Pulmonary hypertension concurrent with pericardial effusion and superior vena cava syndrome: who is the initiator?
Bei-Ning WANG, Yu-Xi LI, Wei MA, Song-Yun CHU, Zhi-Hao LIU, Wen-Hui DING, Jian-Ping LI
1Department of Nephrology, Peking University First Hospital, Beijing, China
2Department of Cardiology, Peking University First Hospital, Beijing, China
3Key Laboratory of Molecular Cardiovascular Sciences of Ministry of Education, Health Science Center, Peking University, Beijing, China
Keywords: Malignancy; Pericardial effusion; Pulmonary hypertension; Superior vena cava syndrome
The diagnosis of pulmonary hypertension (PH) should be made by combining clinical manifestations and echocardiographic probability.[1]Following the confirmation of PH, the classification should begin with the more common groups[group 2 (PH due to left heart disease) and group 3 (PH due to lung diseases and/or hypoxia)], then group 4 (chronic thromboembolic PH and other pulmonary artery obstructions) and finally group 1 (pulmonary arterial hypertension)and group 5 (PH with unclear and/or multifactorial mechanisms).[1]In this case, we demonstrate a rare scenario of obstruction-caused group 4 PH.
A 53-year-old male presented with dyspnea on exertion,unproductive cough, facial swelling, and bilateral edema of lower extremities for one year.There was no fever, night sweats, hemoptysis, weight loss, dizziness, or syncope.Initial laboratory tests showed leukocytosis (white blood count 14.3×109/L; normal range: 3.5-9.5×109/L).The comprehensive metabolic panel, cardiac biomarkers, brain natriuretic peptide, coagulation test and urinalysis were normal.Blood gas analysis (ABG) showed hypoxemia without carbon dioxide retention.Electrocardiogram (ECG) was significant for sinus tachycardia and low QRS voltage without ST-T abnormalities.The chest radiograph showed enlarged cardiac silhouette with clear lung fields.Echocardiogram revealed small pericardial effusion, elevated pulmonary artery systolic pressure (ePASP: 54.5 mmHg) with normal left and right ventricular function and no valvular abnormalities(Supplementary clip 1).He was given Moxifloxacin considering the leukocytosis.Seven months later, he presented again for persistent dyspnea.Repeated echocardiogram revealed much larger pericardial effusion and significant PH(ePASP: 75.0 mmHg).He was referred to our inpatient unit for further investigation.
He was a bachelor and non-smoker, working as a craftsman.There was no history of toxin exposure, medication use, or allergy.His uncle had been diagnosed with pulmonary tuberculosis three years ago but was already cured by antitubercular agents.No family history of heart diseases,connective tissue diseases, or early death was elicited.On admission, he was afebrile, pulse 96/min, respiratory rate 22/min, blood pressure 110/78 mmHg, and SvO290%.Physical examination was significant for cyanotic lips, superficial vascular distension over the chest (Figure 1), and bilateral edema in lower extremities.Tachycardia with regular rhythm and increased intensity of P2were noticed on auscultation without murmurs or crackles.The abdomen was soft and non-tender without organomegaly.No digital clubbing or sclerodactyly was disclosed.
