252例心力衰竭住院患者沙库巴曲缬沙坦的用药分析
孙莹 顾永丽 孙增先

摘 要 目的:分析心力衰竭住院患者沙库巴曲缬沙坦的用药情况。方法:收集我院2019年10月-2020年3月使用沙库巴曲缬沙坦的住院心力衰竭患者的资料,包括其基本信息,如性别、年龄、住院科室、住院时间等;汇总其沙库巴曲缬沙坦使用情况(包括适应证、禁忌证、用法用量、用药疗程)、与血管紧张素转化酶抑制剂(ACEI)/血管紧张素Ⅱ受体拮抗剂(ARB)类药物的转换、药物不良反应等信息。结果:共收集到252例患者的资料,其中男性172例(68.25%)、女性80例(31.75%);平均年龄为(67.02±14.23)岁,有85例(33.73%)的年龄≥75岁;平均住院时间为(12.03±8.19)d,用药前平均左室射血分数(LVEF)为(38.69±10.45)%,平均血钾水平为(4.16±0.65)mmol/L,平均肾小球滤过率估计值(eGFR)为(69.14±32.01)mL/(min·1.73 m2)。分布科室以心内科(59.14%)为主,其次为肾内科(8.73%)、呼吸科(7.14%)、心外科(5.95%)、老年科(5.56%)、急诊内科(3.57%)和神经内科(3.17%)。所有患者均有用药适应证,但有25例(9.92%)存在用药禁忌证,其中6例(2.38%)为血钾>5.4 mmol/L、19例(7.54%)为eGFR<15 mL/(min·1.73 m2)。用法用量以50 mg,bid为主(45.24%);39例(15.47%)给药频次为qd,为不合理给药;平均疗程为(7.80±5.86)d。有7例患者(2.78%)与ACEI转换,其中3例患者(42.86%)的转换间隔时间未达36 h;20例患者(7.93%)与ARB转换,均无明显转换不适宜情况。14例患者(5.56%)出现低血压,其中2例经停药、12例经减少用药剂量后,血压均恢复至正常范围内;所有患者均未出现高血钾、血管神经性水肿、肾功能损害、不自主肌肉颤动和心律失常等不良反应。结论:我院心力衰竭住院患者使用沙库巴曲缬沙坦均有用药适应证,且安全性良好,仅有少数患者出现血压不耐受情况;但存在给药剂量偏小、给药频次不适宜、禁忌证用药、药物转换时机不适宜等情况。临床藥师可开展合理用药知识宣讲,加强对患者的药学监护,及时发现不合理用药情况并监测不良反应,同时积极干预,以确保患者用药的合理性及安全性。
关键词 血管紧张素受体-脑啡肽酶抑制剂;沙库巴曲缬沙坦;心力衰竭;住院患者;合理用药;安全性
中图分类号 R972+.9;R969.3 文献标志码 A 文章编号 1001-0408(2020)22-2801-05
DOI 10.6039/j.issn.1001-0408.2020.22.20
ABSTRACT OBJECTIVE: To analyze the situation of inpatients with heart failure taking sacubitril-valsartan. METHODS: The data of heart failture inpatients using sacubitril-valsartan in our hospital were collected during Oct. 2019 to Mar. 2020, including basic information of patients such as gender, age, inpatient department, length of stay; the application of sacubitril-valsartan, including indications, contraindications, usage and dosage, course of medication; conversion with angiotensin converting enzyme inhibitor (ACEI)/angiotensin Ⅱ receptor antagonist (ARB) and adverse drug reactions, were summarized. RESULTS: A total of 252 cases were collected, including 172 