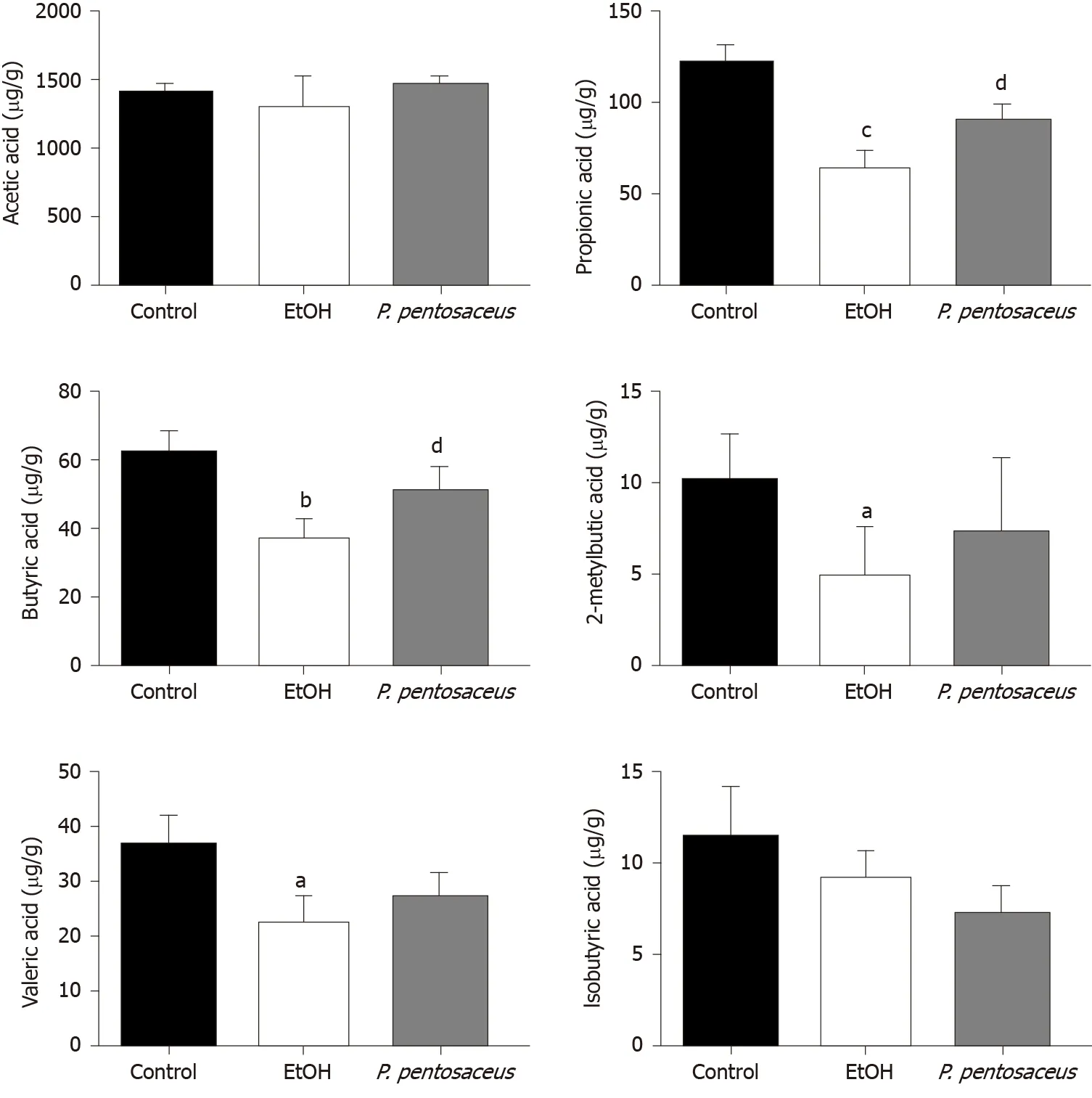
Figure 6 The short-chain fatty acid levels in cecal contents among the three groups. The levels of acetic acid, propionic acid, butyric acid, isobutyric acid, 2-methylbutyric acid and valeric acid. All data are presented as means ± SE. aP < 0.05, bP < 0.01, cP < 0.001 vs the Control group. dP < 0.05 vs the EtOH group. P. pentosaceus: Pediococcus pentosaceus.
