Overlooked risk for needle tract seeding following endoscopic ultrasound-guided minimally invasive tissue acquisition
Ruo-Yu Gao, Ben-Hua Wu, Xin-Ying Shen, Tie-Li Peng, De-Feng Li, Cheng Wei, Zhi-Chao Yu, Ming-Han Luo,Feng Xiong, Li-Sheng Wang, Jun Yao
Abstract Endoscopic ultrasound-guided minimally invasive tissue acquisition can be performed by two approaches as follows: Endoscopic ultrasound-guided fineneedle aspiration (EUS-FNA) and endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB). These have been evolved into leading approaches and widely used for the histological diagnosis of tumors in the gastrointestinal tract and adjacent organs. However, the role of EUS-FNA and EUS-FNB in disease diagnosis and evaluation remains controversial. Although the incidence of surgery-associated complications remains low, the consequences of needle tract seeding can be serious or even life-threatening. Recently, increasing case reports of needle tract seeding are emerging, especially caused by EUS-FNA. This complication needs serious consideration. In the present work, we integrated these case reports and the related literature, and summarized the relevant cases and technical characteristics of needle tract seeding caused by EUS-FNA and EUSFNB. Collectively, our findings provided valuable insights into the prevention and reduction of such serious complication.
Key Words: Endoscopic ultrasound-guided fine-needle aspiration; Endoscopic ultrasoundguided fine-needle biopsy; Needle tract seeding; Gastrointestinal tract; Computed tomography
INTRODUCTION
Endoscopic ultrasound-guided minimally invasive tissue acquisition can be mainly performed by two approaches as follows: Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and endoscopic ultrasound-guided fine-needle biopsy (EUSFNB). Such procedures are safe and accurate to acquire tissue in the pancreas, abdomen, subepithelial masses, pelvis, and lymphoma. In EUS-FNA, the puncture needle is penetrated into the target lesion through the digestive tract under the monitoring of endoscopic ultrasound probe to obtain cell or tissue materials in order to determine the nature, origin, and pathological features of the lesion. Since its first introduction in the 1990s, EUS-FNA has become the standard sampling approach for suspected intra-abdominal and intrathoracic lesions (various masses and lymph node tissues) due to its high accuracy[1]and low complication rate[2], which can further provide staging and diagnostic information[3-6]. It has been reported that the incidence of post-EUS-FNA surgical complications, such as infection, acute pancreatitis, bleeding, and duodenal perforation, ranges from 0.98% to 3.4%[7-9]. However, a technology-related limitation of FNA is the scant cellularity and lack of histological structure provided in samples, leading to the difficulty of diagnosis[10,11]. To overcome these shortcomings, EUS-FNB has been developed. It uses a new type of needle with micro-core acquisition technology, which can obtain histological core samples and cytological aspirates at the same time, achieving a diagnostic sensitivity and specificity > 90%[12-15]. Studies have shown that the number of passes, diagnostic accuracy, and histological yield of EUS-FNB are better compared with those of EUS-FNA[16,17]. Both FNA and FNB are considered relatively safe in most cases, and EUS uses a shorter needle track, which may lead to a lower possibility of needle tract seeding[7,18]. The estimated prevalence of needle tract seeding of FNA is 0.003%-0.009%[2]. Recently, increasing case reports of needle tract seeding following EUS-FNA or EUS-FNB are emerging, especially in the diagnosis of pancreatic cancer[19,20]. In the present work, we integrated these case reports and the related literature, and summarized the relevant cases and technical characteristics of needle tract seeding. Collectively, our findings provided valuable insights into the prevention and reduction of such serious complication.
METHODS
PubMed, MEDLINE ,and Cochrane Library were searched to identify articles published between June 1996 and January 2020 using the search items as follows: “Endoscopic ultrasound (EUS)”, “fine needle aspiration (FNA)”, “fine needle biopsy (FNB)”, “needle tract seeding”, and “seeding”. A total of 140 potentially relevant articles were identified from our initial search, 94 of which were excluded after reviewing the abstracts and titles. Finally, 46 articles focusing on EUS-FNA or EUSFNB related complications met our inclusion criteria, including 34 case reports about needle tract seeding following EUS-FNA or EUS-FNB.
