外泌体ABCA1蛋白在阿尔茨海默病诊断中的价值
刘辰庚 支杨 李莹 赵越 路尧 王培昌
[摘要] 目的 通过小鼠实验和临床标本检测初步研究外泌体ATP结合盒转运体A1(ABCA1)蛋白作为阿尔茨海默病(AD)诊断标志物的价值。 方法 提取9月龄组APP/PS1 AD模型小鼠脑脊液(CSF)和血清外泌体分别进行质谱分析和ABCA1蛋白酶联免疫吸附试验(ELISA)检测。将3、6、9月龄组APP/PS1小鼠CSF外泌体注射至3月龄组野生型(WT)小鼠的第三脑室后分别检测0、2、4、6 h组血清和CSF外泌体ABCA1水平。检测69例主观认知下降(SCD)组、43例轻度认知障碍(MCI)组和35例痴呆期(DAT)组及30例对照组受试者的血清外泌体ABCA1水平。 结果 质谱和ELISA结果示:3、6、9月龄组APP/PS1双转基因小鼠CSF和血清外泌体ABCA1蛋白水平分别高于同月龄WT小鼠(P < 0.05);6月龄组APP/PS1双转基因小鼠CSF和血清中外泌体ABCA1蛋白水平显著高于3月龄组APP/PS1双转基因小鼠(P < 0.05),9月龄组APP/PS1双转基因小鼠CSF和血清中外泌体ABCA1蛋白水平显著高于6月龄组APP/PS1双转基因小鼠(P < 0.05)。外泌体注射后2、4、6 h组的WT小鼠血清外泌体ABCA1蛋白水平显著升高(P < 0.05),4 h组最高;外泌体注射后2、4、6 h组的WT小鼠CSF外泌体ABCA1蛋白水平均显著降低(P < 0.05),6 h组最低。SCD组与对照组血清外泌体ABCA1蛋白水平比较差异无统计学意义(P > 0.05);MCI组和DAT组血清外泌体ABCA1蛋白水平较对照组显著升高(P < 0.05),且DAT组显著高于MCI组(P < 0.05)。当截断值为0.39时,血清外泌体ABCA1蛋白诊断MCI的敏感度为72.5%,特异度为75.4%;当截断值为0.52时,血清外泌体ABCA1蛋白诊断DAT的敏感度为70.1%,特异度为69.2%;血清外泌体ABCA1对MCI和DAT的诊断效能显著高于SCD(P < 0.05)。 结论 ABCA1蛋白外泌体可经透过血脑屏障到达外周血而被有效检测,其可作为AD诊断的候选标志物,但在SCD的诊断中还需积累更多的研究数据。
[关键词] 阿尔茨海默病;外泌体;诊断;ATP结合盒转运体A1
[中图分类号] R742 [文献标识码] A [文章編号] 1673-7210(2020)10(a)-0006-05
The value of exosomal ABCA1 protein in the diagnosis of Alzheimer′s disease
LIU Chengeng1 ZHI Yang2 LI Ying1,3 ZHAO Yue1 LU Yao1 WANG Peichang1
1.Department of Clinical Laboratory, Xuanwu Hospital, Capital Medcial University, Beijing 100053, China; 2.Department of Clinical Laboratory, Beijing Xicheng District Guangwai Hospital, Beijing 100055, China; 3.Department of Clinical Laboratory, PLA Air Force Medical Characteristic Center, Beijing 100142, China