We next performed a correlation analysis of altered bacterial genera, gut barrier markers, inflammatory cytokines and liver injury-related indices (Figure 7). The concentrations of proinflammatory cytokines (IL-5, IL-6, TNF-α, G-CSF, KC, and MIP-1α) were positively correlated with liver injury-related indices (ALT and AST). TLR4 mRNA expression was also positively correlated with the levels of IL-6, TNF-α, G-CSF, KC, MIP-1α and ALT, indicating that TLR activation and inflammatory cytokine release contributed to liver injury. Additionally, the altered microbiota showed positive or negative correlations with gut barrier markers and liver injury-related parameters. The relative abundance of potentially pathogenicEscherichiawas enriched after alcohol feeding and showed positive correlations with the levels of proinflammatory cytokines (IL-5, IL-6, KC, and MIP-1α).PediococcusandLactobacilluswere enriched in theP. pentosaceusgroup and exhibited positive correlations with gut barrier markers (ZO-1 and MUC-2) and negative correlations with ALT levels. The relative abundance ofAkkermansiaandFaecalibacteriumwas significantly higher afterP. pentosaceussupplementation and exhibited positive correlations with SCFA concentrations (propionic acid and butyric acid) and gut barrier markers (Reg3β), but negative correlations with the levels of proinflammatory cytokines (IL-5, G-CSF, TNFα and KC) and liver injury parameters (ALT). Similarly, the proportion ofClostridiumwas negatively correlated with ALT, AST and TNF-α levels and positively correlated with the butyric acid concentration. The gut barrier marker ZO-1 was positively correlated with SCFA concentrations (propionic acid and butyric acid) but negatively correlated with proinflammatory cytokine levels (IL-6, MIP-1α, and TNF-α). Therefore, the correlation network analysis indicated that the modified gut microbiota, SCFAs and the gut barrier contributed to alleviating systemic inflammation and liver injury.
DISCUSSION
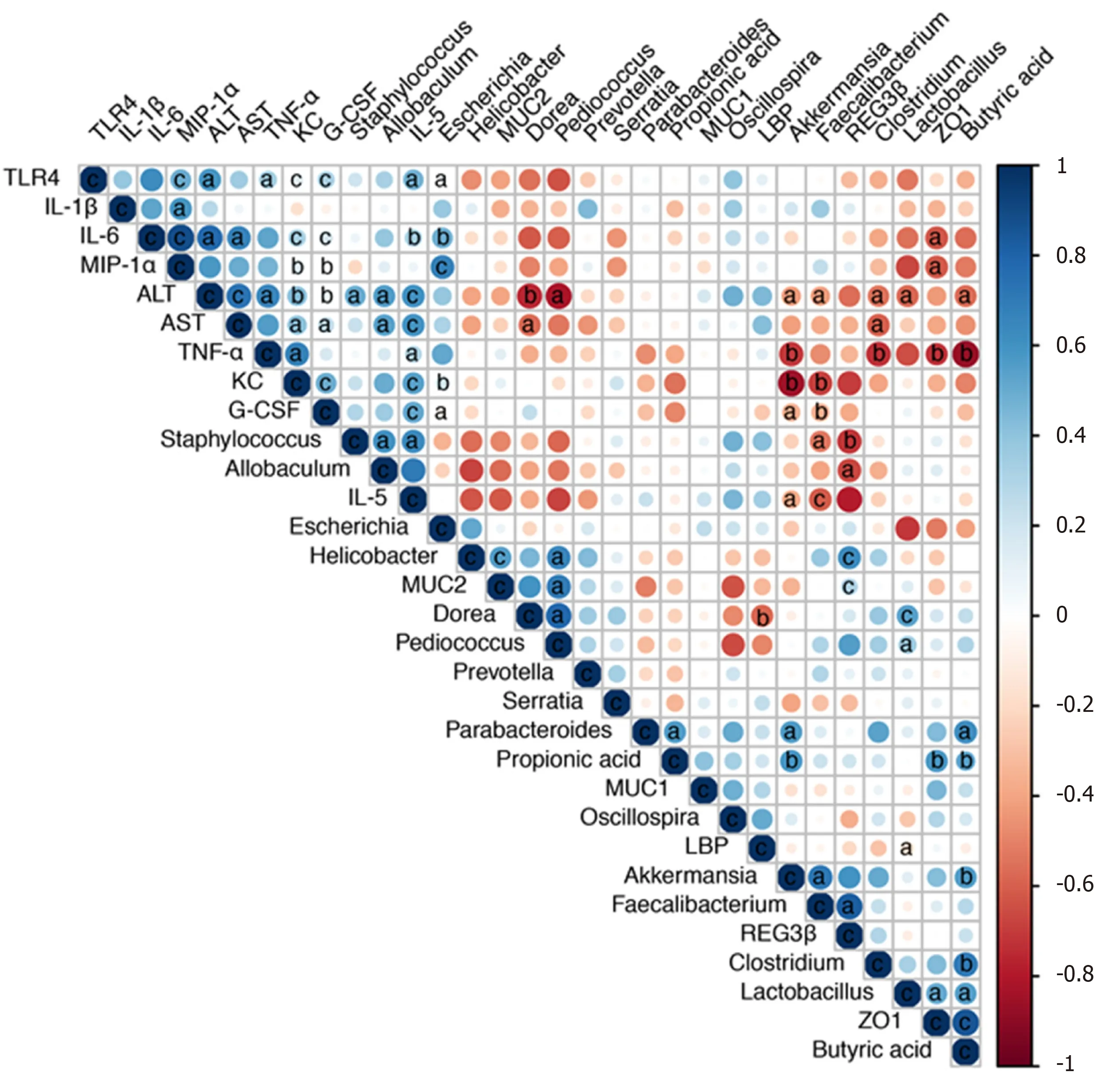
Figure 7 Correlation network analysis of representative microbial genera, gut barrier markers, inflammatory cytokines and liver injuryrelated indices. Spearman’s correlation analysis was performed, and statistical significance was indicated as follows: aP < 0.05, bP < 0.01, cP < 0.001. TLR4: Tolllike receptor 4; IL: Interleukin; MIP-1α: Macrophage inflammatory protein-1α; ALT: Alanine aminotransferase; AST: Aspartate transaminase; TNF-α: Tumor necrosis factor-α; KC: Keratinocyte-derived protein chemokine; G-CSF: Granulocyte colony-stimulating factor; MUC: Mucin; LBP: Lipopolysaccharide-binding protein.
