Role of nutritional status and nutritional support in outcome of hepatitis B virus-associated acute-onchronic liver failure
Yue Chang, Qin-Yu Liu, Qing Zhang, Ya-Mei Rong, Cheng-Zhen Lu, Hai Li
Abstract
Key words: Liver failure; Hepatitis B; Nutrition therapy; Intestinal host defense; Cytokine; Prognosis
INTRODUCTION
Liver failure, in which liver cell function cannot meet the physiological needs of the human body, is a clinically common serious liver disease[1]. Globally, hepatitis B virus (HBV)-related liver failure is an important issue. Due to the high level of HBV infection in China, HBV-associated acute-on-chronic liver failure (HBV-ACLF) is a major burden as well[2]. The condition of patients with HBV-ACLF results in impaired nutrient synthesis and absorption, due to inadequate nutritional intake, abnormal liver structure, and functional failure[3]. At the same time, malnutrition will further aggravate the liver injury, affecting the quality of life and survival time[4].At present, malnutrition in most patients with liver disease progresses slowly, so it is particularly important to screen for nutritional risk in the early stage of the disease[5,6]. Therefore, more attention should be paid to nutritional risk screening and corresponding nutritional support[7]. However, in the past, the treatment of acute-onchronic liver failure relied too much on drugs and artificial liver support[8], ignoring basic nutritional support. In addition, the data on nutritional risk screening and nutritional status assessment in patients with liver failure is still insufficient.
The gastrointestinal tract is not only involved in digestion and absorption but also has an important barrier function[9]. In the gastrointestinal tract, there is the largest pool of bacteria in the human body. Intestinal flora plays an important role in immunity and biological antagonism to the host[10]. The gastrointestinal tract is the central organ of the body to produce a stress response, and for the liver, it is the first organ to be impacted after the intestinal barrier is damaged[11]. The destruction of the intestinal barrier can be seen in the animal model of liver failure, indicating that liver injury will further aggravate the damage to the intestinal function[12]. In addition, intestinal endotoxin and cytokines are important causes of liver failure, that is, these findings indirectly proved that the damage of the intestinal barrier has a serious effect on the liver[13,14]. Thus far, the effect of improving nutritional status on gastrointestinal barrier function in patients with acute-on-chronic liver failure has not been determined.
As the nutritional status of HBV-ACLF patients has been poorly studied, we investigated the nutritional risk and nutritional status of HBV-ACLF patients and evaluated the impact of nutritional support on the gastrointestinal barrier and 28-d mortality in patients with HBV-ACLF.
MATERIALS AND METHODS
Patients
This study consisted of two parts. For Study 1, the changes in serum markers and cytokines related to nutritional risk, nutritional status, and intestinal barrier function were compared between HBV-ACLF patients and patients with compensated cirrhosis (control group). A total of 234 HBV-ACLF patients were recruited and admitted to Characteristic Medical Center of People's Armed Police Force, Tianjin Xiqing Hospital, and Tianjin Second People's Hospital from January 2016 to December 2018. All participants provided written informed consent in line with the Declaration of Helsinki. This study was registered with ClinicalTrials.gov (NCT03108794 and NCT01938820).
The inclusion criteria were: (1) HBV-ACLF diagnosis and staging according to the consensus recommendations of the Asian-Pacific Association for the Study of the Liver (APASL): Jaundice (serum bilirubin ≥ 5 mg/dL) and coagulopathy [international normalized ratio (INR) ≥ 1.5 or prothrombin activity (PTA) < 40%] complicated within four week by ascites and/or encephalopathy[15]; and (2) Hospitalization for more than 48 h. Patients with hepatocellular carcinoma or other tumors; who had undergone hepatectomy; combined with other liver diseases and unconscious; and who could not cooperate with the investigation were excluded from this study.
Propensity score matching was used to screen patients with HBV-related compensated liver cirrhosis at a ratio of 1:1. The matched patients had the same sex and age as the HBV-ACLF group. None of them were complicated with other hepatitis virus infections.
For Study 2, HBV-ACLF patients treated in the same hospital from January 2014 to December 2016 were collected retrospectively as a control group. None of these patients had undergone nutritional risk screening or nutritional status assessment. The 28-d survival of the control group was compared with HBV-ACLF patients in Study 1. The flowchart of this study is shown in Figure 1.