[Abstract] Objective To preliminary study the value of exosome ABCA1 protein as a diagnostic marker for Alzheimer′s disease (AD) through mouse experiments and clinical specimen detection. Methods Cerebrospinal fluid (CSF) and serum exosomes of APP/PS1 AD model mice in nine-month-old group were extracted and analyzed by mass spectrometry and ABCA1 protein detected by enzyme linked immunosorbent assay (ELISA). After three, six, nine months old APP/PS1 mice cerebrospinal fluid exosomes were injected into the third ventricle of three-month-old wild type (WT) mice, serum and CSF of zero, two, four, six-hour-groups were detected respectively exosome ABCA1 level. Serum exosome ABCA1 levels were detected in 69 patients with subjective cognitive decline (SCD group), 43 patients with mild cognitive impairment (MCI group) and 35 patients with dementia (DAT group) and 30 cases of control group. Results The results of mass spectrometry and ELISA showed that the levels of CSF and ABCA1 protein in serum of APP/PS1 double transgenic mice in three-month-old group, six-month-old group and nine-month-old group were higher than those of WT mice at the same month age (P < 0.05). CSF and serum exosomes ABCA1 protein of APP/PS1 double transgenic mice in six-month-old group were significantly higher than those in three-month-old group (P < 0.05). CSF and serum exosomes ABCA1 protein in nine-month-old APP/PS1 double