Based on the results of the present study,P. pentosaceussupplementation is effective at alleviating ethanol-induced liver inflammation and steatosis. The potential mechanisms were further investigated. Ethanol feeding results in intestinal dysbiosis and gut barrier dysregulation, whileP. pentosaceussupplementation modulates the microbiota diversity and composition, improves gut barrier function and SCFA metabolism, and reduces endotoxin and proinflammatory cytokine levels, which might be associated with the protective effect on ethanol-induced liver injury.
Regulation of the intestinal microbiota with probiotics, prebiotics and fecal microbiota transplantation is another promising treatment option for ALD[19,20].P. pentosaceusis widely used as a probiotic and is considered beneficial for health. In our study,P. pentosaceusCGMCC 7049 is a newly isolated strain of bacteria that was shown to be resistant to acid and bile salts, with a high tolerance to ethanol[21]. However, we had not yet determined whetherP. pentosaceusexerts a protective effect on ALD and the potential mechanism. Therefore, this potential protective effect was further evaluated using chronic plus binge animal models[17].
Intestinal dysbiosis and gut barrier disruption result in the translocation of PAMPs, such as endotoxins, from the gut to the liver[22]. An increase in endotoxin levels in the systemic circulation activates the TLR pathway in Kupffer cells, and increased production of proinflammatory cytokines and chemokines result in liver inflammation[18,23]. In the present study,P. pentosaceussupplementation ameliorated liver inflammation and steatosis, decreased ALT, AST and TG levels, and reduced neutrophil infiltration. Alcohol feeding induced gut leakiness, increased the endotoxin level and upregulated the expression of the TLR4 mRNA in the liver. Consistent with these results, the levels of proinflammatory cytokines, including IL-5, IL-6, TNF-α, GCSF, KC, MIP-1α and MCP-1, were significantly increased. Increased levels of cytokines, such as IL-6 and TNF-α, induce liver inflammation and fibrosis, and increased levels of chemokines recruit neutrophils to aggravate liver inflammation in patients with ALD[21]. Based on our findings,P. pentosaceussupplementation decreased endotoxin levels and downregulated TLR4 expression. In addition, the levels of proinflammatory cytokines, including IL-5, TNF-α, G-CSF, KC, MIP-1α and MCP-1, were significantly decreased. Chemokines such as MCP-1 and MIP-1α, which are upregulated in patients with alcoholic hepatitis[21], are responsible for the recruitment and activation of macrophages and monocytes and modulate the production of the proinflammatory cytokines TNF-α, IL-1β and IL-6[24]. Therefore,P. pentosaceussupplementation reduced the circulating endotoxin level and subsequently decreased the levels of proinflammatory cytokines and chemokines, which might explain the protective effect on liver inflammation.