Demographic and clinical characteristics
The venous blood of the subjects included in Study 1 was collected at admission for laboratory tests. Platelet (PLT) was tested using an automatic blood analyzer (Mindray BC-6800, China). The biochemical indexes alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), Total bilirubin level (TBIL), gamma-glutamyl transferase, and creatinine (Cr) were detected by biochemical analysis (Mindray BS-200, China). The coagulation function indicators prothrombin time (PT), INR, and PTA were detected with an automatic coagulation analyzer (SysmexCS5100, Japan). HBVDNA was detected by fluorescence quantitative polymerase chain reaction (ABI 7500FAST, Life Technologies, United states). The model for end-stage liver disease (MELD) score was calculated as follows:R= 9.6 ×loge (Cr, mg/dL)+ 3.8 ×loge (TBIL, mg/dL)+ 11.2 ×loge (INR)+6.4[16]. All these data were also collected or calculated for the non-nutritional support group in Study 2.
Nutritional risk screening and nutritional status assessment
All nutritional evaluation and marker tests were performed at the time of diagnosis and 2 wk and 4 wk after treatment.
Nutritional risk screening (NRS) 2002 was used to assess the nutritional status, severity, and age of the patients. If the three scores added up to more than or equal to 3 points, the patient was considered to have a nutritional risk[17].
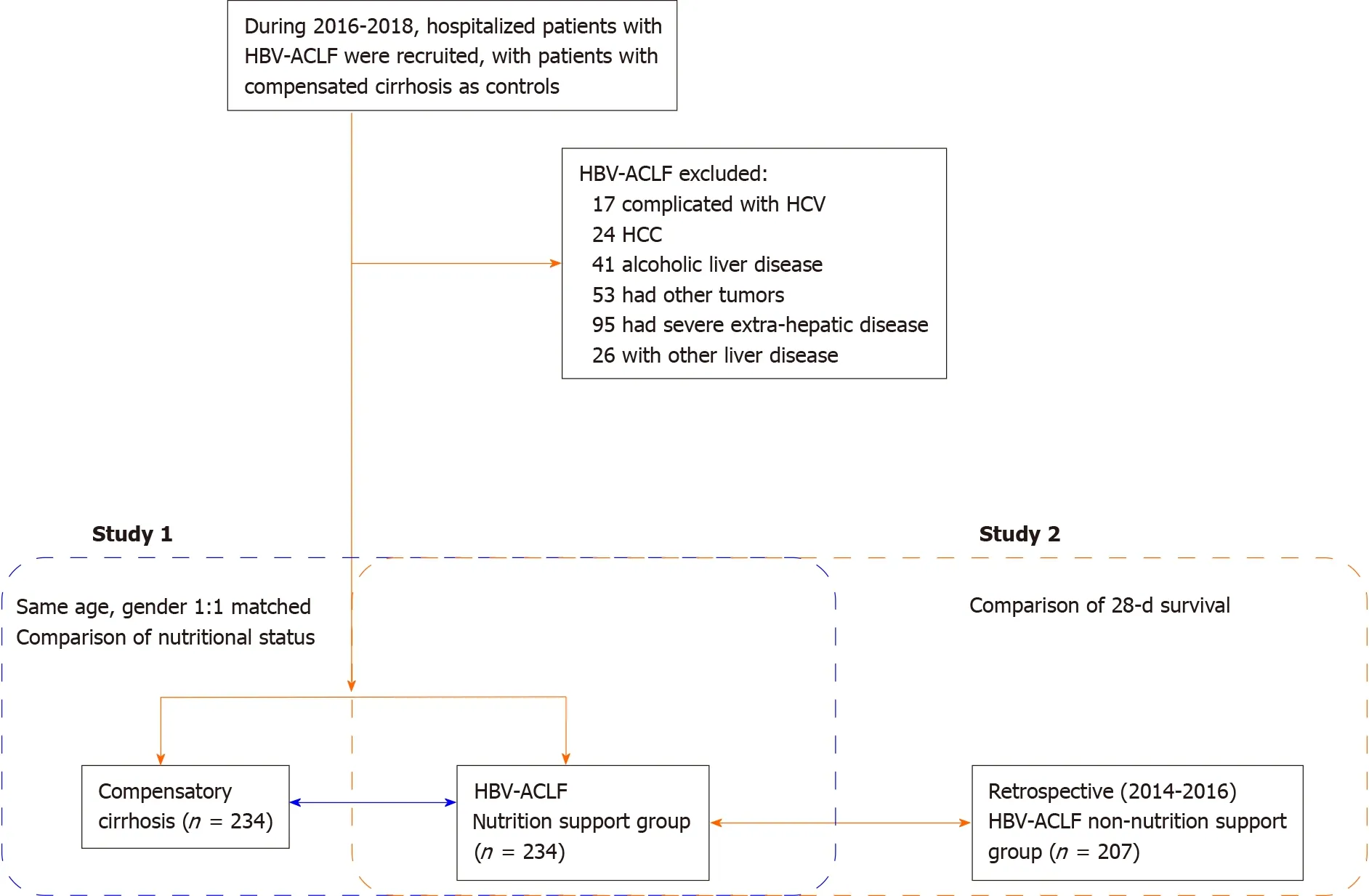
Figure 1 Overview of this study. HBV-ACLF: Hepatitis B virus-associated acute-on-chronic liver failure; HCV: Hepatitis C virus infection; HCC: Hepatocellular carcinoma.