males (68.25%) and 80 females (31.75%). The average age of the patients was (67.02±14.23) years old, and 85 cases were 75 years or older (33.73%). Average hospitalization time was (12.03±8.19)d, the average left ventricular ejection fraction (LVEF) before medication was (38.69±10.45)%, the average blood potassium was (4.16±0.65) mmol/L, and the average estimated value of glomerular filtration (eGFR) was (69.14±32.01) mL/(min·1.73 m2). The main distri- bution departments were cardiology department (59.14%), followed by nephrology department (8.73%), respiration department (7.14%), cardiac surgery department (5.95%), geriatrics department (5.56%), emergency medicine department (3.57%) and neurology department (3.17%). All patients had indications, but 25 cases (9.92%) had contraindications, 6 cases (2.38%) had blood potassium>5.4 mmol/L, 19 cases (7.54%) had eGFR<15 mL/(min·1.73 m2). The usage and dosage was 50 mg/bid (45.24%); 39 cases (15.47%) were given medicine once a day, which was unreasonable. Average treatment course was(7.80±5.86) d. 7 patients (2.78%) converted to ACEI, and 3 patients (42.86%) had a conversion interval less than 36 h; 20 patients (7.93%) were converted to ARB, and there was no obvious inappropriate conversion. Hypotension occurred in 14 patients (5.56%). Blood pressure returned to the normal range in 2 patients after drug withdrawal and 12 patients after dose reduction. No patient had adverse reactions such as hyperkalemia, angioneuro edema, renal impairment, involuntary muscle tremor and arrhythmia. CONCLUSIONS: All the inpatients with heart failure in our hospital have indications and good safety. Only a few patients have blood pressure intolerance. However, there were problems such as low dosage, inappropriate frequency of administration, drug use against contraindications, and inappropriate