Pediatric bowel preparation: Sodium picosulfate, magnesium oxide, citric acid vs polyethylene glycol, a randomized trial
Carmen Cuffari, Steven L Ciciora, Masakazu Ando, Mena Boules, Joseph M Croffie
Abstract
Key Words: Children; Colonoscopy; Colon cleansing; Sodium picosulfate, magnesium oxide, and citric acid; Polyethylene glycol
INTRODUCTION
Colonoscopy in the pediatric population is commonly used to evaluate gastrointestinal (GI) concerns and remains essential to diagnosing certain GI diseases such as inflammatory bowel disease (IBD)[1,2]. Several factors play a role in an optimal colonoscopy, including but not limited to effective bowel preparation for complete visualization of the colonic mucosa[3]. Bowel preparation selection and administration in children can be challenging for a variety of reasons, such as a large volume of preparation to ingest, low tolerability of the preparation, or bothersome side effects[2]. The priority for pediatric bowel preparation should be safety and tolerability of the agent, with efficacy being an important consideration as well[2].
Existing clinical practice position on bowel preparation in children from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition suggests several single-agent best practice regimens for pediatric bowel preparation, including 1-d polyethylene glycol (PEG 3350); 2-d PEG 3350; nasogastric PEG-electrolyte; nasogastric sulfate-free PEG-electrolyte; and magnesium citrate + bisacodyl[4]. However, there is no preferred option, and some preparations are not approved by the FDA for use in children. Additionally, standardized protocols for bowel preparation are lacking, with significant variability in protocols between medical centers and individual practitioners, likely due to the lack of national standards for pediatric bowel preparations for colonoscopy[1,2,4].
Sodium picosulfate, magnesium oxide, and citric acid (SPMC) is a low-volume bowel preparation approved by the US Food and Drug Administration for cleansing of the colon prior to colonoscopy in adults and pediatric patients ages 9 years and older[5]. The objective of this study was to describe the efficacy, safety, and tolerability of SPMC bowel preparation in children. Oral PEG-based bowel preparation solution, per local standard of care, was included as a concurrent reference group.
MATERIALS AND METHODS
Study design
This was a phase 3, randomized, assessor-blinded, multicenter, dose-ranging study of low-volume SPMC (Ferring Pharmaceuticals Inc., Parsippany, NJ, United States) (ClinicalTrials.gov identifier: NCT01928862). The study was conducted at 9 sites in the United States, in accordance with the principles set forth in the Declaration of Helsinki and in compliance with ICH-GCP standards. The study protocol was approved by Institutional Review Boards for each study site (Supplementary Table 1).
Eligibility criteria
Eligible participants were males and females, aged 9 to 16 years, who were undergoing an elective colonoscopy. Females of childbearing potential must have had a negative pregnancy test at screening and randomization. Eligible participants must have had at least 3 spontaneous bowel movements per week for 1 mo prior to colonoscopy, and have been willing, able, and competent to complete the procedure and comply with instructions. Written informed consent (and assent, if applicable) was obtained at screening.
Exclusion criteria included acute surgical abdominal conditions (e.g., acute GI obstruction or perforation); hospitalization for IBD; any prior colorectal surgery (not including appendectomy, hemorrhoid surgery, or prior endoscopic surgery); colon disease (history of colonic cancer, toxic megacolon, toxic colitis, idiopathicpseudoobstruction, hypomotility syndrome, colon resection); ascites; GI disorder (active ulcer, outlet obstruction, retention, gastroparesis, ileus); upper GI surgery; significant cardiovascular disease; or a history of renal insufficiency with current serum creatinine or potassium levels outside of normal limits.
Use of certain medications was prohibited during the study: Lithium, laxatives (suspended 24 h prior to colonoscopy; not including laxatives as institutional standard of care for colonoscopy bowel preparation), constipating drugs (suspended 2 d prior), antidiarrheals (suspended 72 h prior), and oral iron preparations (suspended 1 wk prior).
Randomization
Participants were allocated to treatments according to computer-generated randomization codes that were generated by an independent statistician for all study sites. Participants 9-12 years old were randomized 1:1:1 to SPMC ½ dose × 2, SPMC 1 dose × 2, or PEG. Participants 13-16 years old were randomized 1:1 to SPMC 1 dose × 2 or PEG. Randomization numbers were allocated sequentially to participants at each study site, by the order of enrollment.