EUS-FNA- OR EUS-FNB-INDUCED NEEDLE TRACT SEEDING
According to related reports, the incidence of tumor seeding ranges from 0.005% to 0.009% in percutaneous FNA of gastrointestinal tract (GI) and adjacent organic lesions guided by external ultrasound or computed tomography (CT)[21]. Furthermore, a previously published retrospective study consisting of 73 patients submitted to pancreatic mass FNA has reported that the incidence of needle tract seeding is as high as 1.4%[22]. In recent years, studies have shown that the total complication rate of EUSFNA varies from 0.5% to 3%[13,23,24]. One of the more serious complications following EUS-FNA is tumor seeding, which can lead to the development of peritoneal carcinomatosis and recurrence along the needle tract. Due to the shorter puncture path under the guidance of EUS, the incidence of tumor seeding is considered to be much lower compared with that of the percutaneous FNA[18]. In previous case reports[25-28], the location of the recurrent tumor appearing in the gastric wall is very close to the previous FNA puncture site, supporting the hypothesis that the tumor may spread along the needle track following EUS-FNA. As for EUS-FNB, previous studies have reported that it has a comparable adverse event rate (varied between 0-7.8%) to EUSFNA[29-33], and the recurrence due to needle tract seeding after EUS-FNB is also considered a rare event. Recently, Kawabataet al[34]have reported a case of tumor seeding caused by EUS-FNB.
On the contrary, there are some reports revealing that preoperative EUS-FNA for patients with pancreatic cancer does not affect the postoperative survival, needle tract seeding, or peritoneal recurrence[35-39]. For example, Yaneet al[40]have assessed whether preoperative EUS-FNA in patients with pancreatic cancer increases the risk of stomach or peritoneal recurrence, and whether it affects the patient’s recurrence-free survival and overall survival in a retrospective study. In this retrospective study, there were 301 patients undergoing distal pancreatectomy due to invasive pancreatic cancer from January 2006 to December 2015. A total of 176 patients received preoperative EUSFNA, and 34 patients had a peritoneal recurrence (including 18 patients in the non-EUS-FNA group and 16 patients in the EUS-FNA group). Multivariate analysis did not find a significant correlation between the preoperative EUS-FNA and recurrence of stomach or peritoneal tumors[40]. To date, similar results have been obtained in the other eight retrospective studies[8,35,36,38,39,41-43]. In these studies, preoperative EUS-FNA has no negative impact on the survival of patients with pancreatic cancer and does not lead to tumor seeding through the needle tract.
However, it has been suggested that the risk of needle tract seeding following EUSFNA or EUS-FNB is underestimated. First, most studies are single-center studies with a relatively small number of patients. Therefore, it is impossible to accurately estimate the incidence of needle tract seeding. Second, it is generally believed that it is difficult to diagnose low-volume gastric or peritoneal metastases clinically, because not all patients undergo the same strict diagnostic follow-up after surgery. Third, the followup period is relatively short, and thus the patients with unresectable tumors die before the clinical evidence of tumor spreading. Fourth, a preoperative biopsy may lead to the deposition of cancer cells outside the surgical resection area, which mistakenly classifies the cancer cell growth as a tumor recurrence or incomplete resection. In summary, these characteristics may lead to a significant reduction in the prediction of the number and location of cancer recurrence.
In a prospective study[44], 140 patients underwent lumen fluid aspiration before and after FNA through the suction channel and underwent cytological analysis during EUS. The cytological examination of intraluminal fluid showed that the positive rate of malignant tumors in patients with intraluminal tumor was 48%. This is a normal phenomenon, because cancer cells in the GI may shed. However, what is puzzling is that three (11.5%) patients tested positive for post EUS-FNA luminal fluid cytology in 26 patients with pancreatic cancer (extraluminal cancer). Normally, these three cases of extraluminal cancer should not be positive in cytological examination of intraluminal fluid, because there is no reasonable explanation for the metastatic pathway that allows extraluminal cancer cells into intestinal cavity. Therefore, the post-FNA positive luminal fluid cytology may be related to FNA.
At present, EUS-FNA is performed on intraabdominal and intrathoracic tumors, and surgical resection of these lesions usually does not remove the needle tract site, which may lead to a rare phenomenon of tumor seeding after operation. The incidence of such seeding is significantly low, and published data are only found in case reports and literature reviews. Due to the deficiency of data, it is not possible to determine whether the tumor seeding is caused by the malignant potential of these tumors or a technical defect. In the future, we need to conduct multi-center, large-scale, prospective studies to fully determine the clinical characteristics of needle tract seeding following EUS-FNA and EUS-FNB.