timing of drug conversion. Clinical pharmacists can carry out the knowledge propaganda of rational drug use, strengthen the pharmaceutical care of patients, timely detect the situation of irrational drug use and monitor adverse drug reactions, and actively intervene to ensure the rationality and safety of patients medication.
KEYWORDS Angiotensin receptor-enkephalinase inhibitor; Sacubitril-valsartan; Heart failure; Inpatient; Rational drug use; Safety
心力衰竭是各种心血管疾病的终末阶段,患者预后差且病死率高[1]。临床常用由血管紧张素转换酶抑制剂(ACEI)/血管紧张素Ⅱ受体拮抗剂(ARB)、醛固酮受体拮抗药、β受体阻滞药组成的“金三角”方案治疗,但疗效欠佳[2]。沙库巴曲缬沙坦是全球首个血管紧张素受体-脑啡肽酶抑制剂(ARNI)。有研究证实,与依那普利相比,沙库巴曲缬沙坦可使患者的主要复合终点(心血管死亡和心力衰竭住院)风险降低20%,全因病死率风险降低16%[3]。2016年欧洲心脏病学会《急、慢性心力衰竭诊断与治疗指南》[4]、2017年美国心脏病学会/美国心脏协会/美国心力衰竭学会《心力衰竭管理指南》[5]、2019年我国《心力衰竭合理用药指南(第2版)》[6]均将沙库巴曲缬沙坦作为治疗心力衰竭的Ⅰ类药物推荐。但该药于2017年7月在我国上市,其作为治疗心力衰竭的新型药物,临床应用时间较短,用药经验不足。为了评价该药用于治疗心力衰竭的合理性和安全性,本研究回顾性分析了我院2019年10月-2020年3月住院心力衰竭患者使用沙库巴曲缬沙坦的情况,旨在为该药的临床合理应用提供参考。
1 資料与方法
1.1 纳入与排除标准
纳入标准:(1)年龄≥18岁;(2)应用沙库巴曲缬沙坦;(3)基本信息完整。
排除标准:(1)基本信息不全的患者;(2)死亡患者。
1.2 资料来源
通过医院信息系统收集我院2019年10月-2020年3月使用沙库巴曲缬沙坦的住院心力衰竭患者资料,包括其基本信息,如性别、年龄、住院科室、住院时间等;汇总沙库巴曲缬沙坦使用情况,包括适应证、禁忌证、用法用量、用药疗程;与ACEI/ARB类药物转换情况;药物不良反应;体征及实验室检查指标,包括血压、纽约心脏病协会(NYHA)分级、用药前左室射血分数(LVEF)、肝肾功能、电解质和血凝常规等。
1.3 沙库巴曲缬沙坦用药合理性评价标准
参考沙库巴曲缬沙坦药品说明书、《急、慢性心力衰竭诊断与治疗指南》[4]、《心力衰竭管理指南》[5]、《心力衰竭合理用药指南(第2版)》[6]对沙库巴曲缬沙坦临床应用的合理性进行评价,详见表1。
1.4 数据处理
采用Excel 2013对数据进行处理、分析。
2 结果
2.1 患者基本信息
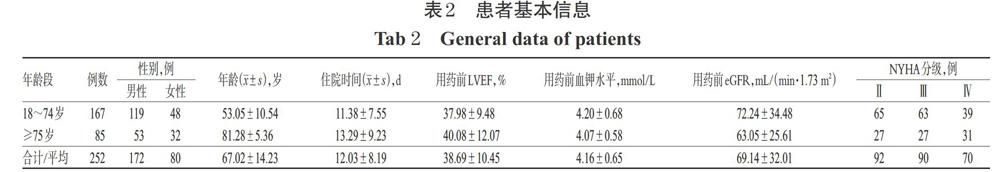
共收集到252例患者资料,其中男性172例(68.25%)、女性80例(31.75%),男、女比例为2.15 ∶ 1;有85例(33.73%)患者的年龄≥75岁,平均年龄为(67.02±14.23)岁;平均住院时间为(12.03±8.19)d。NYHA 分级Ⅱ级92例、Ⅲ级90例、Ⅳ级70例。用药前平均LVEF为(38.69±10.45)%,平均血钾为(4.16±0.65)mmol/L,平均eGFR为(69.14±32.01)mL/(min·1.73 m2);252例患者肝功能及血凝功能均未见明显异常。患者基本信息见表2。
2.2 科室分布
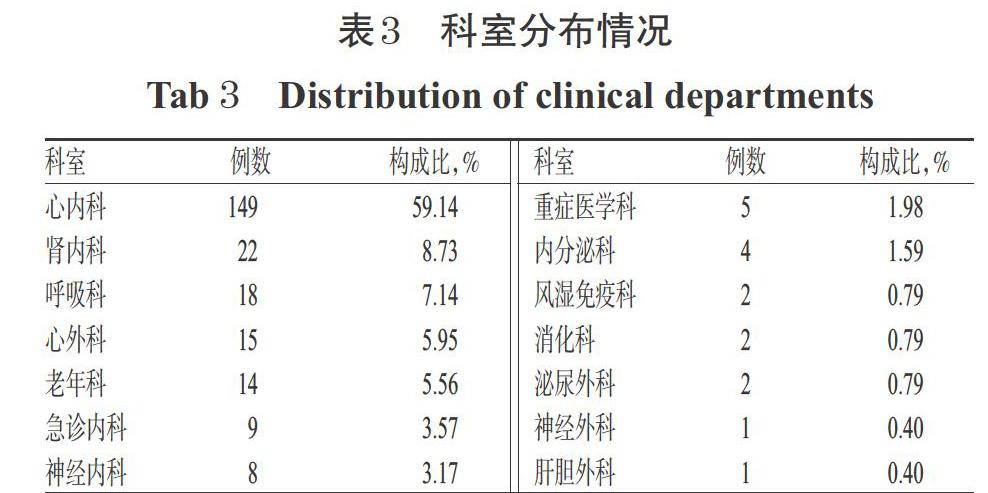
252例患者分布在14个科室,主要以心内科(59.14%)为主,其次为肾内科(8.73%)、呼吸科(7.14%)、心外科(5.95%)、老年科(5.56%)、急诊内科(3.57%)和神经内科(3.17%)。科室分布情况见表3。