transgenic mice were significantly higher than those in six-month-old APP/PS1 double transgenic mice (P < 0.05). The serum level of ABCA1 protein in WT mice was significantly increased in two, four and six hour-groups after exosomes injection (P < 0.05), and the highest level was found in four-hour group. The levels of ABCA1 protein in CSF exosomes of WT mice were significantly decreased in two, four and six hour-group after exosomes injection (P < 0.05), and the lowest in six-hour-group. There was no significant difference in ABCA1 protein between SCD group and control group (P > 0.05). The level of ABCA1 protein in serum of MCI group and DAT group was significantly higher than that of control group (P < 0.05), and that of DAT group was significantly higher than that of MCI group (P < 0.05). When the cut-off value was 0.39, the sensitivity and specificity of ABCA1 protein was 72.5% and 75.4% respectively, when the cut-off value was 0.52, the sensitivity and specificity of ABCA1 protein was 70.1% and 69.2% respectively in the diagnose of MCI. The diagnostic efficiency of ABCA1 protein in MCI and DAT was significantly higher than that in SCD (P < 0.05). Conclusion ABCA1 exosomes can pass through the blood-brain barrier and reach the peripheral blood, which can be used as a candidate marker for AD diagnosis. However, more research data need to be accumulated in the diagnosis of SCD.
[Key words] Alzheimer′s disease; Exosome; Diagnosis; ATP-binding cassette transporter A1