According to the improved subjective global assessment (SGA) score sheet, the nutritional status of the patients was evaluated by specialized medical personnel. Muscle consumption was defined when fatigue, muscle soreness, and elevated blood creatine kinase were present, and the thickness of the triceps skinfold (TSF) was evaluated. Each indicator consisted of A, B, and C corresponding to 1, 2, and 3 points, respectively. Finally, the patient's total SGA score was calculated, which consisted of 7 to 21 points. TSF was measured by two professional trainers using a fixed soft ruler and fold meter. The right hand was measured with a sebum thickness meter three times.
Human body composition was observed in the HBV-ACLF patients and the control group. According to the instructions, the body composition analyzer (Inbody 3.0, BiospaceCo., Ltd., South Korea) was used to determine the protein, fat, and skeletal muscle contents using the multifrequency bioelectrical impedance method.
Biomarkers of the gastrointestinal barrier
Enzyme linked immunosorbent assay (ELISA) was used to detect secretory immunoglobulin A (sIgA) in feces (Abcam, Cambridge, UK). The coccus-bacillus ratio was measured by direct smear in the laboratory department.
Biomarkers of inflammation
ELISA was used to detect D-lactate (Becton, Dickinson and Company, United states) and endotoxin (Abcam, Cambridge, UK) in serum at the time of diagnosis and 2 wk and 4 wk after treatment.
Centralized testing for cytokines was performed by Jingmei Beijing Central Laboratory. Serum interleukin-1 (IL-1), interleukin-10 (IL-10), and tumor necrosis factor alpha (TNF-α) were detected with a Luminex-100 flow fluorescence microsphere detector (Luminex, Austin, TX, United states) according to the detection procedure. The levels of IL-1, IL-10, and TNF-α were detected using a human cytokine commercial kit (Becton, Dickinson and Company, United states).
Nutritional intervention
All HBV-ACLF patients had a light diet, licorice preparation, glutathione, albumin, fresh plasma, and other drugs to protect liver cells. Artificial extracorporeal liver support was considered in critical patients[15].
In Study 1, HBV-ACLF patients were given individualized nutritional support by the nutrition support team. Nutritional support was initiated promptly for malnourished HBV-ACLF patients. Patients without malnutrition were provided with enteral nutrition (preferentially) and/or parenteral nutrition if they were viewed as unlikely to resume normal nutrition within the next 5-7 d. The nutritional composition and calories in the intestine of the patients were calculated by using the principle of small multi-meals and referring to the nutritional composition table. HBV-ACLF patients were encouraged to receive late evening oral nutritional supplementation in dietary habit. During hospitalization, total energy intake for HBV-ACLF patients was not lower than 35 kcal/(kg·d), while protein intake was not lower than 1.2-1.5 g/(kg·d)[18]. Total nutrition, carbohydrate, protein, and lipid were collected and calculated daily by 24-h dietary recalls[19]. Nutrition was supplemented by fresh plasma, albumin, glucose, and medium/long-chain fat emulsion on the second day of deficiency, while water-soluble and fat-soluble vitamins were supplemented to maintain the water-electrolyte balance. Branched-chain amino acids were supplied when adequate nitrogen intake was not achieved. If necessary, parenteral nutrition and calories were combined. The nutritional intake was re-assessed every day. According to the EASL guidelines, sodium intake was restricted to 80 mmol·d in patients with ascites[7]. Vitamin D was supplemented in patients with a vitamin D level < 20 ng/mL until serum 25 (OH) D > 30 ng/mL[18].
In Study 2, the HBV-ACLF non-nutritional support group was also treated with licorice preparation, glutathione, albumin, and fresh plasma to protect liver cells, but no individualized total intake calculation and nutritional support were performed. Only albumin and fresh plasma were given intermittently, and no long-chain fat emulsion was used.