SUMMARY OF CASES ON NEEDLE TRACT SEEDING
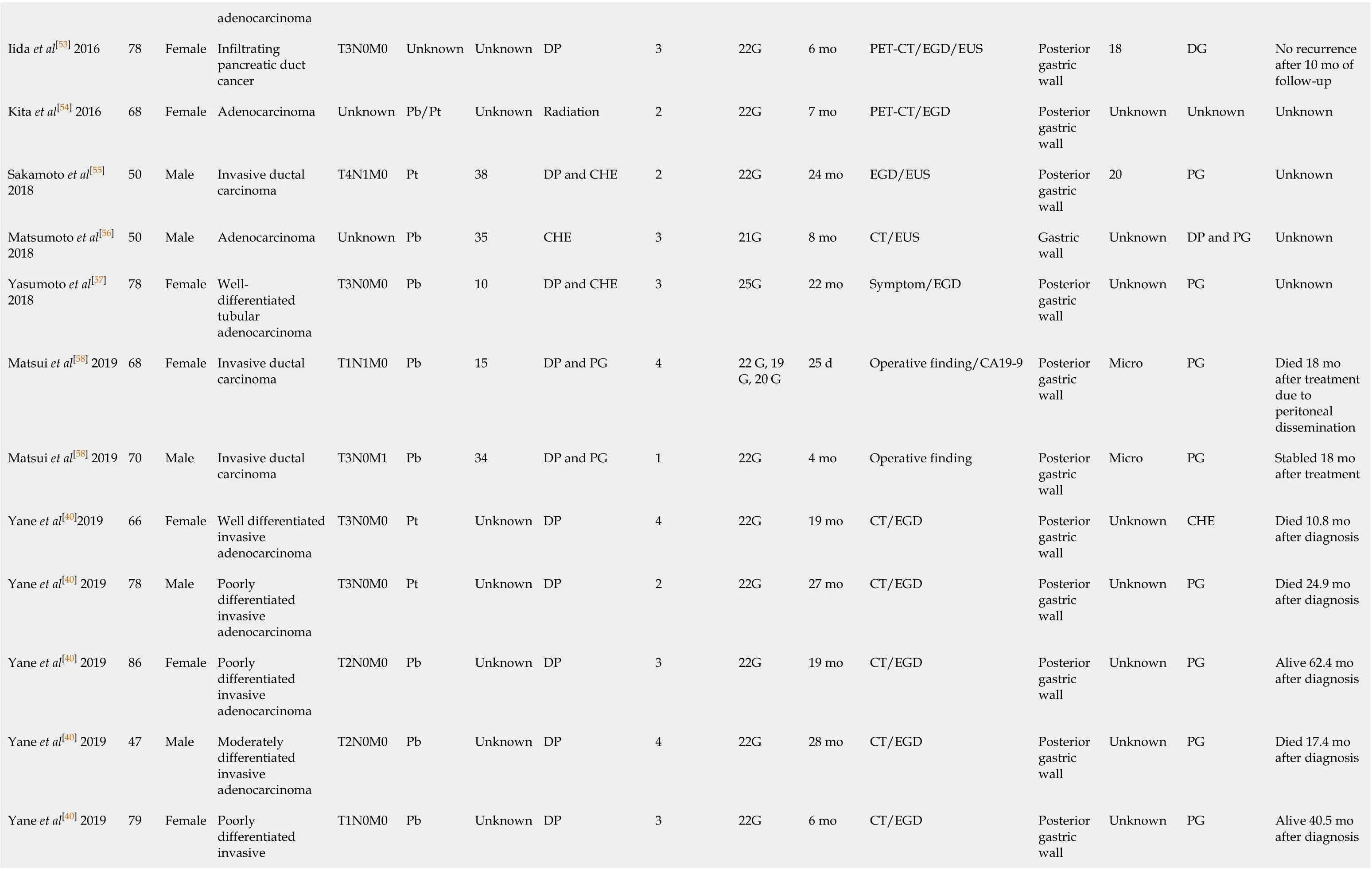
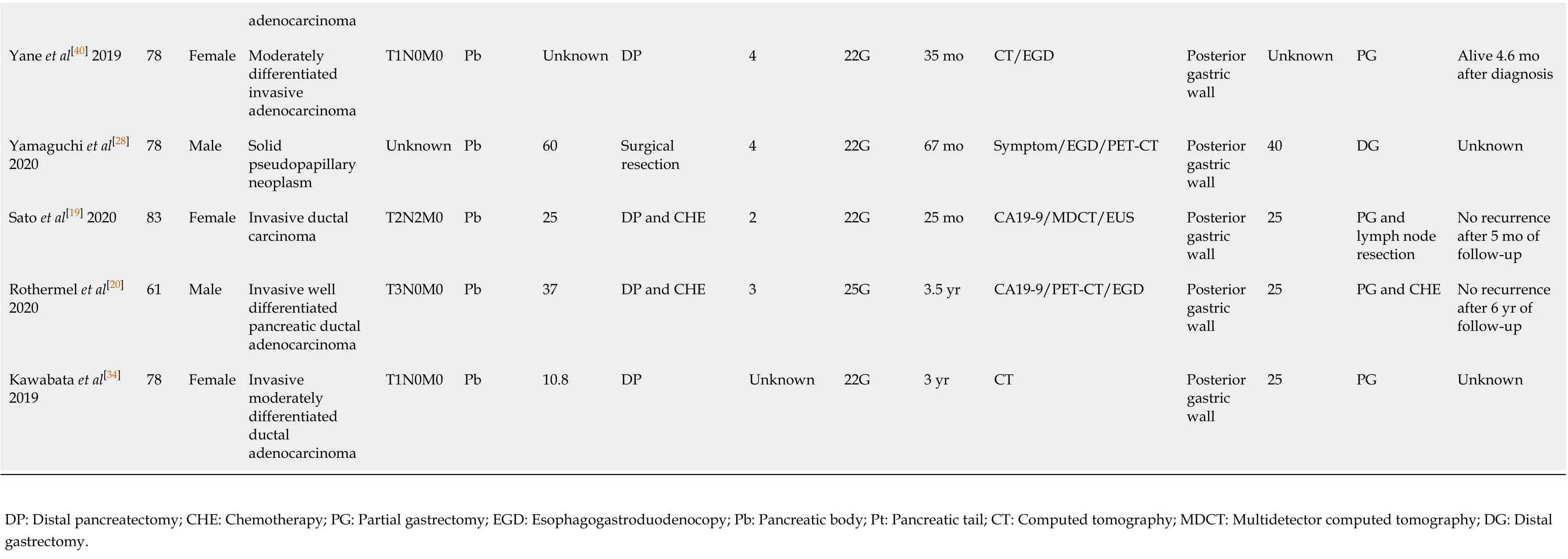
Since the first described case of EUS-FNA-induced needle tract seeding in a patient with intraductal papillary mucinous neoplasm in 2003[45], 33 patients with needle tract seeding following EUS-FNA or EUS-FNB have been reported up to January 2020. Of these cases, 27 were found in pancreatic cancer patients after EUS-FNA[19,20,25,27,28,36,40,46-58], one in a pancreatic cancer patient after EUS-FNB[34](Table 1), and five in patients with other intra-abdominal and intrathoracic tumors after EUS-FNA[59-63](Table 2). A total of 29 cases with pancreatic body or pancreatic tail cancer had needle tract seeding caused by diagnostic FNA or FNB. Tumor seeding following FNA or FNB has not been reported in pancreatic head mass. A possible reason is that patients with pancreatic head lesions need to remove both the primary foci and pancreatico-duodenum (including the needle tract). Therefore, seeding is unlikely to be successful. In contrast, FNA or FNB is usually performed for pancreatic body/tail tumors through the transgastric approach without removing the needle tract during pancreatectomy. Therefore, if the surgical procedure fails to remove the needle tract, we should seriously consider the possibility of tumor seeding following FNA or FNB of resectable thoracoabdominal tumors.
This review included 33 patients (16 males, 17 females; mean age 68.2 ± 12.2 years). Regarding the seeding site, 30 cases (90.9%) in the gastric wall, two in the esophageal wall, and one in the gastroesophageal junction were found to have tumor implantation. Only 18 cases have reported the size of the seeding tumor, with a median of 25 mm (range, 4-50 mm). Most seeding tumors were mainly located in the submucosal muscle layer or serous membrane layer of the GI, and thus they mainly appeared as submucosal tumor-like masses.