2.3 适应证与禁忌证
252例患者均有用药适应证,其中158例(62.70%)为LVEF≤40%,有94例(37.30%)为LVEF>40%;219例(86.90%)为NT-proBNP≥600 pg/mL,14例(5.56%)为400~<600 pg/mL,19例(7.54%)为NT-proBNP<400 pg/mL,详见表4。
2.4 用法用量
252例患者中,沙库巴曲缬沙坦用法用量以50 mg/bid为主(45.24%),其次为25 mg/bid(27.38%);最大单次给药剂量为200 mg/bid(0.40%),最小单次给药剂量为12.5 mg/bid(0.40%);有39例(15.48%)给药频次(qd)不合理。用法用量见表5。
2.5 用药疗程
252例患者中,沙库巴曲缬沙坦使用疗程为1~29 d,平均为(7.80±5.86)d。其中,151例(59.92%)为1~7 d,72例(28.57%)为8~14 d,29例(11.51%)为15~30 d。
2.6 与ACEI/ARB类药物转换
252例患者中,有7例(2.78%)与ACEI类药物转换,其中有3例(42.86%)间隔时间未达36 h;20例(7.93%)与ARB类药物转换,无明显转换不适宜情况。
2.7 不良反应
252例患者中,有14例(5.56%)于用药后出现血压低于90/60 mmHg,其中2例经停药、12例经减少用药剂量后,血压均恢复至正常范围内;所有患者均未出现高血钾、血管神经性水肿、肾功能损害、不自主肌肉颤动及心律失常等不良反应。
3 讨论
3.1 药物适应证与禁忌证分析
沙库巴曲缬沙坦推荐用于LVEF≤40%、NT-proBNP≥600 pg/mL或近12个月内因心力衰竭住院、NT-proBNP≥400 pg/mL的心力衰竭患者[4,6]。但有多项研究证实,沙库巴曲缬沙坦仍可使LVEF保留或中间值的心力衰竭患者获益[8-10]。本研究中,252例患者均有用药适应证;虽然有5例LVEF>50%且NT-proBNP<400 pg/mL,但这些患者均有心功能不全既往史,且长期应用抗心力衰竭药物治疗,有用药适应证。有研究表明,即使在慢性肾脏疾病患者中,沙库巴曲缬沙坦也可改善其eGFR,发挥肾脏保护作用[11]。本研究中,有25例患者存在用药禁忌证,包括6例血钾>5.4 mmol/L、19例eGFR<15 mL/(min·1.73 m2),这些患者虽无高钾血症、肾功能恶化等不良反应发生,但仍需注意定期复查其肾功能及电解质水平,权衡利弊以确保用药安全。
3.2 藥物用法用量分析
《心力衰竭合理用药指南(第2版)》推荐,沙库巴曲缬沙坦应从小剂量开始使用,每2~4周剂量加倍,逐渐增至200 mg/bid以目标维持;对于中度肝功能损伤(Child-Pugh分级B级)、年龄≥75岁的患者,该药的起始剂量要小[6]。本研究纳入的年龄≥75岁的患者中,有37例沙库巴曲缬沙坦给药剂量以50 mg/bid为主,其次为25 mg/bid(28例)。但在<75岁的患者中,给药剂量仍以50、25 mg/bid为主,提示本研究中患者用药剂量普遍偏低。
本研究中,有39例患者给药频次不合理,均为qd。有研究认为,沙库巴曲缬沙坦在实际临床应用过程中的给药剂量可受多种因素的影响,而我国人群可能对该药更为敏感,加之自身基础血压及耐受剂量均偏低,使得剂量增加至目标维持剂量较为困难,但滴定速度慢的患者耐受性更好[8,12]。有研究表明,患者应用低剂量的沙库巴曲缬沙坦,虽未达目标维持剂量,但仍可使其心血管获益[13]。因此,医师可根据患者耐受情况,逐渐增加用药剂量直至目标维持剂量或最大可耐受剂量,以改善患者的远期预后。
3.3 用药疗程分析
本研究中,患者的平均住院天数为(12.03±8.19)d,平均用药天数为(7.80±5.86)d,均为在确诊为心力衰竭后加用沙库巴曲缬沙坦。有研究表明,及早使用沙库巴曲缬沙坦,可降低患者NT-proBNP水平以及心血管死亡或心力衰竭住院风险[14];使用沙库巴曲缬沙坦6~12个月可改善其左室肥厚并减低室性心律失常的发生率[15-16]。但由于该药在我国上市时间较短,长期服用沙库巴曲缬沙坦是否可改善患者的远期预后、降低其病死率及再住院率,尚需临床长期观察随访进一步证实。
3.4 不良反应分析