Statistical analysis
Statistical analyses were performed using Student’st-test, Welch’st-test, Mann-Whitney U test, chi-square test, or ANOVA, as appropriate. A receiver operating characteristic (ROC) curve was constructed using different markers to predict death in patients with HBV-ACLF. Youden’s index was used to determine the best cut-off value. The prognosis of patients with HBV-ACLF in the nutritional support group and the non-nutritional support group was analyzed using the Kaplan-Meier method. All statistical analyses were performed using Statistical Product and Service Solutions version 22.0 (IBM Corp., New York State, United states) and GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, United states).P< 0.05 was accepted as statistically significant.
RESULTS
Nutritional status of the two groups
According to the NRS 2002 assessment, the nutritional risk of the HBV-ACLF group at admission was significantly higher than that of the control group (214/234vs158/234,P< 0.001) (Figure 2A). The average energy intake [18.1 ± 5.0 kcal / (kg·d)] was significantly lower in the HBV-ACLF group than in the control group [36.6 ± 4.8 kcal/(kg·d)] (P< 0.001; Figure 2B). According to the results of the body composition analysis, the skeletal muscle (25.3 ± 2.4 kgvs26.8 ± 2.6 kg), protein (5.9 ± 0.9 kgvs7.8 ± 1.12 kg), and body fat (16.1 ± 1.3 kgvs21.4 ± 3.1 kg) levels in the HBV-ACLF group were significantly lower than those of the control group, while the decrease in skeletal muscle and fat content was more pronounced (Figure 2C). At hospital admission, the intake of calories in patients with HBV-ACLF (13.9 ± 1.5 kgvs26.7 ± 3.2 kg), protein (1.7 ± 0.3 kgvs3.0 ± 0.3 kg), and fat (2.8 ± 0.5 kgvs7.4 ± 1.2 kg) were lower than those of the control group, and the deficiency of fat intake was more obvious (Figure 2D). The baseline characteristics of the HBV-ACLF group and the control group are shown in Table 1.
The SGA scores in the HBV-ACLF group were significantly higher than those in the control group (P< 0.001), while the TSF in the HBV-ACLF group was lower (P< 0.001), indicating that there was excessive fat consumption in patients with HBVACLF (Figure 3).

Table 1 Baseline characteristics of compensated cirrhosis and hepatitis B virus-associated acute-on-chronic liver failure patients enrolled in Study 1, n (%)
Difference in biomarkers of the gastrointestinal barrier and inflammation
Compared with the control group, the coccus-bacillus ratio in feces, sIgA in stool, and serum D-lactate were significantly higher in the HBV-ACLF group (Figure 4A-C), which indicated that the intestinal barrier was impaired in HBV-ACLF patients.
For biomarkers of inflammation, the levels of serum endotoxin, IL-1, IL-10, and TNF-α in the HBV-ACLF group were significantly higher than those of the control group (Figure 4D).
Nutritional support and prognosis of HBV associated acute-on-chronic liver failure
According to the survival conditions of the patients at 2 d, the HBV-ACLF patients were divided into a survival group and a death group. The survival group had a lower nutritional risk (SAG scores) (P< 0.001), lower serum D-lactate (P= 0.007), and lower levels of cytokines (P< 0.05) at admission (Table 2). The differences in biomarkers were used to establish an ROC for predicting 28 d mortality. IL-10 had the best result in predicting the death of HBV-ACLF patients (Figure 5A). The cutoff value was 80.5 µg/mL. Other detailed results of ROC are shown in Supplemental Table 1.
After individualized nutritional support for HBV-ACLF patients, the TSF of survivors did not continue to decrease (P= 0.673). By week 4, the SGA score was significantly improved (P< 0.001). There was no significant change in the coccusbacillus ratio, but the levels of serum D-lactate (P< 0.001) and fecal sIgA (P< 0.001) decreased gradually. Serum endotoxin (P= 0.021), TNF-α (P< 0.001), and IL-1 (P< 0.001) decreased gradually as well, but IL-10 had no significant change (P= 0.402) (Figure 5B).
In Study 2, the non-nutritional support group had similar age, liver function, renal function, complications, and other clinical characteristics to the nutritional support group (P< 0.05; Table 3). A total of 91 patients (38.9 %) patients died within 28 d in the HBV-ACLF nutritional support group and 102 (49.3%) died in the non-nutritionalsupport group. There was a difference in 28-d survival between the two groups (χ2= 5.812,P= 0.016; Figure 6).

Table 2 Characteristics of nutritional and gastrointestinal barrier between survival group and death group in hepatitis B virusassociated acute-on-chronic liver failure patients at admission, n (%)
DISCUSSION
This study comprehensively assessed the nutritional status, nutritional risk, and gastrointestinal barrier function in patients with HBV-ACLF. We found that improving the nutritional status of HBV-ACLF patients can improve gastrointestinal barrier function and associate with a better prognosis.