In terms of the FNA/FNB procedure, 25 (75.8%) cases used 22-G puncture needle during the execution of surgery, and the puncture process in two cases were not described. In most cases, a syringe was used for vacuum suction during the puncture process. The average number of needle pass was 2.8 ± 1.0.
As for the treatment of EUS-FNA or FNB-related needle tract seeding, chemotherapy has been performed in four cases, and the patients died at 10.8 mo, 12 mo, 26 mo, and 2 years, respectively, after chemotherapy. Surgical resection was selected in 21 cases, of which eight were followed for an average of 23.2 mo without tumor recurrence, three died about 20 mo after surgery, and detailed results and follow-up information are not obtained from the other ten cases undergoing surgical resection. In one case of tumor seeding in the esophageal wall, the lesion disappeared at 2 mo after radiation therapy[60]. In one case receiving surgery and chemotherapy at the same time, no tumor recurred after 6 years of follow-up[20]. There are no reports for the treatment methods for the remaining six cases. Additionally, Iidaet al[26]have reported that a patient with pancreatic cancer had an EUS-FNA-related tumor seeding on the lower posterior gastric wall. At 21 mo after distal gastrectomy, a recurrent lesion was found on the upper posterior gastric wall. The author has suggested that the patient should undergo total gastrectomy rather than simple surgical resection[26]. Based on the above-mentioned results, the long-term prognosis of simple surgical resection of seeding tumors remains unclear. It is possible that radical surgery in combination with chemotherapy can improve the prognosis.