The gut barrier plays a crucial role in preventing endotoxin translocation from the gut to the liver, and alcohol consumption disrupts the epithelial barrier and gutvascular barrier and results in increased intestinal permeability in animal models and patients with ALD[22,25]. In our study, the mRNA expression of the tight junction protein ZO-1 was decreased after alcohol consumption, but the probioticP. pentosaceustreatment increased the level of the ZO-1 protein. On the other hand, the intestinal mucus layer, which consists of mucins that are synthesized and secreted by goblet cells, is critical for protecting the epithelial barrier against colonization by pathogenic bacteria[25-27]. In our study, the mRNA expression of intestinal mucins, including MUC-1, MUC-2 and MUC-4, was significantly increased afterP. pentosaceussupplementation. Antimicrobial peptides such as Reg3β and Reg3γ are secreted by epithelial cells and Paneth cells to inhibit bacterial overgrowth, and Reg3β peptides exert bacteriostatic effects on gram-negative bacteria and protect against pathogenic bacterial colonization[10,28,29]. Reg3β-deficient mice exhibit restricted bacterial overgrowth and translocation to prevent ethanol-induced liver injury[21]. Treatment with the probioticP. pentosaceussignificantly increased the expression of Reg3β mRNA in the present study, which may explain the inhibitory effect of this probiotic on intestinal bacterial overgrowth. Thus, the levels of tight junction proteins, mucin proteins and antimicrobial peptides were upregulated by probiotic supplementation to improve gut barrier function.
Excessive alcohol consumption induces changes in the gut microbiota composition and bacterial overgrowth and increases intestinal permeability and bacterial translocation from the gut to the liver to promote alcoholic steatohepatitis[22,23]. In the present study, the microbiota diversity and richness were reduced, and the microbial composition was also altered after alcohol consumption; the change in microbial diversity was partially reversed byP. pentosaceustreatment. The reduction in bacterial diversity was consistent with the finding that patients with alcoholic hepatitis have lower Shannon diversity and Chao richness indices[30]. In the present study, alcohol feeding increased theFirmicutes-to-Bacteroidetesratio (F/B ratio), andP. pentosaceussupplementation restored the F/B ratio to a value similar to that of the control group. These results are consistent with the finding thatFirmicutesandBacteroidetesare the predominant phyla detected in patients with alcoholic hepatitis, and the F/B ratio is 127% higher than in healthy controls[30]. At the genus level, the abundance ofEscherichiaandStaphylococcusincreased with alcohol consumption, which was related to intestinal inflammation.Escherichiaare also enriched in patients with nonalcoholic steatohepatitis, liver cirrhosis and hepatocellular carcinoma[31-33].Escherichiaincrease the production of endotoxins, secondary bile acids and endogenous ethanol to promote liver inflammation[34], and one possible explanation for the increase ofEscherichiain patients with ALD might be its tolerance to ethanol[35].Staphylococcusis an important pathogen that causes infection in patients with alcoholic liver cirrhosis and increases the risk of mortality[36]. According to a previous study, antimicrobial substances fromP. pentosaceusare effective at inhibiting pathogenicStaphylococcus[37]. Consistent with this finding, the relative abundance ofStaphylococcuswas significantly decreased afterP. pentosaceussupplementation in the present study. On the other hand, the relative abundance ofLactobacillus, Prevotella, Faecalibacterium, andClostridiumdecreased with alcohol feeding. Supplementation withLactobacillus rhamnosusGG promotesLactobacillusexpansion and is a beneficial treatment for alcohol-induced liver injury[38-41]. Likewise,P. pentosaceusadministration restored the relative abundance ofLactobacillusandPediococcusin the present study. In addition,Prevotella, FaecalibacteriumandClostridiumare considered SCFA-producing bacteria, and their levels are also decreased in patients with alcoholic liver cirrhosis[42]. In the present study,P. pentosaceussupplementation partially restored the abundance ofPrevotellaandClostridium. Akkermansiahas been reported to be depleted and negatively correlated with the severity of ALD, and anAkkermansiatreatment increases the number of goblet cells and the expression of mucin proteins to alleviate ethanolinduced liver injury[43]. An increasing trend inAkkermansiaabundance was observed afterP. pentosaceussupplementation, which exhibited a positive correlation with SCFA concentrations and gut barrier markers. Thus, probiotic supplementation may attenuate intestinal dysbiosis.