We found that most patients with HBV-ACLF had inadequate nutrient intake and malnutrition at admission or diagnosis. Clinical nutritionists believe that patients with liver cirrhosis should be given nutritional treatment when there is a nutritional risk[20,21]. Thus, the determination of the nutritional status and various nutritional components of the patients at the time of diagnosis or admission is necessary. On this basis, an individualized nutritional support treatment plan has been developed for each patient to meet the needs of the human body and promote the recovery from the disease[7,18].
We further verified malnutrition in patients with HBV-ACLF by comparing TSF and SGA score. Although anthropometric indexes such as TSF have limitations, they are not affected by ascitic or other factors. Due to their ease of use, anthropometric indexes are used as the evaluation criteria for nutritional status in many studies of malnutrition in liver diseases[22]. However, the time lag is certain regardless of what type of physiologic parameters. In contrast, SGA is a comprehensive index to reflect the nutritional status, which is widely used in the field of liver disease and recommended by the European Association of Parenteral Nutrition for comprehensive nutritional assessment[18]. In addition, Kyleet al[23]screened 995 patients and found that NRS 2002 had higher sensitivity and specificity. NRS 2002 and SGA scores were both used in our study.
The presence of gastrointestinal barrier impairment in patients with HBV-ACLF is also one of our main findings. Gastrointestinal function and liver function are closely related. Gastrointestinal dysfunction can occur in patients with liver failure in the early stage. At the same time, portal hypertensive gastropathy, portal hypertensive enteropathy, increased abdominal pressure caused by ascites, and intestinal flora disorders, which are the results of liver failure, are also the causes of malnutrition in patients[24]. Finally, a vicious circle is formed, which further affects the function of the gastrointestinal barrier.
Moreover, this study confirmed that the level of serum D-lactate in patients with chronic liver failure was significantly increased. When the barrier function of thegastrointestinal tract is impaired, intestinal bacteria show excess propagation or intestinal flora translocation occurs, and the production and absorption of endotoxin increase, which further leads to damage to the liver and other organs[25]. Endotoxin has a variety of physiological and pathological effects, such as causing a febrile reaction, activating the complement system, and causing local or systemic Shwartzman reactions and disseminated intravascular coagulation[26]. In addition to the direct effect of endotoxin, the toxic mechanism can also induce the cascade release of cytokines, strongly increase tumor necrosis factor, result in an Th1/Th2 imbalance, and further aggravate the disease and cause death[27]. D-lactate is the product of bacterial metabolism and lysis, which can be produced by a variety of intestinal bacteria[28]. When intestinal permeability increases abnormally, high levels of D-lactate produced by intestinal bacteria enter the circulation through the intestine. The liver cannot metabolize D-lactate, so the level of serum D-lactate can reflect the extent of the impaired gastrointestinal barrier[29].

Table 3 Baseline characteristics of hepatitis B virus-associated acute-on-chronic liver failure patients enrolled in Study 2, n (%)
In addition to the intestinal mechanical barrier and biological barrier, there are many plasma cells in the lamina propria of the intestinal mucosa, which mainly secrete sIgA. Intestinal sIgA plays an important role in maintaining intestinal immune surveillance, clearing bacteria, and preventing bacteria from adhering to the mucosa[30]. The detection of intestinal mucosa sIgA content requires intestinal mucosa biopsies for radioimmunity, which is not applicable for patients with HBV-ACLF[31]. Thus, sIgA was measured in the feces. In Study 1, the TSF of the surviving patients did not continue to decrease, indicating that the nutritional status of the patients did not deteriorate further. The further decreases of biomarkers of impaired gastrointestinal function and inflammation in survival were also found after nutritional support. Furthermore, we found that IL-10 is a potential predictor of 28-d death.