In these 33 case reports, the discovering process of seeding tumors also greatly varies. A total of 13 (39.3%) cases had abnormalities detected during regular CT/positron emission tomography-computed tomography (PET-CT) examinations, seven (21.2%) had clinical symptoms and received further examination, four (12.1%) showed a submucosal mass by regular esophagogastroduodenoscopy examination, six (18.2%) were found to have elevated CA19-9 levels and underwent further examination, and three (9.1%) were accidentally discovered during the operation. In addition, the interval time from EUS-FNA or EUS-FNB to detection of needle tract seeding greatly varies, with a median interval of 22 mo (range, 1-67 mo).
Since most seeding tumors are located in the gastric submucosal layer, it may be difficult to detect these early lesions by gastroscopy unless they are large enough to form a raised mass that can be visualizedviagastroscopy. Paquinet al[46]have reportedthat a 3 cm mass was found on the stomach wall by EUS. However, endoscopy revealed a normal gastric mucosa without ulcers or other abnormalities[46]. If tumor seeding is suspected in the GI, EUS may be useful in the early detection of these lesions, while the predicted probability may be too low to be cost-effective in most cases. Since EUS-FNA or EUS-FNB is more commonly used in the diagnosis of pancreatic diseases, most cases of tumor seeding are related to pancreatic tumors. Early detection is significantly crucial for needle tract seeding by surgical procedures. Of the 28 pancreatic cancer patients included in this review, 23 (82.2%) had no clinical symptoms at the time of recurrence. On the contrary, ten of these patients underwent CA19-9 testing, and eight (80.0%) exhibited increased CA19-9 levels. Therefore, during postoperative follow-up, CA19-9 may be helpful for the early detection of seeding recurrence. In addition, research shows that changes in the levels of CA19-9 are frequently more than 6 mo earlier than radiological recurrence in patients with pancreatic cancer[64]. In addition, PET-CT represents a more sensitive approach compared with the traditional imaging methods (CT and magnetic resonance imaging [MRI]), and it is also used for the early detection of tumor recurrence after pancreatic cancer surgery[65,66]. Six (100%) cases receiving PET showed that the seeding tumors increased the uptake of fluorodeoxyglucose. However, PET/CT examinations areusually just to clarify the unclear manifestations of CT and MRI.