Laboratory data on admission were as follows: white blood cells 10.4×109/L (normal range: 3.5-9.5×109/L), hemoglobin 139 g/L (normal range: 137-179 g/L), platelet 176×109/L (normal range: 100-300×109/L), neutrocyte percentage 74.4% (normal range: 40-75%).Comprehensive metabolic panel, cardiac biomarkers, brain natriuretic peptide, coagulation test, and thyroid function test were normal.Urinalysis was unremarkable.Erythrocyte sedimentationrate, C-reactive protein, and procalcitonin were all normal.Blood gas analysis (ABG) showed pH = 7.47, PO2= 65 mmHg, PCO2= 38 mmHg, HCO3-= 27.9 mmol/L, and SaO294%.Electrocardiogram still indicated sinus tachycardia and low QRS voltage without ST-T abnormalities (Figure 2).The chest radiograph showed cardiac silhouette enlargement without lung field lesions or pleural effusion(Figure 3).Detailed echocardiographic data: normal left and right ventricular structure and function, normal atrial dimensions, no valvular abnormalities, E/e’ 22.5, e’ med 5.0 cm/s, peak tricuspid regurgitant velocity (TRV) 4.0 m/s,estimated pulmonary artery systolic pressure (ePASP) 75.0 mmHg, pulmonary artery (PA) diameter 25 mm, inferior vena cava diameter 20.9 mm with inspiratory collapse <50%, large pericardial effusion (widest echo-free space 28 mm) with no signs of cardiac tamponade or pulmonary embolism (Supplementary clip 2).No abnormal finding such as portal hypertension, hepatomegaly, or splenomegaly was disclosed via abdominal ultrasonography.Pulmonary function testing (PFT) showed forced expiratory volume in one second (FEV1) %predicted 46.6%, forced vital capacity(FVC) %predicted 56.8%, FEV1/FVC 67.1%, total lung capacity (TLC) %predicted 59.7% and diffusing capacity for carbon monoxide (DLCO) %predicted 40.5%, suggesting ventilation and diffusion impairment.The bronchial