An unblinded study coordinator enrolled participants electronically, distributed the study drug, and instructed the participant and caregiver(s) about proper use of the study drug. The endoscopist, who performed the colonoscopy and assessed bowel preparation efficacy, and any assistant(s), were blinded to the participant’s treatment group.
Interventions
Participants and caregivers were instructed to prepare SPMC according to the package insert instructions, as described previously in the SEE CLEAR studies[6,7]. The preferred method was as a split dose, with the first dose administered the evening before (between 5:00p and 9:00p) and second dose administered the morning of colonoscopy (between 5 h and 9 h before the colonoscopy). The alternative dosing method was daybefore dosing, with the first dose administered the day before the colonoscopy during the afternoon or early evening, and the second dose administered 6 h later and before midnight. Oral PEG-based bowel preparation solutions were administered per local protocol/standard of care at each study site. The exact preparation administered was recorded by the unblinded study coordinator.
Endpoints
The primary efficacy endpoint was overall quality of colon cleansing by the modified Aronchick Scale (AS) prior to irrigation of the colon (Supplementary Table 2)[8]. The secondary efficacy endpoint was the participant’s tolerability and satisfaction, as measured by a 7-item questionnaire (a version of the Mayo Clinic Bowel Prep Tolerability Questionnaire[9]that was modified for pediatric use; Supplementary Table 3).
Safety assessments included adverse events (AEs), laboratory evaluations, and physical examination. Blood draws for laboratory evaluations were obtained at Screening (within 21 d before colonoscopy), on Day 0 (colonoscopy), and at Day 5 (follow-up). AEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) version 20.1.
Statistical analyses
A total of at least 45 participants were to be exposed to SPMC. In studies of SPMC for bowel preparation in adults, 81.7% to 87.7% had a successful colon cleansing[6,7,10]. The planned sample size would have provided an exact 95% confidence interval (CI) of 65% to 90% if 80% of the participants receiving SPMC were deemed to have successful colon cleansing.
The efficacy analyses were conducted on the intent-to-treat (ITT) analysis set, which included all participants who were randomized. All summaries for the ITT analysis set were made per the randomized treatment group. The primary efficacy endpoint was also summarized on the per-protocol (PP) analysis set by excluding participants who had major protocol deviations. Safety assessments were conducted on the safety analysis set, which included all participants who consumed at least 1 dose of study drug. All summaries for the safety analysis set were made according to actual treatment received.
The primary efficacy outcome (‘responders’) by AS was the proportion of participants receiving an ‘excellent’ or ‘good’ rating. The proportion of responders was summarized by treatment group within each age group, with a conventional twosided 95%CI as well as a 90%CI. Considering the small sample size, the 90% CI was intended as the more appropriate estimate to present, but the 95%CI was also calculated as it is more widely used. For the secondary efficacy endpoint, participants were considered to have a tolerable experience if they responded ‘never’ or ‘rarely’ to the relevant questions; likewise, they had a satisfactory experience if they responded ‘very easy/well’ or ‘easy/well’ on the relevant questions (Supplementary Table 3).
RESULTS
The trial was conducted between June 2014 (first participant enrolled) and March 2017 (last follow-up visit). The trial ended after the expected number of participants had enrolled and completed the trial. A total of 78 participants were randomized, with 48 aged 9-12 years, and 30 aged 13-16 years (Figure 1, Table 1). Of the 48 participants receiving SPMC (safety population), 46 (95.8%) completed both doses of the bowel preparation. Of the 30 participants randomized to PEG arm, 27 received a PEG-based bowel preparation and the remaining 3 received a non-PEG-based preparation (magnesium citrate). All 30 participants randomized to the PEG arm were included in the efficacy analysis set, however only the 27 patients actually ingesting PEG were included in the safety analysis set.