阿尔茨海默病(AD)是一种以神经元细胞进行性损伤和凋亡为主要细胞学表现的退行性神经系统疾病,临床表现主要为进行性的记忆、语言和认知能力障碍。随着我国人口老龄化进程的加快,AD患病人数的绝对值呈升高趋势,我国AD患病人数已超800万,65岁以上人群患病率为4%~6%。AD的发展一般可分为主观认知下降(SCD)、轻度认知障碍(MCI)和痴呆期(DAT)。现已证实,DAT患者脑组织已经发生了不可逆的损伤,且尚无有效的治疗药物,故在损伤相对可逆且可进行有效医疗干预的SCD和MCI时发现和治疗疾病尤为重要[1-3]。本研究应用质谱技术检测上述3个疾病阶段受试者和小鼠模型的血清和脑脊液(CSF)标本外泌体蛋白的表达,并就呈升高趋势的ATP结合盒转运体A1(ABCA1)在AD诊断中的临床价值做初步研究。
1 资料与方法
1.1 一般资料
随机选取2018年5月—2019年10月就诊于首都医科大学宣武医院、北京市西城区广外医院和中国人民解放军空军总医院检验科的受试者共177例,其中SCD组受试者69例,男36例,女33例,平均年龄(61.2±5.2)岁;MCI组受试者43例,男22例,女21例,平均年龄(66.7±5.6)岁;DAT组受试者35例,男18例,女17例,平均年龄(70.8±6.2)岁。另随机选取表面健康受试者30例为对照组,男15例,女15例,平均年龄(65.2±6.1)岁。所有受试者的诊断均依据相关指南[3-5],未接受降血脂治疗。对照组受试者排除神经系统、内分泌系统、肝肾及心脑血管疾病,未接受降血脂治疗。所有受试者于上午空腹抽取静脉血,为尽量避免血细胞外泌体释放的影响,标本静置30 min血细胞凝集后立即以3000 g离心7 min分离血清,血清标本置于液氮保存,在进行ABCA1检测前仅允许冻融一次。
1.2 实验动物
3、6、9月龄组APP/PS1双转基因小鼠[SPF级饲养,生产许可证号:SYXK(京)2014-0029]及3、6、9月龄组野生型(WT)小鼠购自中国医学科学院实验动物研究所,使用脑立体定位仪(Stoelting,美国伊利诺伊,型号:51600)于前囟前2.0 mm、中縫旁2.0 mm、硬膜下4 mm处抽取CSF[6];使用眼球摘除法留取全血,静置30 min血细胞凝集后立即以3000 g离心7 min分离血清。按照下文所述提取外泌体,将CSF和血清外泌体送中国北京百奥公司进行外泌体蛋白质谱分析。同时,使用立体定位仪将9月龄小鼠CSF中提取的外泌体PBS悬液100 μL注射至3月龄WT小鼠的第三脑室,分别于0、2、4、6 h留取CSF和血清(分别为0、2、4、6 h组)。标本保存于液氮,在进行ABCA1检测前仅允许冻融一次。每组标本量为5份。以上研究方案已通过首都医科大学宣武医院伦理审核。
1.3 外泌体提取
使用快速血清/体液外泌体提取试剂盒(Umibio,中国上海,货号:UR52141)进行CSF和血清外泌体的提取,CSF标本的上样量为200 μL,血清的上样量为100 μL,严格按照试剂盒说明书的操作进行。用于小鼠注射的外泌体重悬于100 μL的PBS中,用于蛋白检测的外泌体使用细胞膜蛋白与细胞浆蛋白提取试剂盒(百奥莱博,中国北京,货号:YT042)按说明书操作提取总蛋白。
1.4 ABCA1检测