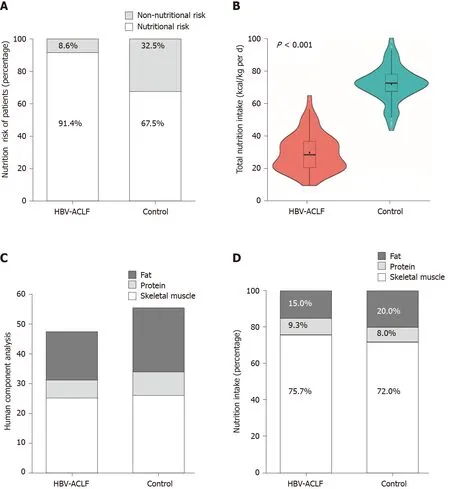
Figure 2 Comparison of nutritional status between the hepatitis B virus-associated acute-on-chronic liver failure group and the control group. A: According to the Nutritional risk screening 2002 assessment, 91.4% of hepatitis B virus-associated acute-on-chronic liver failure (HBV-ACLF) patients and 67.5% of compensated cirrhosis patients had a nutritional risk; B: The energy intake in HBV-ACLF patients was lower than that in compensated cirrhosis patients (P < 0.001); C: The body composition analysis in HBV-ACLF and control group. Significant decreases in skeletal muscle and fat in HBV-ACLF patients were observed; D: The intake of calories, protein, and fat in HBV-ACLF were lower than those in the control group at hospital admission (P < 0.001). HBV-ACLF: Hepatitis B virusassociated acute-on-chronic liver failure.
In Study 2, a retrospective cohort, we demonstrated that individualized and dynamic nutritional support reduced 28-d mortality. In practice, we found that intravenous infusion of albumin and medium/long-chain fat emulsion can be well tolerated in HBV-ACLF patients. Protein and fat are the dominant source of energy for liver regeneration and hepatocyte turnover. So protein and fat should not be overly restricted in the diet. The administration of parenteral nutrition, including fat emulsion, did not worsen liver function. However, Pierceet al[32]noted that too much fat increases the burden on the liver in mice. This change leads to or aggravates jaundice, increases transaminase and blood glucose, and decreases immune function. Therefore, the supplemental dose of fat should be limited to < 1 g/(kg·d)[33].
There are some limitations and shortcomings in this study: (1) There are some differences in the definition of acute-on-chronic liver failure among the American Association for the Study of the Liver Diseases, the European Association for the Study of the Liver, and APASL[15]. The subjects were selected according to the consensus recommendations of the APASL; (2) The non-nutritional support group with liver failure was from a retrospective cohort, which may not reflect the situation of all hospitalized patients at the same time; and (3) Some biomarker tests for predicting death in HBV-ACLF patients were based on ELISA, and this result may be affected by the manufacturers of test reagents and measurement errors.
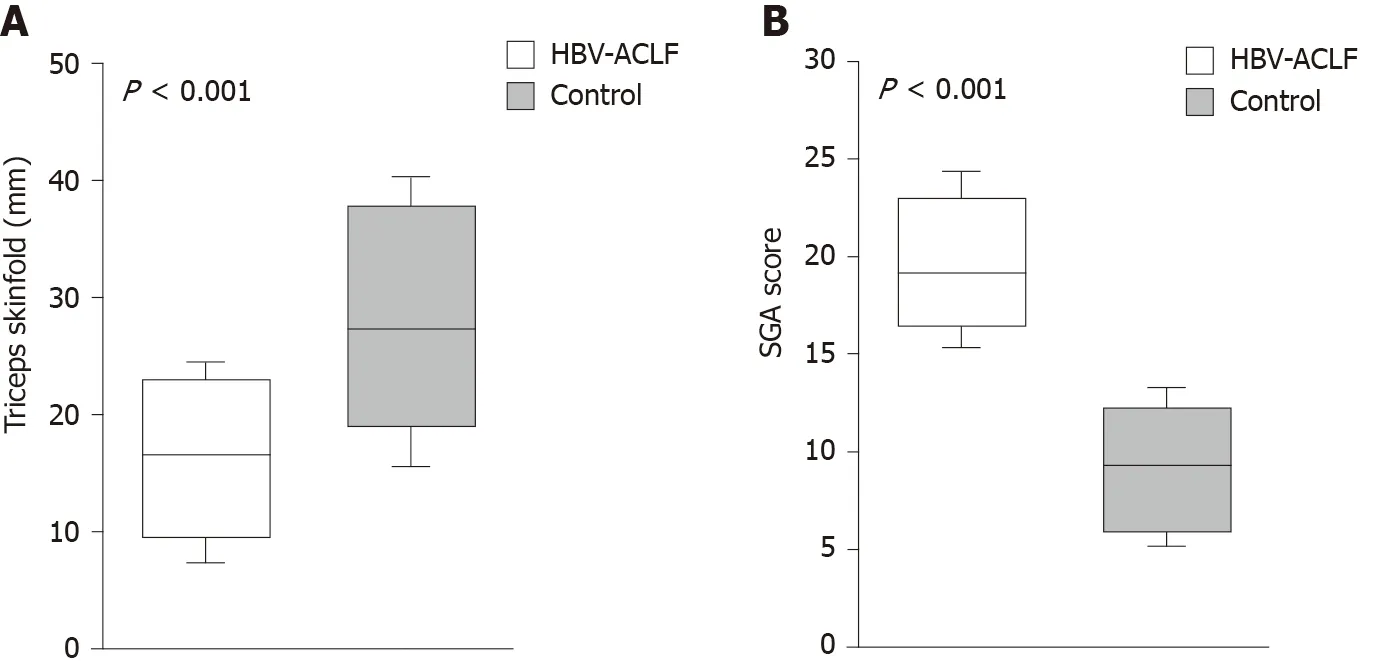
Figure 3 Difference in triceps skinfold thickness and subjective global assessment score. A: Patients with hepatitis B virus-associated acute-onchronic liver failure (HBV-ACLF) had a higher subjective global assessment score; B: Patients with HBV-ACLF had a lower triceps skinfold thickness. SGA: Subjective global assessment; HBV-ACLF: Hepatitis B virus-associated acute-on-chronic liver failure.