A medical history of diarrhea was reported by 27% (13/48) and 27% (8/30) of participants receiving SPMC (any dose) and PEG, respectively; likewise, constipation was reported by 19% (9/48) and 30% (9/30) of participants. In the SPMC treatment arms, split dosing was used for 13/48 (27.1%) participants, and day-before dosing for 35/48 (72.9%). Data on the PEG dosing regimen was available for 22/27 participants, all of whom used a day-before regimen.
For the primary efficacy endpoint, responders by AS, SPMC 1 dose × 2 showed consistent efficacy compared to PEG in both age groups (Figure 2). In the 9-12 years group, 87.5% (90%CI: 65.6%, 97.7%) were responders for SPMC 1 dose × 2 treatment arm, and 81.3% (90%CI: 58.3%, 94.7%) were responders for PEG treatment arm. In the 13-16 years group, 81.3% (90%CI: 58.3%, 94.7%) were responders for SPMC 1 dose × 2 treatment arm, and 85.7% (90%CI: 61.5%, 97.4%) were responders for PEG treatment arm. In the SPMC ½ dose × 2 arm (9-12 years only), 50.0% (90%CI: 27.9%, 72.1%) of participants were responders.
From the tolerability and satisfaction questionnaire, in both age groups, a greater number of participants receiving SPMC 1 dose × 2 found it ‘very easy’ or ‘easy’ to ingest than those receiving PEG (Figure 3). Likewise, fewer patients receiving SPMC 1dose × 2 reported abdominal discomfort happened ‘often’ or ‘very often’ compared to those receiving PEG (Figure 4). Feeling nausea ‘often’ or ‘very often’ during the bowel preparation was reported by 40% (12/30) of participants receiving PEG and by 18.6% (6/32) of participants receiving SPMC 1 dose × 2. A greater percentage of participants who received SPMC were ‘never’ or ‘rarely’ bothered about going to the bathroom compared to those receiving PEG (43.8%vs13.3%). No relevant differences were reported between PEG and SPMC for taste or how often the participant woke during the night.
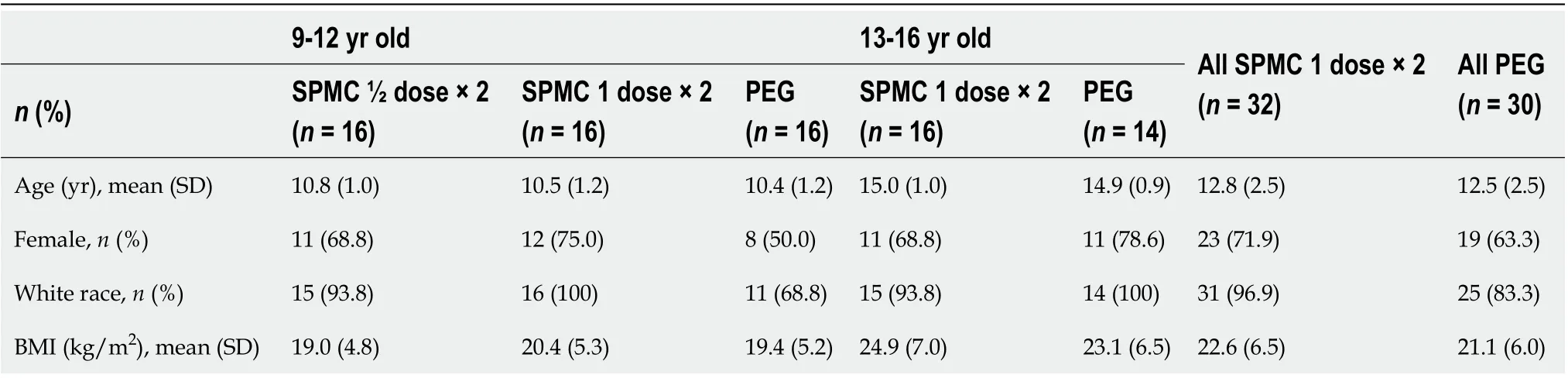
Table 1 Demographic and baseline characteristics, intent-to-treat population
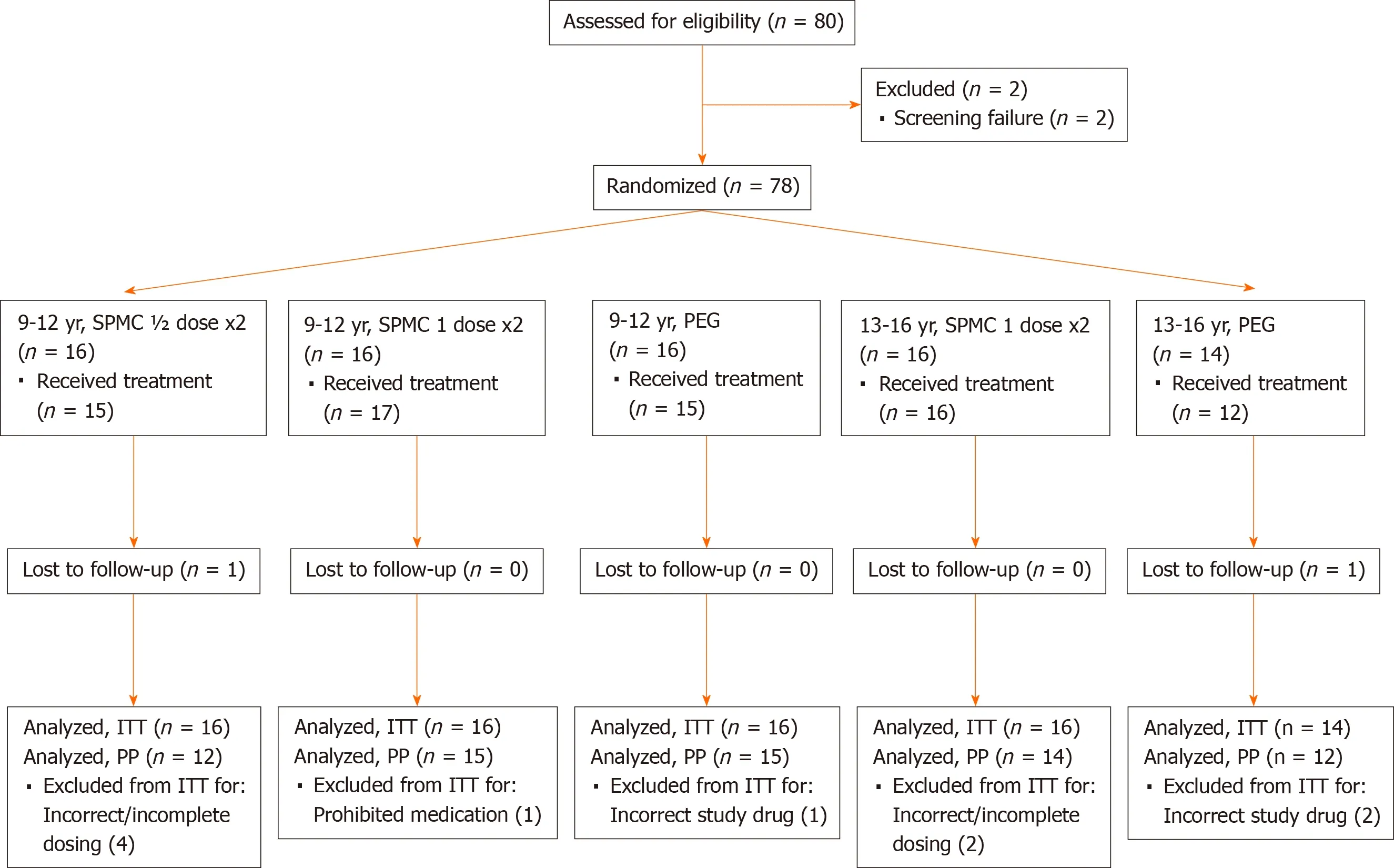
Figure 1 Consort diagram of study population. One participant in the SPMC ½ dose x2 group received SPMC 1 dose x2 treatment. ITT: Intent-to-treat; PEG: Polyethylene glycol; PP: Per protocol; SPMC; Sodium picosulfate, magnesium oxide, and citric acid.
Treatment-emergent AEs (TEAEs) were reported by 45.5% (15/33) of participants who received SPMC 1 dose x2 and 63.0% (17/27) of participants who received PEG (Table 2). One participant receiving SPMC 1 dose × 2 reported severe AEs: Abdominal pain (considered related to study drug, participant did not receive second dose, AE resolved), GI inflammation (Crohn’s disease, unrelated to study drug), and intestinal ulcer (unrelated to study drug).