dilation test was negative.Considering the large pericardial effusion (PE) and the suspected exposure to tuberculosis(TB), PE workup was initiated and ruled out active infection(bacteria, TB, common viruses, and syphilis) and connective tissue diseases.As to the malignant tumor, another common cause of PE, serum tumor biomarker disclosed the slight elevation of cytokeratin-19 fragment CYFRA 21-1(11.3 ng/mL; normal range: < 3.3 ng/mL) and there was no peripheral lymphadenopathy via ultrasound.
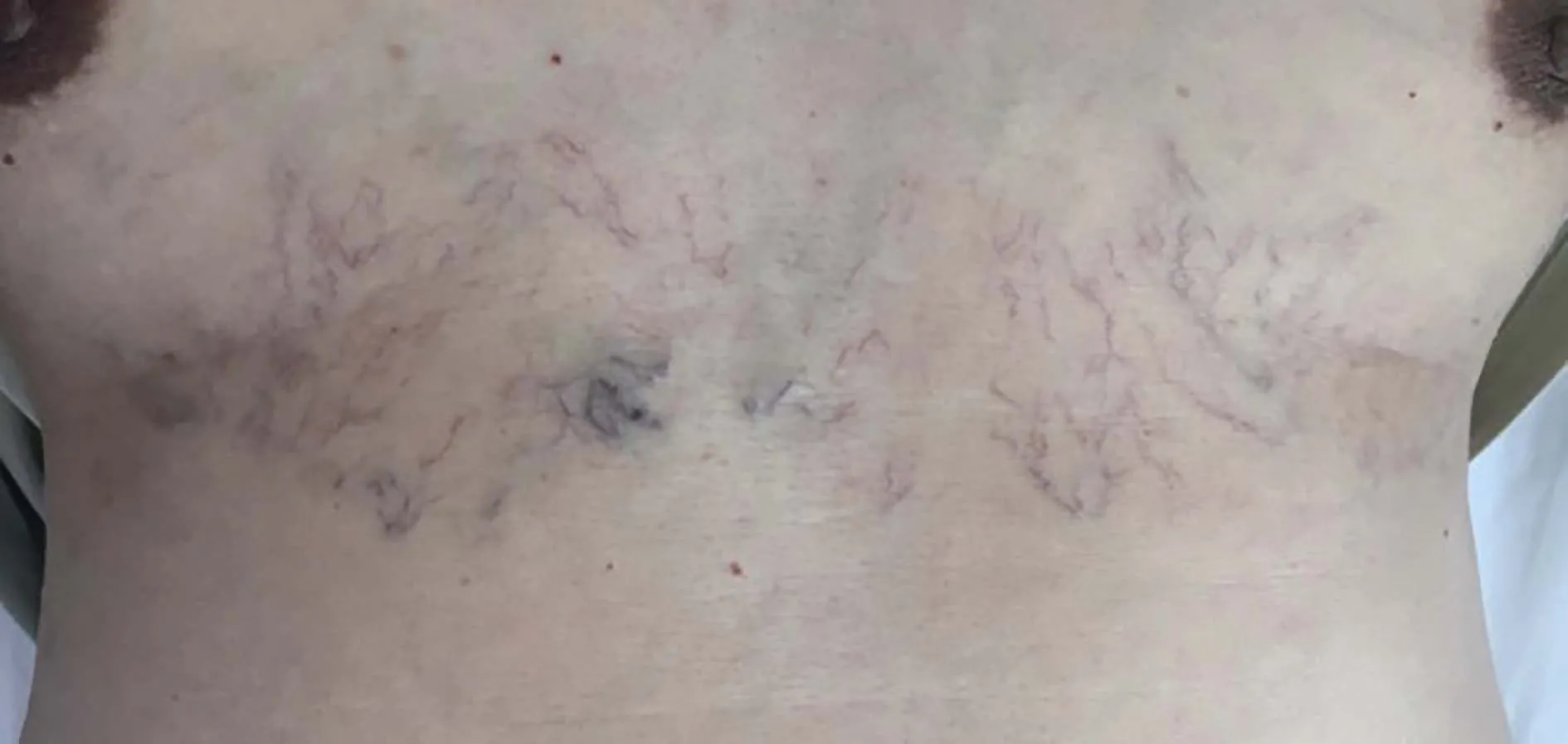
Figure 1.Superficial vascular distention.Physical examination was significant for vascular distension over the patient’s chest wall.
The diagnosis of PH was made on this patient based on echocardiogram (TRV > 3.4 m/s), so the diagnosticalgorithm was prompted.According to the ESC/ERS guideline,[1]PH induced by left heart disease (group 2) and lung diseases (group 3) should be taken into consideration first;however, there was no evidence of left ventricular systolic dysfunction.The noticeable restrictive ventilation and diffusion impairment on PFT would lead to the consideration of group 3 PH, which needs to be confirmed by further lung imaging otherwise.Chronic thromboembolic pulmonary hypertension (CTEPH, the primary part of group 4 PH)could be ruled out due to normal D-dimer, but there has not been an appropriate exclusion of other uncommon obstructions of the pulmonary artery.No sufficient evidence of group 1/1’ or group 5 PH was established thus far.Based on the discussion above, further investigations and analyses are needed to figure out a specific group of PH.
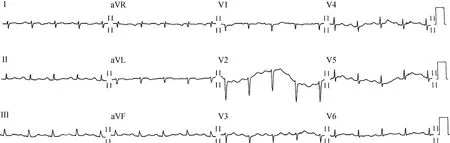
Figure 2.Electrocardiogram (ECG) on admission.The ECG on admission was significant for sinus tachycardia and low QRS voltage without ST-T abnormalities in this patient.