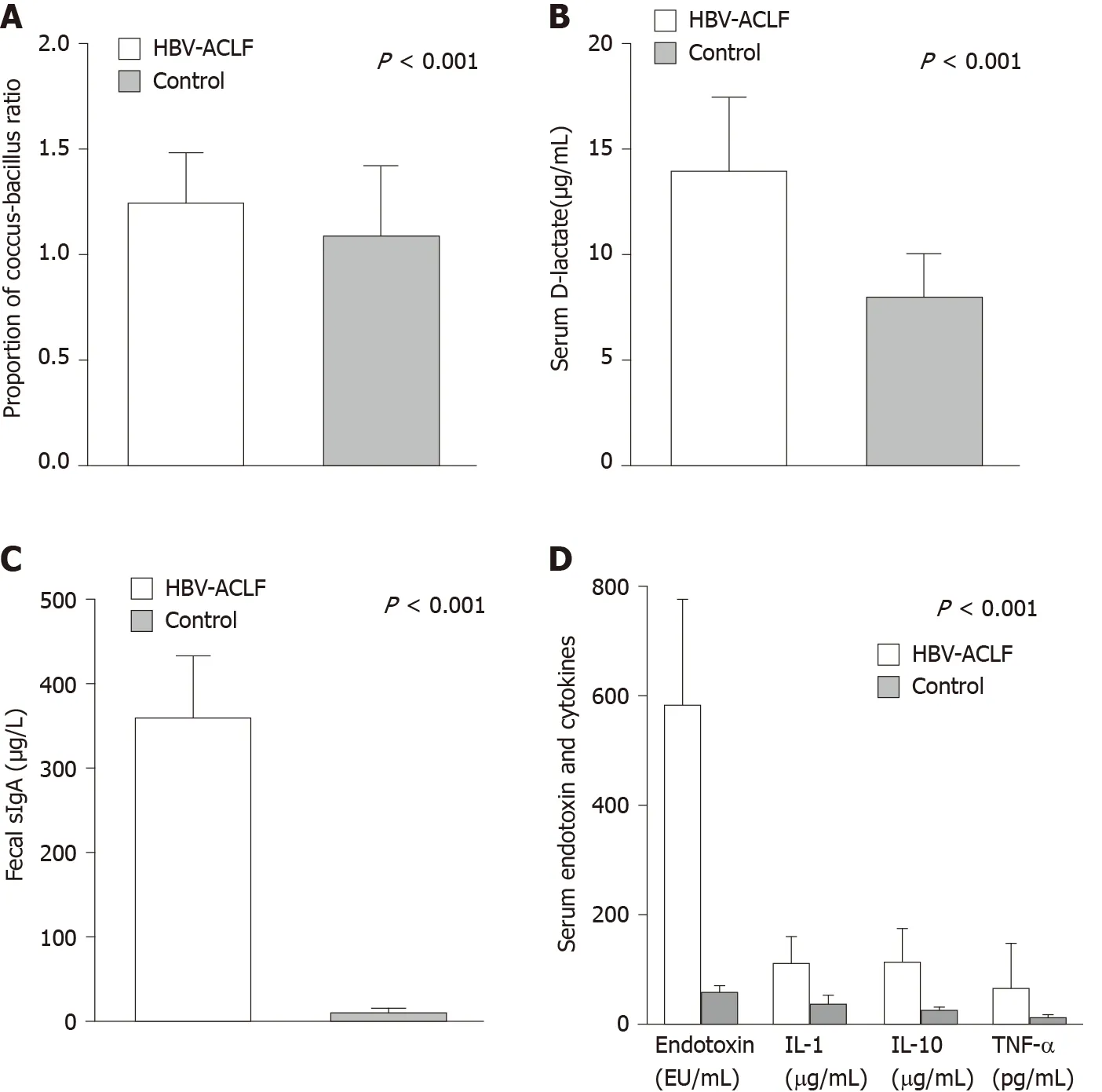
Figure 4 Comparison of biomarkers of the gastrointestinal barrier and inflammation. A: The coccus-bacillus ratio in feces in the hepatitis B virusassociated acute-on-chronic liver failure (HBV-ACLF) group (1.2 ± 0.2) was higher than that of the control group (1.1 ± 0.3; P < 0.001); B: The level of serum Dlactate in the HBV-ACLF group (358.1 ± 74.6 µg/mL) was higher than that of the control group (8.0 ± 2.1 µg/mL; P < 0.001); C: The level of sIgA in the HBV-ACLF group (358.1 ± 74.6 mg/L) was higher than that of the control group (12.5 ± 4.2) mg/L; P < 0.001); D: The levels of endotoxin, IL-1, IL-10, and TNF-α in HBV-ACLF patients were higher than those in the control group (P < 0.001). HBV-ACLF: Hepatitis B virus-associated acute-on-chronic liver failure; IL-1: Interleukin-1; IL-10: Interleukin-10; TNF-α: Tumor necrosis factor alpha.
Overall, we performed the nutritional assessment of HBV-ACLF patients for the first time and found that their intestinal peristaltic ability decreased and the intestinal barrier function was damaged. In addition, individualized and dynamic nutritional support was associated with a better prognosis of 28-d mortality in HBV-ACLF patients and can improve the nutritional status of survivors.

Figure 5 Role of biomarkers in hepatitis B virus-associated acute-on-chronic liver failure patients. A: Value of biomarkers in predicting death in HBV-ACLF patients. The area under the receiver operating characteristic curve of IL-10 was the largest (area under the curve = 0.907, P < 0.001); B: Trends of biomarkers in survivors. HBV-ACLF: Hepatitis B virus-associated acute-on-chronic liver failure; SGA: Subjective global assessment; SIgA: Secretory immunoglobulin A; IL-1: Interleukin-1; IL-10: Interleukin-10; TNF-α: Tumor necrosis factor alpha.
ARTICLE HIGHLIGHTS

Research objectives
To investigate the nutritional risk and nutritional status of HBV-ACLF patients and evaluated the impact of nutritional support on the gastrointestinal barrier and 28-d mortality.
Research methods