Treatment-emergent adverse drug reactions (ADRs) were reported by 12.1% (4/33) of participants for SPMC and 18.5% (5/27) for PEG (Table 2). The most commonlyreported ADRs were vomiting (6.1%vs3.7%) and nausea (3.0%vs14.8%) for SPMC 1 dose × 2 and PEG groups, respectively.
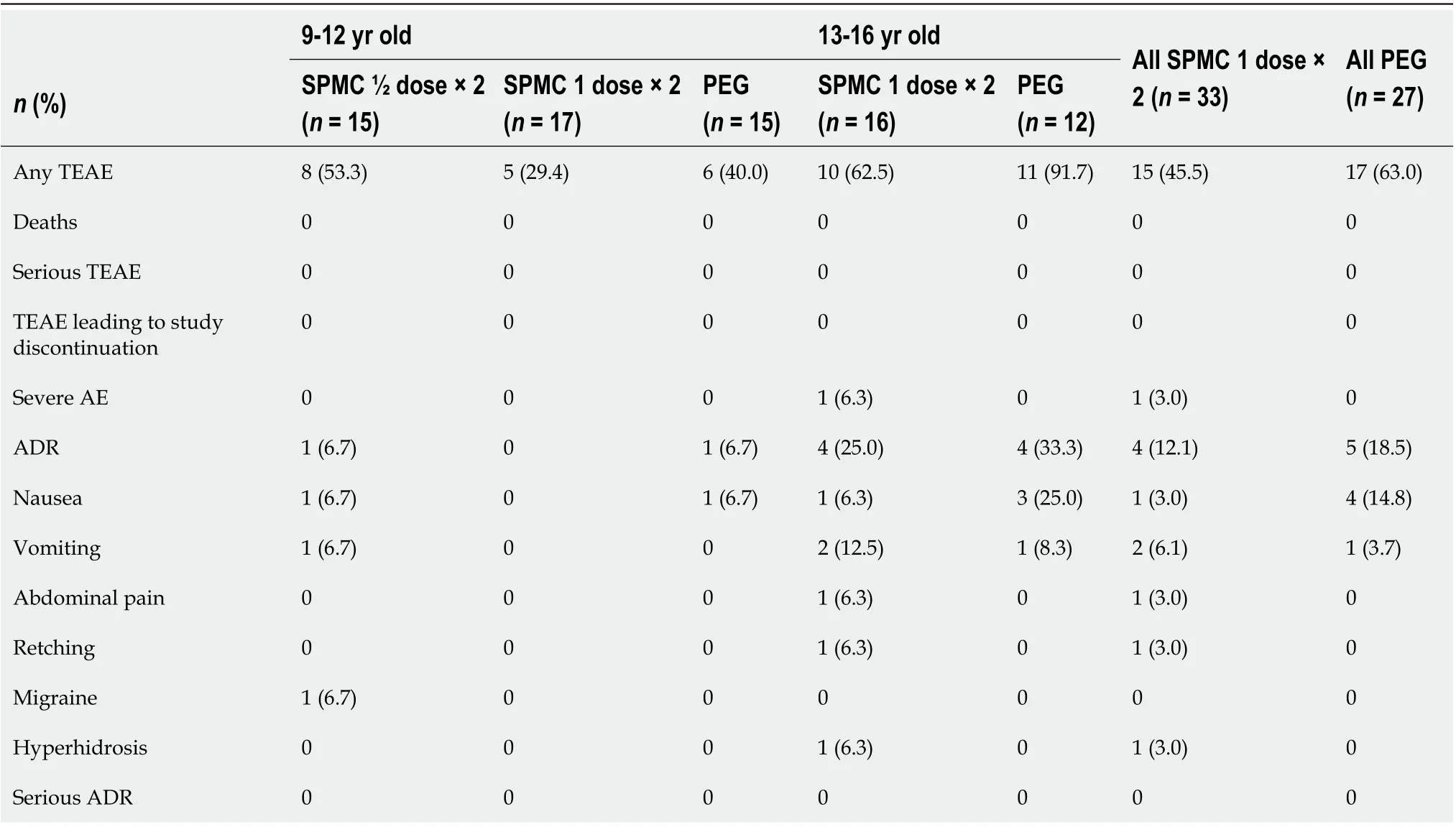
Table 2 Summary of adverse events, safety population
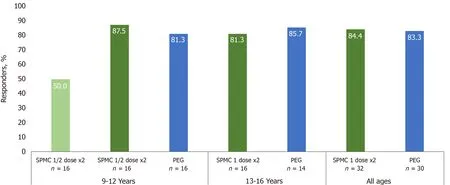
Figure 2 The majority of participants receiving sodium picosulfate, magnesium oxide, and citric acid 1 dose x2 in both age groups were responders for overall colon cleansing on the modified Aronchick scale (AS; ‘excellent’ or ‘good’), rated by a treatment-blinded endoscopist. The responder rates of SPMC 1 dose x2 group were similar to PEG group. SPMC; Sodium picosulfate, magnesium oxide, and citric acid; PEG: Polyethylene glycol.