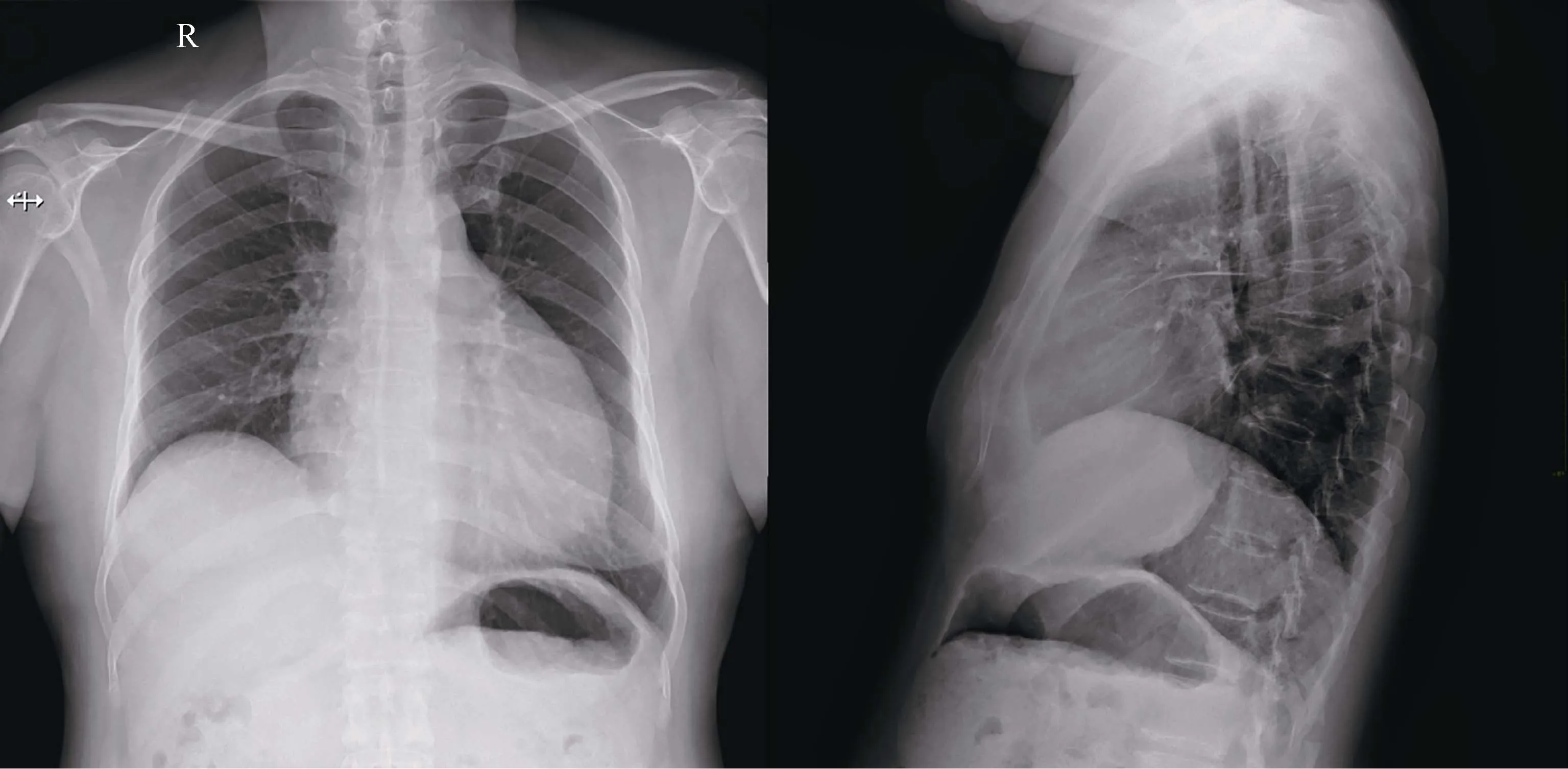
Figure 3.Chest radiograph.Chest radiograph of this patient showing cardiac silhouette enlargement without lung field lesions or pleural effusion.
Taking PE and PH into a full picture, while PE can be seen in chronic PH as a resultant indicator of right ventricle dysfunction, there was no decent evidence of right heart overload on this patient.Moreover, several intriguing hints(facial swelling and superficial vascular distension) implied superior vena cava (SVC) syndrome that was often seen in malignant tumor cases; thus, a specific subset of group 4 PH due to tumor obstruction was highly suspected.We immediately repeated echocardiogram and found a suspicious mass at the right atrioventricular groove (Figure 4A).The chest enhanced CT scan was then ordered and confirmed a mediastinal mass already invaded the right pulmonary artery(PA), SVC and, pericardium (Figure 4B & C).On the other hand, no explicit lung diseases were disclosed.Given the malignant PE, the diagnostic pericardiocentesis was performed according to PE triage algorithm.[2]The biochemical,immunologic, and bacteriologic studies and polymerase chain reaction of drained fluid ruled out purulent and tuberculous causes, while pathology studies revealed some atypical cells (Figure 5).All things considered, the diagnosis of a mediastinal mass invading PA, SVC, and pericardium and causing obstructive PH, SVC syndrome, and large PE was established.Besides giving symptom-relieving diuretics and pericardial fluid drainage, oncology was consulted.The patient was soon referred to oncology and wasfinally diagnosed with thymic carcinoma (nonkeratinizing squamous cell carcinoma) through transthoracic needle biopsy.Chemotherapy was initiated later.
