Watch and wait approach in rectal cancer: Current controversies and future directions
Fernando Lopez-Campos, Margarita Martin-Martin, Roberto Fornell-Perez, Juan Carlos Garcia-Perez, Javier Die-Trill, Raquel Fuentes-Mateos, Sergio Lopez-Duran, Jose Dominguez-Rullan, Reyes Ferreiro, Alejandro Riquelme-Oliveira, Asuncion Hervas-Moron, Felipe Counago
Abstract According to the main international clinical guidelines, the recommended treatment for locally-advanced rectal cancer is neoadjuvant chemoradiotherapy followed by surgery. However, doubts have been raised about the appropriate definition of clinical complete response (cCR) after neoadjuvant therapy and the role of surgery in patients who achieve a cCR. Surgical resection is associated with significant morbidity and decreased quality of life (QoL), which is especially relevant given the favourable prognosis in this patient subset. Accordingly, there has been a growing interest in alternative approaches with less morbidity, including the organ-preserving watch and wait strategy, in which surgery is omitted in patients who have achieved a cCR. These patients are managed with a specific follow-up protocol to ensure adequate cancer control, including the early identification of recurrent disease. However, there are several open questions about this strategy, including patient selection, the clinical and radiological criteria to accurately determine cCR, the duration of neoadjuvant treatment, the role of dose intensification (chemotherapy and/or radiotherapy), optimal followup protocols, and the future perspectives of this approach. In the present review, we summarize the available evidence on the watch and wait strategy in this clinical scenario, including ongoing clinical trials, QoL in these patients, and the controversies surrounding this treatment approach.
Key words: Watch and wait; Rectal cancer; Clinical complete response; Organ preservation; Dose intensification
INTRODUCTION
According to the most recent GLOBOCAN data (2018), colorectal cancer is the 4thmost common cancer worldwide, with an annual incidence of more than 700000 cases and the 3rdhighest mortality rate[1]. In patients with locally-advanced rectal cancer (LARC), the most effective treatment, in terms of efficacy and toxicity, is long-course neoadjuvant chemoradiotherapy (CRT) followed by total mesorectal excision (TME)[2]. An important disadvantage of this approach is a high risk of surgical complications, with a postoperative mortality rate at 6-months ranging from 2%-8%, and as high as 30% in older patients (> 85 years)[3].
Given this context, in recent years there has been a growing awareness of the need to strike a balance between curative treatment and quality of life (QoL). As a result, the application of radical surgery in all patients diagnosed with LARC is increasingly being questioned. The rising interest in organ preservation strategies reflects the need to prevent, whenever possible, the significant postoperative morbidity (intestinal, urinary and sexual dysfunction) associated with TME. The risk of postoperative dysfunction is particularly evident in surgical procedures such as abdominoperineal resection, which requires a permanent ostomy, which has a severe negative impact on QoL.
According to the available data, from 10%-25% of patients with LARC achieve a pathologic complete response (pCR) - defined as the absence of viable residual tumour cells in the surgical specimen - after neoadjuvant treatment[4]. The response rate is higher in patients who receive high-dose radiotherapy[5]and/or optimized chemotherapy[6]. Research is currently underway to identify predictors of pCR after standard neoadjuvant treatment in order to improve response rates. In this context, the organ-preserving treatment approach that has come to be known as "watch and wait", in which surgery is omitted after CRT, has become increasingly relevant.
The watch and wait strategy was originally proposed by Dr. Habr-Gama and her group, who have supported the non-surgical treatment of LARC for nearly two decades in patients who achieve a complete clinical response (cCR), defined as the absence of clinically-detectable residual tumour, after neoadjuvant therapy. The findings of the studies conducted by this group[7-11]suggest that overall survival (OS) rates in selected patients who undergo observation with regular follow-up after neoadjuvant treatment are comparable to those obtained in patients who achieve a pCR after radical surgery. The main advantage of the watch and wait approach is that it avoids all of the significant morbidity and mortality risks associated with abdominoperineal resection.
Subsequent studies carried out by other groups support these data, as shown in a recent systematic review[12]that evaluated a total of 23 studies (867 patients), concluding that there are no significant differences in OS and local recurrence between surgically-treated patients and those managed with the watch and wait protocol. However, larger prospective studies are needed to confirm long-term outcomes and to resolve controversies surrounding the selection of candidates for watch and wait, the accurate determination of cCR, and the optimal follow-up protocols.