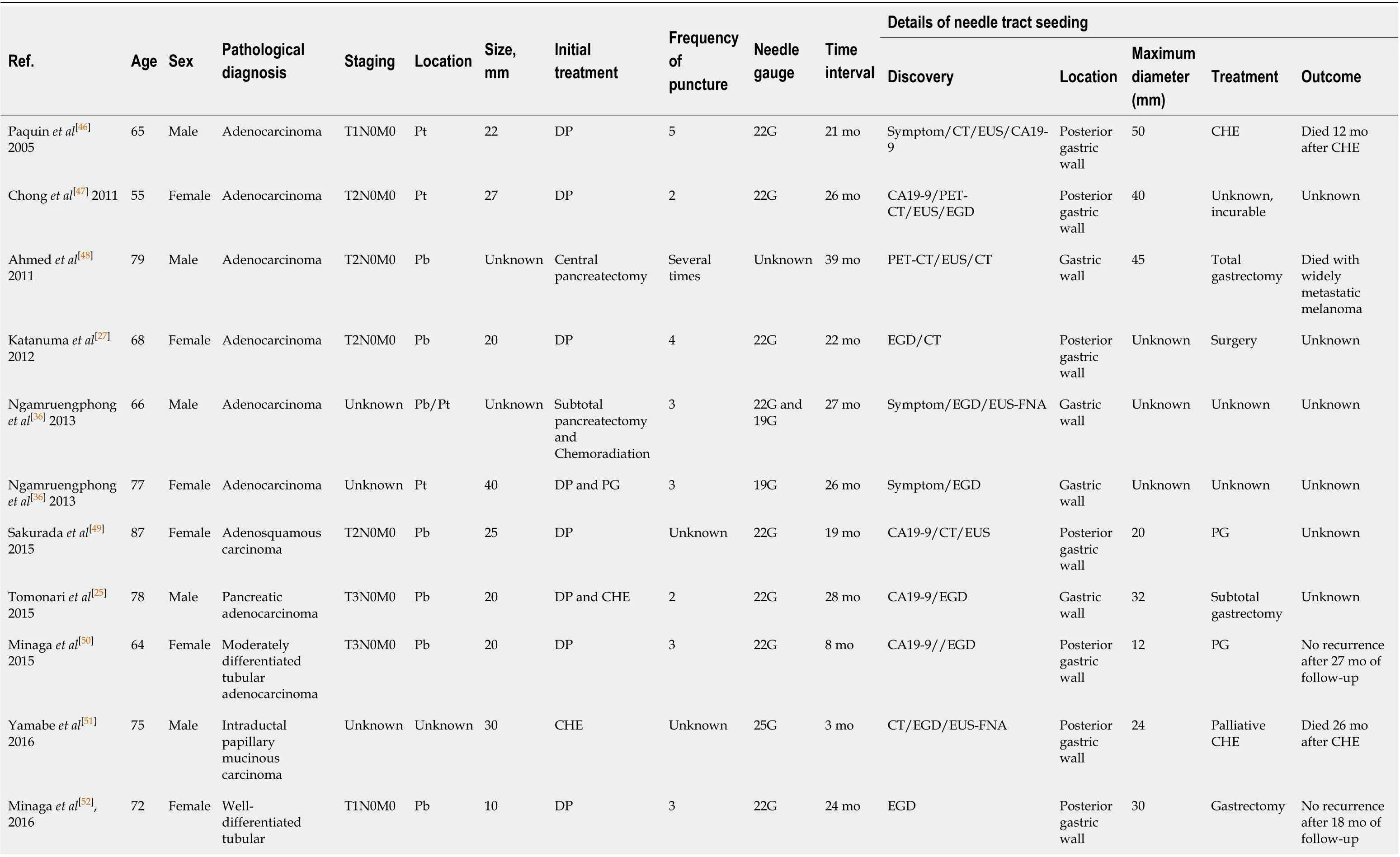
Table 1 Characteristics of reported needle tract seeding of pancreatic cancer
adenocarcinoma Iida et al[53] 2016 78 Female Infiltrating pancreatic duct cancer T3N0M0 Unknown Unknown DP 3 22G 6 mo PET-CT/EGD/EUS Posterior gastric wall 18 DG No recurrence after 10 mo of follow-up Kita et al[54] 2016 68 Female Adenocarcinoma Unknown Pb/Pt Unknown Radiation 2 22G 7 mo PET-CT/EGD Posterior gastric wall Unknown Unknown Unknown Sakamoto et al[55] 2018 50 Male Invasive ductal carcinoma T4N1M0 Pt 38 DP and CHE 2 22G 24 mo EGD/EUS Posterior gastric wall 20 PG Unknown Matsumoto et al[56] 2018 50 Male Adenocarcinoma Unknown Pb 35 CHE 3 21G 8 mo CT/EUS Gastric wall Unknown DP and PG Unknown Yasumoto et al[57] 2018 78 Female Welldifferentiated tubular adenocarcinoma T3N0M0 Pb 10 DP and CHE 3 25G 22 mo Symptom/EGD Posterior gastric wall Unknown PG Unknown Matsui et al[58] 2019 68 Female Invasive ductal carcinoma T1N1M0 Pb 15 DP and PG 4 22 G, 19 G, 20 G 25 d Operative finding/CA19-9 Posterior gastric wall Micro PG Died 18 mo after treatment due to peritoneal dissemination Matsui et al[58] 2019 70 Male Invasive ductal carcinoma T3N0M1 Pb 34 DP and PG 1 22G 4 mo Operative finding Posterior gastric wall Micro PG Stabled 18 mo after treatment Yane et al[40]2019 66 Female Well differentiated invasive adenocarcinoma T3N0M0 Pt Unknown DP 4 22G 19 mo CT/EGD Posterior gastric wall Unknown CHE Died 10.8 mo after diagnosis Yane et al[40] 2019 78 Male Poorly differentiated invasive adenocarcinoma T3N0M0 Pt Unknown DP 2 22G 27 mo CT/EGD Posterior gastric wall Unknown PG Died 24.9 mo after diagnosis Yane et al[40] 2019 86 Female Poorly differentiated invasive adenocarcinoma T2N0M0 Pb Unknown DP 3 22G 19 mo CT/EGD Posterior gastric wall Unknown PG Alive 62.4 mo after diagnosis Yane et al[40] 2019 47 Male Moderately differentiated invasive adenocarcinoma T2N0M0 Pb Unknown DP 4 22G 28 mo CT/EGD Posterior gastric wall Unknown PG Died 17.4 mo after diagnosis Poorly differentiated invasive Yane et al[40] 2019 79 Female T1N0M0 Pb Unknown DP 3 22G 6 mo CT/EGD Posterior gastric wall Unknown PG Alive 40.5 mo after diagnosis