Laboratory values and vital signs showed no meaningful changes associated with study drug administration. Three participants had abnormally low blood glucose (40-47 mg/dL) (2 in the SPMC 1 dose × 2 cohort; 1 in the PEG arm), which occurred on Day 0 for 1 participant receiving SPMC, and on Day 5 for 1 participant receiving SPMC and 1 receiving PEG; participants did not experience clinically-meaningful symptoms related to the hypoglycemia. Participants receiving SPMC 1 dose × 2 showed small and transient increases in magnesium, from a mean (SD) of 0.89 (0.07) mmol/L at baseline to 1.04 (0.14) mmol/L on Day 0, which returned to 0.94 (0.22) mmol/L on Day 5 (follow-up), with no clinically-meaningful symptoms.
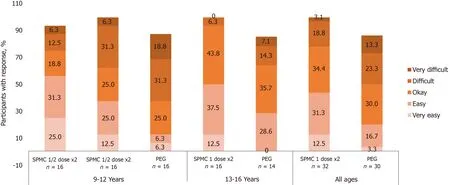
Figure 3 Participants were asked “How easy was it to drink the bowel cleanout regimen?”. Overall, 43.8% of participants receiving SPMC 1 dose x2 reported it was ‘very easy’ or ‘easy’ to drink, compared with 20.0% receiving PEG.
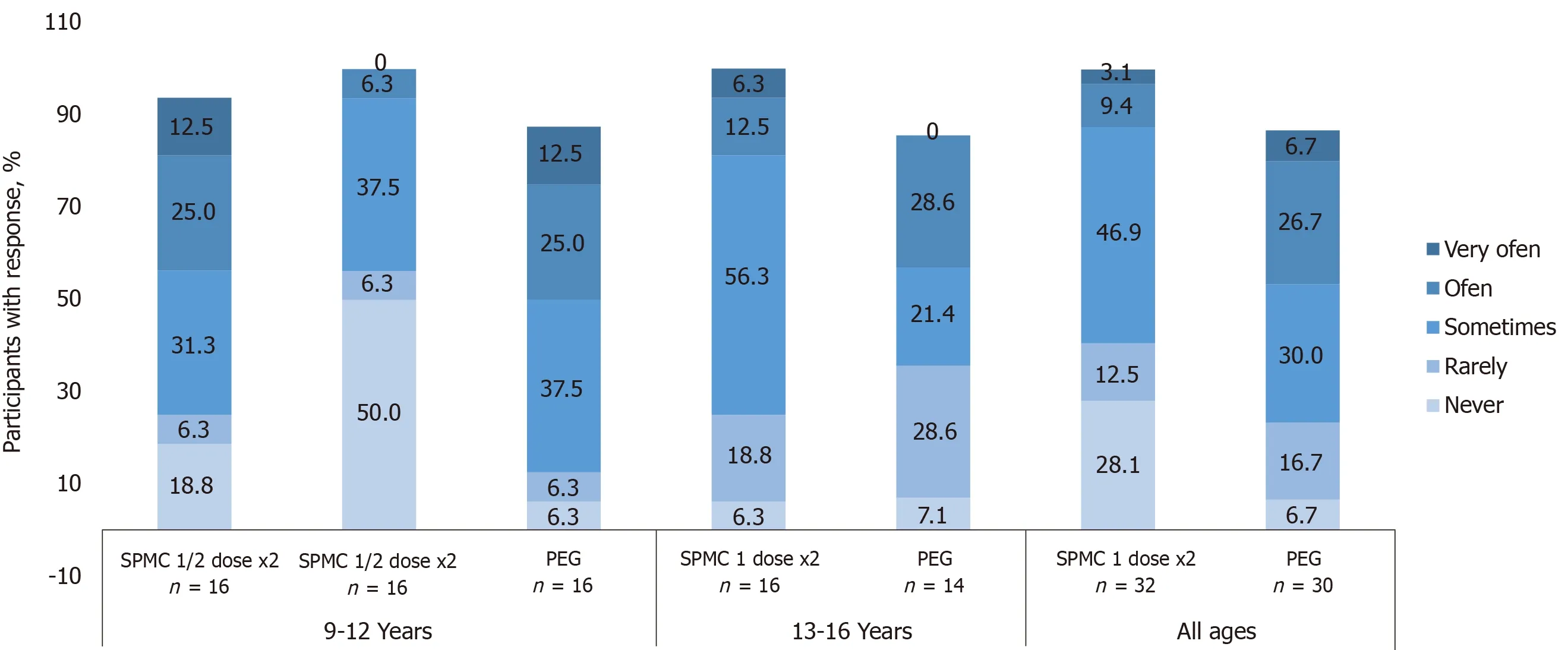
Figure 4 Participants were asked “How often did your tummy hurt since you started the cleanout?”. 28% of participants receiving sodium picosulfate, magnesium oxide, and citric acid (SPMC) 1 dose x2 reported ‘never’ hurting, compared with 6.7% receiving polyethylene glycol (PEG). Only 12.5% of those receiving SPMC 1 dose x2 reported abdominal discomfort ‘often’ or ‘very often’, whereas 33.4% receiving PEG did. Participants with no response are not shown on the graphs and, therefore, numbers may not add to 100%. SPMC: Sodium picosulfate, magnesium oxide, and citric acid; PEG: Polyethylene glycol.
DISCUSSION
SPMC was safe for bowel cleansing in children, with no reports of serious adverse events. Numerically, SPMC was associated with fewer reports of any treatmentemergent adverse event or adverse drug reaction compared to PEG, including a much lower rate of nausea (3.0%vs14.8%). Glucose and magnesium imbalances that were measured by laboratory assessments were transient, not clinically significant, and similar to those reported for adults receiving SPMC[5]. The finding of transient magnesium imbalance is not surprising given the presence of magnesium oxide in SPMC.