使用人和小鼠ABCA1酶联免疫吸附试验(ELISA)试剂盒(Bio-Swamp,中国湖北,货号:MU30899)检测受试者和小鼠样本中ABCA1的OD值,使用人和小鼠CD63 ELISA检测试剂盒(CUSABIO,中国湖北,货号:CSB-E14107h)检测受试者和小鼠样本中CD63的OD值。使用CD63作为内参计算样本中ABCA1的相对含量。
1.5 统计学方法
采用SPSS 21.0中文版统计软件进行统计学分析和接受者操作特性曲线(ROC)分析。计量资料用均数±标准差(x±s)表示,采用重复测量方差分析和单因素方差分析,计数资料比较采用χ2检验。以P < 0.05为差异有统计学意义。
2 结果
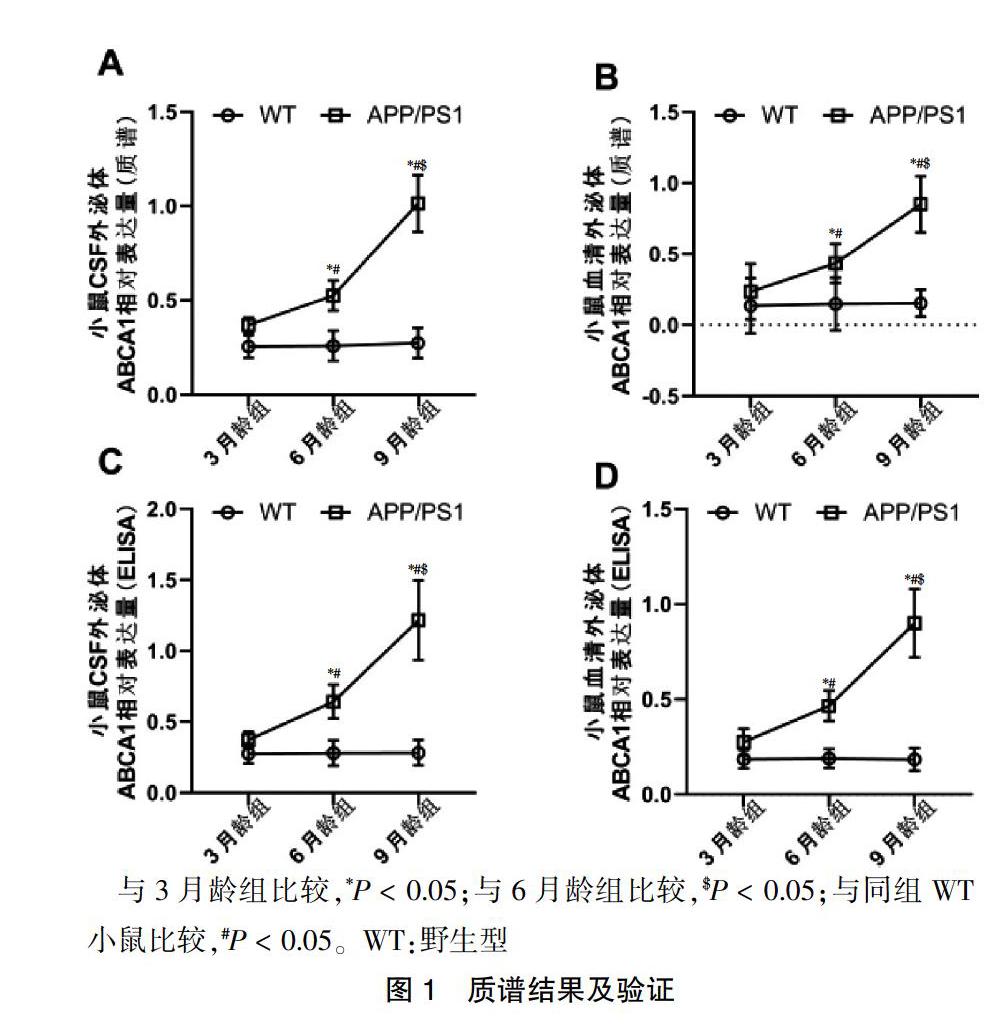
2.1 质谱结果及验证
蛋白质谱结果显示,3、6、9月龄组APP/PS1双转基因小鼠CSF和血清外泌体ABCA1水平分别高于同组WT小鼠(P < 0.05);6月龄组APP/PS1双转基因小鼠CSF和血清中外泌体ABCA1显著高于3月龄组APP/PS1双转基因小鼠(P < 0.05),9月龄组APP/PS1双转基因小鼠CSF和血清中外泌体ABCA1蛋白水平显著高于6月龄组APP/PS1双转基因小鼠(P < 0.05)。ELISA实验得到了与蛋白质谱一致的结果。见图1。
与3月龄组比较,*P < 0.05;与6月龄组比较,$P < 0.05;与同组WT小鼠比较,#P < 0.05。WT:野生型
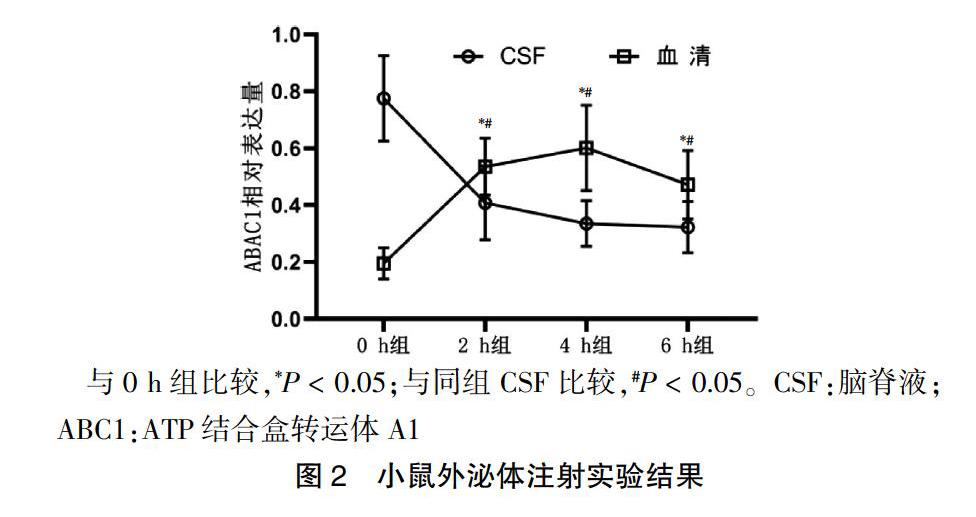
2.2 小鼠外泌体注射实验结果
外泌体注射后2、4、6 h组的WT小鼠血清外泌体ABCA1水平较0 h组显著升高,且高于CSF外泌体ABCA1水平(P < 0.05),4 h组最高;外泌体注射后2、4、6 h组的WT小鼠CSF外泌体ABCA1蛋白水平均显著降低(P < 0.05),6 h组最低。见图2。
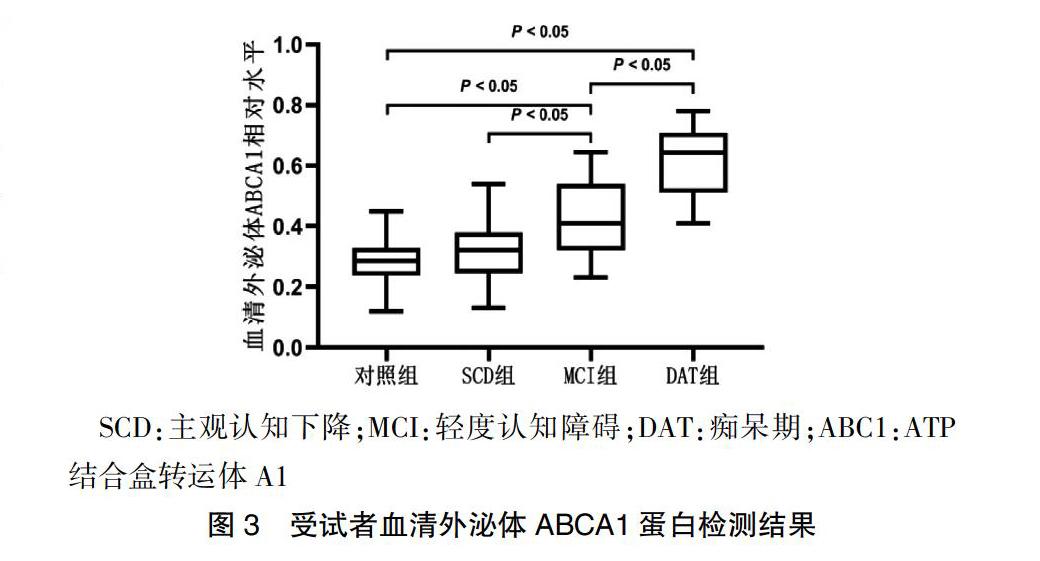
2.3 受试者血清外泌体ABCA1蛋白水平检测结果
SCD组与对照组血清外泌体ABCA1蛋白水平比较差异无统计学意义(P > 0.05);MCI组和DAT组血清外泌体ABCA1蛋白水平较对照组显著升高(P < 0.05),且DAT组显著高于MCI组(P < 0.05),MCI组高于SCD组(P < 0.05)。见图3。
SCD:主观认知下降;MCI:轻度认知障碍;DAT:痴呆期;ABC1:ATP结合盒转运体A1
2.4 ROC和诊断效能分析结果
当截断值为0.39时,血清外泌体ABCA1蛋白诊断MCI的敏感度为72.5%,特异度为75.4%;当截断值为0.52时,血清外泌体ABCA1诊断DAT的敏感度为70.1%,特异度为69.2%;血清外泌体ABCA1对MCI和DAT的诊断效能显著高于SCD(P < 0.05)。见表1。
3 讨论
随着我国人口老龄化的不断演进,AD在老年人群中发病率、死亡率和致残率逐渐升高的特点,对其陪护和治疗的过程给患者本人及家庭和社会带来较大压力[7-8]。同时,AD对脑组织造成的损伤为不可逆的过程,目前尚无有效的药物对其进行治疗和逆转,患者一旦进入痴呆期就只能进行对症治疗和支持性治疗。而对于SCD和MCI患者,如果能及时发现,则有较为系统的方法对其进行早期干预,以阻止或延缓其进入DAT期,故对AD早期发现显得尤为关键[9-11]。