adenocarcinoma Yane et al[40] 2019 78 Female Moderately differentiated invasive adenocarcinoma T1N0M0 Pb Unknown DP 4 22G 35 mo CT/EGD Posterior gastric wall Unknown PG Alive 4.6 mo after diagnosis Yamaguchi et al[28] 2020 78 Male Solid pseudopapillary neoplasm Unknown Pb 60 Surgical resection 4 22G 67 mo Symptom/EGD/PET-CT Posterior gastric wall 40 DG Unknown Sato et al[19] 2020 83 Female Invasive ductal carcinoma T2N2M0 Pb 25 DP and CHE 2 22G 25 mo CA19-9/MDCT/EUS Posterior gastric wall 25 PG and lymph node resection No recurrence after 5 mo of follow-up Rothermel et al[20] 2020 61 Male Invasive well differentiated pancreatic ductal adenocarcinoma T3N0M0 Pb 37 DP and CHE 3 25G 3.5 yr CA19-9/PET-CT/EGD Posterior gastric wall 25 PG and CHE No recurrence after 6 yr of follow-up Kawabata et al[34] 2019 78 Female Invasive moderately differentiated ductal adenocarcinoma T1N0M0 Pb 10.8 DP Unknown 22G 3 yr CT Posterior gastric wall 25 PG Unknown DP: Distal pancreatectomy; CHE: Chemotherapy; PG: Partial gastrectomy; EGD: Esophagogastroduodenocopy; Pb: Pancreatic body; Pt: Pancreatic tail; CT: Computed tomography; MDCT: Multidetector computed tomography; DG: Distal gastrectomy.
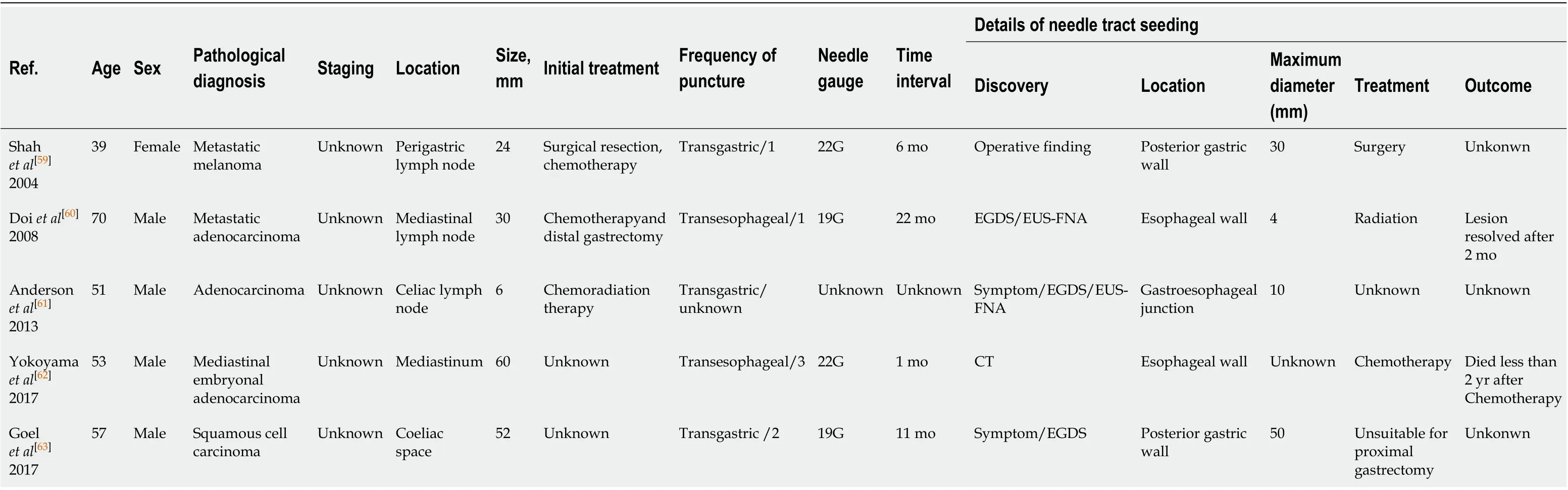
Table 2 Characteristics of reported needle tract seeding of other tumors
According to this review, the best treatment method and detection strategy for needle tract seeding have not yet been clarified at present. Among these cases, some patients achieved a good prognosis after surgery[50,52]. However, some reports have shown that the delayed discovery of needle tract seeding has caused metastases in both the stomach wall and lymph nodes[48]. In addition, it has been reported that rerecurrence occurred after partial gastrectomy due to gastric wall metastasis caused by needle tract seeding[26]. Therefore, early diagnosis and surgical resection of these lesions are an effective method for patients with needle tract seeding after EUS-FNA or EUS-FNB to achieve a long-term survival. Moreover, the examination of CA19-9, endoscopy, and imaging modalities (especially PET-CT) may be of great significance for the early detection of tumor recurrence along needle tract.