The nutritional risk screening assessment, baseline characteristics, and biomarkers of the gastrointestinal barrier of patients with HBV-ACLF (n= 234) and patients with compensatory cirrhosis (n= 234) were analyzed. The 28-d survival of the nutritional support group (n= 234) and non-nutritional support group (n= 207) was compared.
Research results
The nutritional risk of the HBV-ACLF patients was significantly higher than that of the control group. The coccus-bacillus ratio, secretory immunoglobulin A, and serum Dlactate were significantly increased in HBV-ACLF patients. Interleukin-10 may be a potential predictor of death in HBV-ACLF patients. The 28-d survival of the nutritional support group was better than that of the non-nutritional support group (P= 0.016).
Research conclusions
This is the first study to investigate the nutritional risk and status of HBV-ACLF patients and evaluated the impact of nutritional support. We found that nutritional support initiated promptly for malnourished HBV-ACLF patients was associated with a better prognosis of 28-d mortality and improved the status of the gastrointestinal barrier.
Research perspectives
More attention should be paid to nutritional risk screening and corresponding nutritional support for HBV-ACLF patients. Nutritional support initiated promptly for malnourished HBV-ACLF patients is associated with a better prognosis.
ACKNOWLEDGEMENTS
We kindly thank all of the participants in this study.
 World Journal of Gastroenterology2020年29期
World Journal of Gastroenterology2020年29期
- World Journal of Gastroenterology的其它文章
- Endoscopic management of gastrointestinal leaks and fistulae: What option do we have?
- Watch and wait approach in rectal cancer: Current controversies and future directions
- Evaluation of intrahepatic manifestation and distant extrahepatic disease in alveolar echinococcosis
- Multivariate predictive model for asymptomatic spontaneous bacterial peritonitis in patients with liver cirrhosis
- Clinicopathological characteristics and surgical outcomes of sarcomatoid hepatocellular carcinoma
- Patients' perspectives on smoking and inflammatory bowel disease: An online survey in collaboration with European Federation of Crohn's and Ulcerative Colitis Associations