AD的发病机制多样,目前主流的有淀粉样蛋白学说、基因学说、tau蛋白学说和脂质代谢学说等。在脂质代谢学说中,载脂蛋白ApoE4占有重要地位,其在淀粉样蛋白生成及tau蛋白磷酸化中均发挥作用[12-13]。与ApoE4类似,ABCA1广泛分布在包括脑组织的各个组织器官,其可在消耗腺嘌呤核苷三磷酸的基础上对胆固醇等脂质进行由细胞内向细胞外的运输,在脂质代谢中具有重要作用[14-15]。已有研究表明,ABCA1能与ApoE4相互作用,并作为淀粉样蛋白清除通道的组成部分,故ABCA1表达的改变可能参与AD的发生和发展过程[11]。APP/PS1模型小鼠主要的造模原理即为促使淀粉样蛋白的过量沉积,故本课题组发现其外泌体ABCA1表达出现显著性改变可能是由于淀粉样蛋白代谢异常所导致。外泌体是细胞主动分泌的微小囊泡,其中携带了细胞向外界传递的物质及生物学信息,已有研究表明,这类信息可通过外泌体的携带由一个细胞转移至另一个细胞或由一个组织传递至另一个远隔组织,且这个过程可能是主动和特异的[16]。外泌体本身为膜性结构,其具备多种细胞膜和细胞内细胞器膜的膜蛋白成分,ABCA1作为一种膜蛋白,可能是在外泌体生成的阶段即被细胞主动或被动装载到外泌体膜上向外界分泌;但其分泌的增加可能导致细胞本身ABCA1的减少,由于ABCA1在脂代谢中的作用,可能导致细胞内脂代谢的某些障碍,从而参与AD的发生和发展进程[17-19]。
長期以来,脑脊液的β-淀粉样蛋白和tau蛋白被认为是AD诊断的“金标准”,由于其检测对象为CSF,临床应用相对有限,寻找血液、尿液等无创检查的AD生物标志物是目前的研究热点[20-21]。检测小鼠脑室注射实验结果显示,高ABCA1外泌体的脑室注射可导致外周血外泌体中ABCA1蛋白水平的升高,提示其来自于CSF,即高ABCA1外泌体可经透过血脑屏障到达外周血而被有效检测,这对于无创检测而言是十分有利的。同时,受试者的检测结果表明随着AD的进展,其血清外泌体ABCA1蛋白水平逐步升高,提示其可作为AD诊断和分期的标志物。本研究结果提示,血清外泌体ABCA1蛋白水平在SCD组受试者中升高并不显著,其原因可能是SCD是一个较为漫长的疾病演进过程,部分患者可能处于疾病极早期阶段,亦或是由非AD类型的SCD患者影响所导致的假阴性[22],值得进一步研究。
综上,小鼠高ABCA1外泌体可透过血脑屏障到达外周血而被有效检测,可作为AD诊断的候选标志物,但在SCD的诊断中还需积累更多的研究数据。
[参考文献]
[1] Cortes-Canteli M,Iadecola C. Alzheimer′s disease and vascular aging:JACC focus seminar [J]. J Am Coll Cardiol,2020,75(8):942-951.
[2] Brent RJ. Behavioral versus biological definitions of dementia symptoms:recognizing that worthwhile interventions already exist [J]. OBM Geriat,2019,3(4):10.21926/obm.geriatr.1904079.
[3] 韩璎.中国阿尔茨海默病临床前期主观认知下降的诊治策略[J].中国临床医学影像杂志,2018,29(8):534-538.
[4] 贾建平,陆璐,张逸驰,等.美国国立老化研究所与阿尔茨海默病协会诊断指南写作组:阿尔茨海默病源性轻度认知障碍诊断标准推荐[J].中华神经内科杂志,2012, 45(5):345-351.