RISK FACTORS AND PREVENTION STRATEGIES FOR NEEDLE TRACT SEEDING
It has been previously thought that several factors, such as the number of punctures, needle size, needle movement, and tumor characteristics (cystic tumors or poorly differentiated tumors) may affect the development of seeding[50,60,62]. However, due to the small number of reported cases of tumor seeding, it remains unknown whether tumor factors or FNA/FNB procedures are significantly correlated with the occurrence of needle tract seeding. Sakamotoet al[55]have conducted an experiment using an agar model, and they considered that the slow-pull technique and the use of puncture needle with a side hole may result in needle tract seeding. Although these results may not reflect the actual clinical situations, it also gives us a warning.
As a rare but serious complication, needle tract seeding following EUS-FNA or EUSFNB may worsen the prognosis of patients. Therefore, several strategies have been proposed to reduce the tumor seeding. Yaneet al[40]have reported that EUS-FNA should only be used in patients requiring a pathological diagnosis to develop more accurate treatment strategies (for example, patients with pancreatic cancer who are scheduled to undergo preoperative neoadjuvant therapy or difficult to diagnose by imaging). In addition, the distance between the endoscope and the target site should be as short as possible[67]. Tomonariet al[25]has suggested that if the surgical resection does not include puncture needle tract or puncture results cannot change treatment options, EUS-FNA should be avoided or the number of puncture should be limited[25,67]. Therefore, we can consider replacing EUS-FNA with EUS-FNB to diagnose suspected intra-abdominal and intrathoracic lesions. Because some studies have shown that the FNB needle can produce more accurate diagnoses, a better histological yield, and a lower number of passes compared with the FNA needle[17,68]. This is probably a change which will impact also the already low rate of seeding. Moreover, only one case of needle tract seeding following FNB has been reported so far. Tyagi and Dey believed that a puncture needle with a covering sleeve should be used to avoid the needle tract seeding[69].
As the needle site is not within the scope of surgical resection, we should carefully consider the risk-benefit ratio of EUS-FNA or EUS-FNB. If we perform puncture and early radical resection in this situation, regular detection of blood tumor markers, imaging, and endoscopy are essential. Moreover, it has been suggested that neoadjuvant chemotherapy can provide survival benefits for patients with resectable pancreatic cancer[70].
CONCLUSION
More than 25 years after its introduction, endoscopic ultrasound-guided minimally invasive tissue acquisition has replaced percutaneous FNA guided by external ultrasound or CT. Due to its higher effectiveness and lower complications, it has achieved a crucial role in the diagnosis of GI and adjacent organic lesions. Although needle tract seeding following EUS-FNA or EUS-FNB is a rare and easily ignored complication, it can bring serious consequences to patients. Endosonographers should determine the indications for surgery according to the characteristics of different cases. If the location of the needle tract is not within the scope of surgical resection, we should be aware of the risk of tumor metastasis along the needle tract following EUSFNA or EUS-FNB. We should also pay attention to several aspects, such as shortening the puncture path, limiting the number of puncture, paying attention to the procedure method, and adding seeding needle sleeve. For example, EUS-FNB has greatly improved the diagnostic efficacy of EUS guided tissue acquisition with fewer passes so that we may consider replacing EUS-FNA with EUS-FNB to diagnose suspected intraabdominal and intrathoracic lesions. In addition, regular detection of blood tumor markers, imaging, and endoscopy are required to diagnose tumor seeding at an early stage. Furthermore, it is necessary to accumulate more cases of needle tract seeding and conduct a large-scale prospective cohort study to confirm its detailed clinical characteristics in order to actively prevent or early detect the risk of needle tract seeding.
 World Journal of Gastroenterology2020年40期
World Journal of Gastroenterology2020年40期
- World Journal of Gastroenterology的其它文章
- Cirrhotic portal hypertension: From pathophysiology to novel therapeutics
- New strain of Pediococcus pentosaceus alleviates ethanol-induced liver injury by modulating the gut microbiota and short-chain fatty acid metabolism
- Prediction of clinically actionable genetic alterations from colorectal cancer histopathology images using deep learning
- Compromised therapeutic value of pediatric liver transplantation in ethylmalonic encephalopathy: A case report
- Predicting cholecystocholedochal fistulas in patients with Mirizzi syndrome undergoing endoscopic retrograde cholangiopancreatography
- Novel endoscopic papillectomy for reducing postoperative adverse events (with videos)