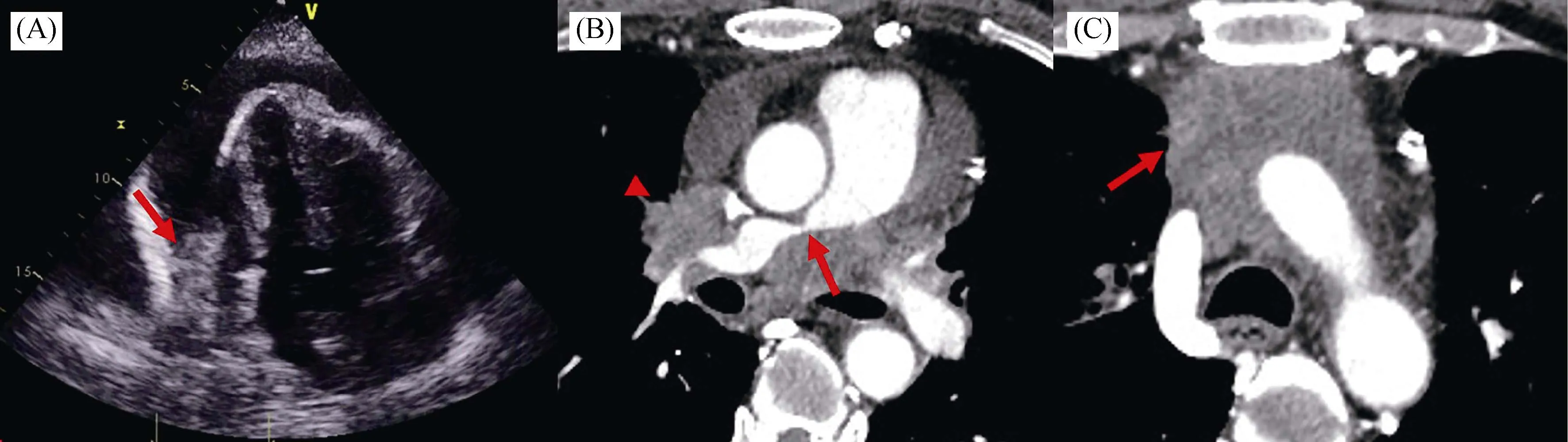
Figure 4.Repeated echocardiogram and enhanced chest computed tomography (CT).(A): Repeated echocardiogram showing a suspected mass (arrow) at the right atrioventricular groove; (B-C): enhanced chest CT showing a mediastinal mass already invading the right pulmonary artery (B, arrow), superior vena cava (B, arrowhead), and pericardium (C, arrow).

Figure 5.Pathological findings of drained pericardial fluid.Histopathology (A) and cytopathology (B) of drained pericardial fluid showing atypical cells (arrow).
According to current retrospective studies, the most common cause of the pericardial effusion is malignancy.[3-7]The direct cancer involvement of the pericardium was seen in 7%-44% of pericardial effusions.[8]However, the etiological spectrum may vary among geographical regions and medical centers.A 149-patient series undergoing pericardiocentesis in Singapore revealed malignancy, followed by iatrogenic postsurgical complications were the most common causes of pericardial effusions.[7]In the Western Cape Province of South Africa, tuberculous pericarditis (confounded by HIV co-infection or not) was reported to be the most common cause of pericardial effusion (69.5%, 162/233).[9]Uremic and tuberculous pericarditis were the most common causes of pericardial disease in southern Saudi Arabia,[10]while a study in the western coast of Saudi Arabia revealed malignancy the leading cause (48.7%, 19/39),followed by infection and uremia.[11]The differences above might due to variations in economic development, disease spectrum, and health care availability.The varied etiologies of the pericardial effusion may also differ in prognosis,among which the malignant pericardial effusion is associated with poor prognosis.[12-14]Although malignant pericardial effusion is the most common, physicians still need to bear a broad differential spectrum.For the patient in this case, who had large pericardial effusion with possible exposure to TB in China, infectious diseases, including TB should be ruled out first.Pulmonary arterial hypertension and chronic heart disease are also listed as common etiologies of pericardial diseases.[2]The patient presented with concurrent pericardial effusion and PH, with which we need to consider right heart dysfunction.However, with no evidence of right heart overload, but with superior vena cava syndrome, we should seek other etiologies and malignancy was finally established.