[5] 贾建平,陆璐,张逸驰,等.美国国立老化研究所与阿尔茨海默病协会诊断指南写作组:阿尔茨海默病痴呆诊断标准的推荐[J].中华神经内科杂志,2012,45(5):352-355.
[6] Shahpasand-Kroner H,Klafki HW,Bauer C,et al. A two-step immunoassay for the simultaneous assessment of Aβ38,Aβ40 and Aβ42 in human blood plasma supports the Aβ42/Aβ40 ratio as a promising biomarker candidate of Alzheimer′s disease [J]. Alzheimers Res Ther,2018,10(1):121.
[7] Zhang S,Huang SY,An XB,et al. Medical histories of control subjects influence the biomarker potential of plasma Aβ in Alzheimer′s disease:a meta-analysis [J]. J Mol Neurosci,2020,70(6):861-870.
[8] das Nair R,Bradshaw LE,Carpenter H,et al. A group memory rehabilitation programme for people with traumatic brain injuries:the ReMemBrIn RCT [J]. Health Technol Assess,2019,23(16):1-194.
[9] 劉辰庚,杨婷婷,王培昌.血清外泌体miRNA-193b诊断阿尔茨海默病的价值[J].实用医学杂志,2018,34(21):3621-3624.
[10] Clare L,Kudlicka A,Oyebode JR,et al. Goal-oriented cognitive rehabilitation for early-stage Alzheimer′s and related dementias:the GREAT RCT [J]. Health Technol Assess,2019,23(10):1-242.
[11] Sun R,Wang H,Shi Y,et al. A pilot study of urinary exosomes in Alzheimer′s disease [J]. Neurodegener Dis,2019,19(5-6):184-191.
[12] Qian H,Zhao X,Cao P,et al. Structure of the human lipid exporter ABCA1 [J]. Cell,2017,169(7):1228-1239.
[13] 吴迪,许凤燕,孙亮,等.外伤性脑损伤诱发阿尔茨海默病的相关机制研究进展[J].卒中与神经疾病,2019,26(3):381-384.
[14] Yin Q,Ji X,Lv R,et al. Targetting exosomes as a new biomarker and therapeutic approach for Alzheimer′s Disease [J]. Clin Interv Aging,2020,15:195-205.
[15] Lee S,Mankhong S,Kang JH. Extracellular vesicle as a source of Alzheimer′s biomarkers:opportunities and challenges [J]. Int J Mol Sci,2019,20(7):E1728.
[16] Pulliam L,Sun B,Mustapic M,et al. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease [J]. J Neurovirol,2019,25(5):702-709.
[17] Kodidela S,Gerth K,Haque S,et al. Extracellular vesicles:a possible link between HIV and Alzheimer′s disease-like pathology in HIV subjects [J]. Cells,2019,8(9):E968.
[18] Crespo-Castrillo A,Arevalo MA. Microglial and astrocytic function in physiological and pathological conditions:estrogenic modulation [J]. Int J Mol Sci,2020,21(9):E3219.
[19] Paulavicius AM,Mizzaci CC,Tavares DRB,et al. Bilingualism for delaying the onset of Alzheimer′s disease:a systematic review and meta-analysis [J]. Eur Geriatr Med,2020,11(4):651-658.
[20] Mantzavinos V,Alexiou A. Biomarkers for Alzheimer′s disease diagnosis [J]. Curr Alzheimer Res,2017,14(11):1149-1154.
[21] Lombardi G,Crescioli G,Cavedo E,et al. Structural magnetic resonance imaging for the early diagnosis of dementia due to Alzheimer′s disease in people with mild cognitive impairment [J]. Cochrane Database Syst Rev,2020,3(3):CD009628.
[22] Birks JS,Harvey RJ. Donepezil for dementia due to Alzh-eimer′s disease [J]. Cochrane Database Syst Rev,2018,6(6):CD001190.
(收稿日期:2020-03-10)