沙库巴曲缬沙坦常见的不良反应包括低血压、高钾血症、咳嗽、肾功能损害及血管性水肿等[3]。有报道提示,沙库巴曲缬沙坦可使收缩压降低3~15 mmHg[17]。本研究中,有14例患者出现低血压,其中2例经停药、12例经减少用药剂量后,其血压均恢复至正常范围内。另有研究发现,沙库巴曲缬沙坦可抑制脑啡肽酶的活性,从而影响中枢神经系统中的β淀粉蛋白的降解,而β淀粉蛋白的沉积与聚集与阿尔茨海默病的发生、发展密切相关,因此沙库巴曲缬沙坦的应用可能会导致患者出现痴呆相关的认知障碍症状[18],但尚需相关实验研究及长期临床观察进一步证实。此外,沙库巴曲缬沙坦引起的不良反应多发生于用药后12 h~10个月[19-20],故患者出院后仍需监测有无不适,并注意定期复查肾功能、电解质等相关指标。
3.5 药物转换分析
沙库巴曲缬沙坦与ACEI联用可抑制缓激肽的降解,增加血管神经性水肿的发生风险,故与ACEI类药物转换需间隔至少36 h[21];而ARB类对缓激肽的降解无明显影响,不会增加血管性水肿的发生率,但沙库巴曲缬沙坦具有拮抗血管紧张素Ⅱ受体的活性,不应同时与ARB类药物合用[7],故沙库巴曲缬沙坦与ARB类药物的转换需间隔至少24 h。本研究中,有7例患者存在与ACEI类药物转换的情况,其中有3例间隔时间未达36 h,虽然这些患者均未见相关不良反应发生,但考虑到未达转换时间,患者仍有发生血管神经性水肿的风险[7],因此临床需重视警惕。本研究中有20例患者与ARB类药物转换,无明显转换不适宜情况发生。
4 结语
沙库巴曲缬沙作为治疗心力衰竭的新型药物,具有舒张血管、预防和逆转心室重构、促尿钠排泄的作用,可显著改善患者症状,降低心力衰竭住院率和病死率,且安全性良好[22]。但因该药在我国上市时间较短,临床应用经验有限,故使用时需更加谨慎。同时,针对用药不规范的情况,临床药师可及时总结,开展合理用药知识宣讲,以提高医师对药物用法用量、适应证、禁忌证、与ACEI/ARB类药物转换和不良反应等药物应用方面的认识;加强对患者的药学监护,及时发现不合理用药情况并监测不良反应,同时积极干预,以确保患者用药的合理性和安全性。
参考文献
[ 1 ] PONIKOWSKI P,ANKER SD,ALHABIB KF,et al. Heart failure:preventing disease and death worldwide[J]. ESC Heart Fail,2014,1(1):4-25.
[ 2 ] Correction to:heart disease and stroke statistics:2017 update:a report from the American Heart Association[J]. Circulation,2017,136. DOI:10.1161/CIR.0000000000000530.
[ 3 ] MCMURRAY JJV,PACKER M,DESAI AS,et al.Angiotensin-neprilysin inhibition versus enalapril in heart failure[J]. N Engl J Med,2014,371(11):993-1004.
[ 4 ] PONIKOWSKI P,VOORS AA,ANKER SD,et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure:the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology(ESC)developed with the special contribution of the Heart Failure Association(HFA)of the ESC[J]. Eur Heart J,2016,37(27):2129- 2200.
[ 5 ] YANCY CW,JESSUP M,BOZKURT B,et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure:a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America[J]. J Card Fail,2017,23(8):628-651.