Likewise, pericardial effusion is a common complication of malignancy and might even be the initial presentation,like this case.The incidence of pericardial involvement in malignant tumors ranged from 5% to 20%.[15-17]A study in 2020 screened and reviewed 43 articles on malignant pericardial effusion in the past 10 years and showed lung cancer to be the most common tumor responsible for pericardial effusion in both men and women.Other common tumor types include breast, hematological and gastrointestinal malignancy.The mean survival time for patients with malignant pericardial effusion rarely exceeded 12 months.[8]Thymic carcinoma, in this case, is an unusual tumor type for pericardial effusion.A 6-year single-center study from the US in 2020 showed the most common malignant origin resulting in pericardial effusion was breast (37.7%, 20/57),followed by lung (29.8%, 17/57) and hematologic (10.5%,6/57) in a 57-patient series with malignant pericardial effusion.Only one patient (1.8%, 1/57) had thymic carcinoma.[13]Another 6-year Italian single-center study on malignant pericardial effusion in 2019 enrolled 29 patients, among which 21 patients had lung cancer, five with breast cancer,and three with other tumors, including one thymus and two larynx tumor.[12]Pericardial fluid analysis is recommended when there is a clinical suspicion of purulent, tuberculous,or neoplastic etiology.[2]Moreover, one of the most consistent factors related to poor prognosis is positive cytology of the pericardial effusion.[8]In this case, the diagnostic pericardiocentesis was prompted when malignancy was highly suspected.
Malignancy is also the most common cause (up to 90%)of superior vena cava (SVC) syndrome in the contemporary setting.Among them, non-small cell lung cancer is the most common primary tumor, accounting for 50% of cases, followed by small cell lung cancer (SCLC, 25%) and non-Hodgkin lymphoma (NHL, 10%).Lung cancer and NHL account for approximately 95% of all malignant SVC syndrome cases.[18-20]
Here we reported a male patient with pulmonary hypertension concurrent with pericardial effusion and SVC syndrome who was finally diagnosed with thymic carcinoma invading the pulmonary artery, SVC, and pericardium.This case demonstrates a tumor-caused unusual group 4 pulmonary hypertension and reminds us that pulmonary hypertension without right heart overload but with incompatible pericardial effusion and SVC syndrome should lead to consideration of specific obstructive causes.Malignancy is responsible for most pericardial effusion and SVC syndrome,lung, breast, and hematological malignancy being the most common.Thymic carcinoma, in this case, is an unusual tumor for pericardial effusion and SVC syndrome.
Acknowledgements
The authors report no conflict of interest.Written informed consent was obtained from the patient for publication of the case and any accompanying images.
 Journal of Geriatric Cardiology2020年11期
Journal of Geriatric Cardiology2020年11期
- Journal of Geriatric Cardiology的其它文章
- ARNI and SGLT2i: a promising association to be used with caution
- Geriatric issues in patients with or being considered for implanted cardiac rhythm devices: a case-based review
- Medical management of symptomatic severe aortic stenosis in patients non-eligible for transcatheter aortic valve implantation
- Ablation strategies for arrhythmogenic right ventricular cardiomyopathy:a systematic review and meta-analysis
- Gender differences in clinical outcomes of acute myocardial infarction undergoing percutaneous coronary intervention: insights from the KAMIR-NIH Registry
- Plasma levels of Elabela are associated with coronary angiographic severity in patients with acute coronary syndrome