The tolerability for SPMC was higher compared to PEG, with more than double the proportion rating the bowel preparation as ‘very easy’ or ‘easy’ to ingest. In children, the tolerability and safety of bowel preparation carries equal or greater importance to the efficacy. Administering bowel preparations in children, and achieving compliance with administration, remains challenging. The tolerability of the pediatric standard of care for bowel preparation, PEG, is recognized to be less than optimal[5]. Here, SPMC was more tolerable than the standard of care for bowel preparation, and almost all participants receiving SPMC ingested both doses. One possible factor for the favorable tolerability for SPMC is the volume of bowel preparation ingested (active medication 5.4 oz per 1 dose, or 10.8 oz in total for both doses) relative to a typical volume of PEG for bowel preparation (approximately 64-72 oz for children 9-16 years)[4,11]. Participants receiving SPMC ingested additional liquid of their choice to complete the bowel preparation. The actual volume of PEG ingested by participants in this study was not available, which may be variable in the pediatric population. A randomized trial showed that split-dosing of PEG (vssingle dosing) led to a more tolerable bowel preparation experience in children[11].
SPMC was efficacious in children 9 to 16 years old, and comparable to the bowel cleansing efficacy of PEG. SPMC 1 dose × 2 displayed high and consistent efficacy across the two age groups, 9-12 years and 13-16 years. SPMC demonstrated a doseresponse relationship in the 9-12 years group, with SPMC ½ dose × 2 arm showing a 50% responder rate, while the SPMC 1 dose × 2 arm had an 87.5% responder rate.