SCFAs, which are derived from dietary fiber fermentation by the commensal gut microbiota, provide an energy source for intestinal epithelial cells and regulate hepatic glucose and energy metabolism[43-45]. Butyrate-producing probiotic or butyrate supplementation improve intestinal permeability and inhibit HDAC1 expression to alleviate hepatic steatosis and injury[46-49]. In human studies, a reduction in SCFA concentrations, including acetic acid, propionic acid and butyric acid, was observed in patients with severe alcoholic hepatitis[43]. Similarly, the levels of SCFAs, including propionic acid, butyric acid, valeric acid, and 2-methylbutyric acid, were markedly decreased in the present study, accompanied by a reduction in the abundance of SCFA-producing bacteria, includingPrevotella, FaecalibacteriumandClostridium, after alcohol feeding. However,P. pentosaceusadministration increased the production of propionic acid and butyric acid by modifying the abundance of SCFA-producing bacteria, includingPrevotella, ClostridiumandAkkermansia, and the higher abundance ofClostridiumandAkkermansiawas also positively correlated with the SCFA concentrations. Based on these results, the increase in the abundance of SCFAproducing bacteria might increase the production of propionic acid and butyric acid, which are associated with an improvement in gut barrier function.
Nevertheless, our study had some potential limitations. Although the chronic plus binge ethanol feeding animal model mimics ethanol-induced liver inflammation and steatosis, liver fibrosis was not induced in this model[17]. The results of animal experiments have shown the protective effect of probiotic supplementation, but further human clinical studies are needed to confirm its safety and efficacy. Differentially abundant bacteria were identified at the genus level, but the exploration of taxa at the species level was not possible due to the depth of 16S rRNA sequencing[50]. AlthoughP. pentosaceussupplementation modified the gut microbiota composition and SCFA metabolism, the causal relationship between the modified microbiota and the alleviation of liver injury was unclear, and the host microbiota and metabolism interaction requires further investigation[51].
CONCLUSION
In conclusion,P. pentosaceussupplementation was effective at alleviating ethanolinduced liver inflammation and steatosis by reversing gut microbiota dysbiosis, regulating SCFA metabolism, improving intestinal barrier function, and reducing circulating endotoxin and proinflammatory cytokine and chemokine levels. These results provide a theoretical basis for the use ofP. pentosaceusCGMCC 7049 as a prospective probiotic treatment option for patients with ALD.
ARTICLE HIGHLIGHTS

Research methods
P. pentosaceusCGMCC 7049 was isolated from healthy adults in our laboratory. The chronic plus binge NIAAA model was used to evaluate the protective effects. Mice were randomly divided into three groups: the control group received a pair-fed control diet and oral gavage with sterile phosphate buffer saline (PBS), the EtOH group received 5% ethanol Lieber-DeCarli diet and oral gavage with PBS, and theP. pentosaceusgroup received a 5% ethanol Lieber-DeCarli diet andP. pentosaceustreatment. Gut and liver tissue samples were harvested to assess the gut barrier function and liver injury-related parameters. Fresh cecal contents were collected for the 16S rRNA gene sequencing and short-chain fatty acid (SCFA) analyses.
Research results
TheP. pentosaceustreatment improved ethanol-induced liver injury by reducing alanine aminotransferase, aspartate transaminase and triglyceride levels, and neutrophil infiltration, which was accompanied by decreased levels of endotoxin and inflammatory cytokines. In addition,P. pentosaceusadministration increased the expression of a tight junction protein, mucin proteins and antibacterial peptides to improve the gut barrier function. Ethanol administration induced intestinal dysbiosis and increased the relative abundance of pathogenicEscherichiaandStaphylococcusbut depleted SCFA-producing bacteria. In contrast,P. pentosaceustreatment increased the microbial diversity and restored the relative abundance of SCFA-producing bacteria, such asPrevotella, ClostridiumandAkkermansia, and increased the production of propionic acid and butyric acid.
Research conclusions
Based on the results of the present study, the newly isolated strain ofP. pentosaceuswas an effective treatment that protected against ethanol-induced liver injury by modulating the gut microbiota and improving SCFA metabolism and gut barrier function.
Research perspectives
An ethanol-resistant strain of probioticP. pentosaceusalleviated ethanol-induced liver injury in a chronic plus binge animal model, which might represent a promising microbe-based therapy for patients with ALD.
 World Journal of Gastroenterology2020年40期
World Journal of Gastroenterology2020年40期
- World Journal of Gastroenterology的其它文章
- Cirrhotic portal hypertension: From pathophysiology to novel therapeutics
- Prediction of clinically actionable genetic alterations from colorectal cancer histopathology images using deep learning
- Compromised therapeutic value of pediatric liver transplantation in ethylmalonic encephalopathy: A case report
- Predicting cholecystocholedochal fistulas in patients with Mirizzi syndrome undergoing endoscopic retrograde cholangiopancreatography
- Novel endoscopic papillectomy for reducing postoperative adverse events (with videos)
- Pediatric bowel preparation: Sodium picosulfate, magnesium oxide, citric acid vs polyethylene glycol, a randomized trial