[ 6 ] 國家卫生计生委合理用药专家委员会,中国药师协会.心力衰竭合理用药指南:第2版[J/CD].中国医学前沿杂志:电子版,2019,11(7):1-78.
[ 7 ] YANCY CW,JANUZZI JL,ALLEN LA,et al. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment:answers to 10 pivotal issues about heart failure with reduced ejection fraction:a report of the American College of Cardiology task force on expert consensus decision pathways[J]. J Am Coll Cardiol,2018,71(2):201-230.
[ 8 ] 陈存芳,贾博,江珊,等.沙库巴曲缬沙坦治疗射血分数中间值心力衰竭患者的疗效及预后[J].中国新药与临床杂志,2020,39(2):88-92.
[ 9 ] SOLOMON SD,ZILE M,PIESKE B,et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction:a phase 2 double-blind randomised controlled trial[J]. Lancet,2012,380(9851):1387-1395.
[10] VADUGANATHAN M,CLAGGETT BL,DESAI AS,et al. Prior heart failure hospitalization,clinical outcomes,and response to sacubitril/valsartan compared with valsartan in HFpEF[J]. J Am Coll Cardiol,2020,75(3):245-254.
[11] PACKER M,CLAGGETT B,LEFKOWITZ MP,et al. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin-angiotensin system:a secondary analysis of the PARADIGM-HF trial[J]. Lancet Diabetes Endo,2018,6(7):547-554.
[12] DU AX,WESTERHOUT CM,MCALISTER FA,et al. Titration and tolerability of sacubitril/valsartan for patients with heart failure in clinical practice[J]. J Cardiovasc Pharmacol,2019,73(3):1-18.
[13] MARTENS P,LAMBEETS S,LAU CW,et al. Impact of sacubitril/valsartan on heart failure admissions:insights from real-world patient prescriptions[J]. Acta Cardiol,2019,74(2):115-122.
[14] WACHTER R,SENNI M,BELOHLAVEK J,et al. Initiation of sacubitril/valsartan in haemodynamically stabilized heart failure patients in hospital or early after discharge:primary results of the randomised TRANSITION study[J]. Eur J Heart Fail,2019,21(8):998-1007.
[15] WANG Y,ZHOU R,LU C,et al. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling:meta-analysis[J]. J Am Heart Assoc,2019,8(13):1-20.
[16] VALENTIM GONCALVES A,PEREIRA-DA-SILVA T,GALRINHO A,et al. Antiarrhythmic effect of sacubitril-valsartan:cause or consequence of clinical improvement?[J]. J Clin Med,2019. DOI:10.3390/jcm8060869.
[17] MARTENS P,BELI?N H,DUPONT M,et al. Insights into implementation of sacubitril/valsartan into clinical practice[J]. ESC Heart Fail,2018,5(3):275-283.
[18] CANNON JA,SHEN L,JHUND PS,et al. Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction[J]. Eur J Heart Fail,2017,19(1):129-137.
[19] BEJAN-ANGOULVANT T,GENET T,VRIGNAUD L,et al. Three case reports of involuntary muscular movements as adverse reactions to sacubitril/valsartan[J]. Br J Clin Pharmacol,2018,84(5):1072-1074.
[20] ALMUFLEH A,MIELNICZUK LM,ZINOVIEV R,et al.Profound vasoplegia during sacubitril/valsartan treatment after heart transplantation[J]. Can J Cardiol,2018,34(3):e5-e7.
[21] VON LUEDER TG,SANGARALINGHAM SJ,WANG BH,et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation:novel therapeutic concepts to combat heart failure[J]. Circ Heart Fail,2013,6(3):594-605.
[22] B?HM M,YOUNG R,JHUND PS,et al. Systolic blood pressure,cardiovascular outcomes and efficacy and safety of sacubitril/valsartan(LCZ696)in patients with chronic heart failure and reduced ejection fraction:results from PARADIGM-HF[J]. Eur Heart J,2017,38(15):1132- 1143.
(收稿日期:2020-07-27 修回日期:2020-10-16)
(編辑:陈 宏)