This study adds new data to the sparse literature on bowel preparation in children. Very few studies have evaluated the use of SPMC for bowel preparation in the pediatric population, and not all commonly used bowel preparations are FDA approved for use in children[12-15]. The results of this study are consistent with earlier studies of sodium picosulfate/SPMC in children, which demonstrated good efficacy of colon cleansing and improved tolerability compared to bisacodyl or PEG[12-15,16].
Existing guidelines suggest PEG as the standard of care for bowel preparation in children, with the caveat that many of the studies used to support the suggestion implemented a 4-d bowel preparation regimen, and some added a stimulant to the preparation (e.g., bisacodyl)[17,18]. Realistically, feasibility of a 4-d preparation regimen becomes more cumbersome and inconvenient, with the potential to reduce cleansing efficacy as patients are more likely to be noncompliant for a 4-d regimen, when compared to a low-volume 2-d regimen[2,4]. Here, the SPMC protocol was a 2-d bowel preparation without the addition of a stimulant, which has been shown to improve patient satisfaction with other preparations. Guidelines also suggest that pediatric bowel preparation regimens should prioritize safety and tolerability and the SPMC protocol seems to achieve such[2].
CONCLUSION
As the tolerability was higher and the efficacy and safety were consistent with the standard of care for pediatric bowel preparation, SPMC 1 dose × 2 should be considered as a more feasible and easier-to-consume option compared to PEG for all bowel preparations in children 9 to 16 years old.
ARTICLE HIGHLIGHTS
Research results
A total of 78 participants were randomized, with 48 aged 9-12 years, and 30 aged 13-16 years. In the 9-12 years group, 87.5% (90%CI: 65.6%, 97.7%) were responders for SPMC 1 dose × 2 treatment arm, and 81.3% (90%CI: 58.3%, 94.7%) were responders for PEG treatment arm. In the 13-16 yr group, 81.3% (90% CI: 58.3%, 94.7%) were responders for SPMC 1 dose × 2 treatment arm, and 85.7% (90%CI: 61.5%, 97.4%) were responders for PEG treatment arm. In the SPMC ½ dose × 2 arm (9-12 years only), 50.0% (90%CI: 27.9%, 72.1%) of participants were responders. In both age groups, a greater number of participants receiving SPMC 1 dose × 2 found it ‘very easy’ or ‘easy’ to ingest than those receiving PEG. Treatment-emergent AEs were reported by 45.5% of participants receiving SPMC 1 dose x2 and 63.0% receiving PEG.
Research conclusions
Sodium picosulfate, magnesium oxide, and citric acid low volume bowel preparation had higher tolerability in children 9-16 years compared to polyethylene glycol-based preparations, potentially due to a lower volume of bowel preparation to ingest. SPMC bowel preparation efficacy and safety were comparable to PEG.
Research perspectives
As the tolerability was higher and the efficacy and safety were consistent with the standard of care for pediatric bowel preparation, SPMC 1 dose x2 should be considered as a more feasible and easier-to-consume option compared to PEG for all bowel preparations in children 9 to 16 years old.
ACKNOWLEDGEMENTS
The authors would like to thank the investigators, study staff, and participants who were involved in the trial.
 World Journal of Gastroenterology2020年40期
World Journal of Gastroenterology2020年40期
- World Journal of Gastroenterology的其它文章
- Cirrhotic portal hypertension: From pathophysiology to novel therapeutics
- New strain of Pediococcus pentosaceus alleviates ethanol-induced liver injury by modulating the gut microbiota and short-chain fatty acid metabolism
- Prediction of clinically actionable genetic alterations from colorectal cancer histopathology images using deep learning
- Compromised therapeutic value of pediatric liver transplantation in ethylmalonic encephalopathy: A case report
- Predicting cholecystocholedochal fistulas in patients with Mirizzi syndrome undergoing endoscopic retrograde cholangiopancreatography
- Novel endoscopic papillectomy for reducing postoperative adverse events (with videos)