DIAGNOSIS AND REASSESSMENT
Imaging studies in patients with a recent diagnosis of rectal cancer are primarily performed for TNM staging to select the optimal therapeutic strategy, whereas the main aim of imaging after neoadjuvant therapy is to evaluate treatment response and to identify areas of tumour infiltration for surgical planning. These same images are used to determine eligibility for the watch and wait approach[13].
Although several different imaging modalities are available for locoregional staging of rectal cancer, the standard technique is magnetic resonance imaging (MRI), which provides visualization of the entire pelvis as well and offers the best assessment of the circumferential resection margin and other prognostic factors[14-17]. In previouslytreated patients, MRI can differentiate between foci of tumour persistence (residual disease) and changes secondary to treatment, an important advantage over other imaging techniques[18-20]. Endorectal ultrasound also provides good results, but its efficacy is limited by a loss of resolution at depth, and difficulties associated with stenotic, bulky or localized rectal tumours[15,21-23]. Notwithstanding these disadvantages, endorectal ultrasound remains the technique of choice to differentiate between early stage tumour (T1vsT2), where its diagnostic accuracy is superior to MRI[14-16,24]. In cases in which MRI is contraindicated (due to a pacemaker or non-MRI-compatible metal implants), ultrasound is the technique of choice[25,26]. Other imaging modalities such as positron-emission tomography (PET) have also shown good results, but these are either not recommended for routine use (e.g., PET) or not yet commercially available, as is the case with specific MRI contrast agents such as ultra-small superparamagnetic particles of iron oxide (USPIO) and gadofosveset[27-29].
MRI: Techniques and sequences
The MRI protocol for primary staging and post-treatment follow-up is the same, despite the different aims[13,16]. MRI scanners of at least 1.5 Tesla with 8-32 channel coils are recommended[30-32]. Endorectal gel can be administered to increase distension, which may facilitate detection of polypoidal or small lesions[16,31,33-35]; however, the use of these gels is controversial because displacement secondary to the compression of the mesorectal fat could theoretically induce false positives (invasion of the mesorectal fascia) or impede the accurate assessment of nodal disease[30,33,36,37]. Nonetheless, this has not been demonstrated[35]. The use of spasmolytics such as glucagon and butylscopolamine is highly variable, although decreased intestinal peristalsis may be useful in assessing tumours located in the upper rectum or when using 3T MRI, which is more sensitive to motion artefacts[16,31,33,36]. The optimal interval between completion of neoadjuvant therapy and follow-up MRI remains controversial, although recent data appear to support an interval of approximately 8 wk[16,17].
Oblique T2-weighted sequences are recommended to locate pelvic lesions. Highresolution T2 imaging should be obtained in different planes with respect to the longitudinal axis of the tumour, with a maximum slice of 3 mm. At present, the T2-weighted sequence is the most commonly used in staging rectal cancer[13,17,38,39]. The use of T1-weighted imaging with intravenous contrast administration is not considered necessary, although some authors suggest that it could facilitate the detection of tumour foci or vascular involvement[13,16,40-42].
The value of diffusion-weighted imaging (DWI) for rectal cancer is also unclear, as no definitive conclusions can be made due to the heterogeneity of the available studies[43,44]. Currently, it is thought that combining DWI with high-resolution T2 imaging could facilitate assessment of the primary tumour after neoadjuvant therapy, especially to help differentiate between partial and complete response[15,16,21]. However, in tumours with mucinous differentiation, this capacity may be limited due to the difficulty of distinguishing between residual tumour and mucin foci[13,17,45]. Some authors have suggested that the quantitative evaluation of the apparent diffusion coefficient (ADC) could be beneficial; however, the results to date have been variable and-given the overlap between benign and malignant ADC values and the complex extrapolation between MRI scanners - no clear recommendations can be made at present[16,46-48]. Although ADC has other potential uses (primary staging, assessment of nodal disease and extramural vascular infiltration) the current evidence base is insufficient to draw any definitive conclusions; that said, some authors have suggested that ADC may be useful in certain well-defined cases[16,49,50].
MRI in watch and wait
Many recent studies of MRI in rectal cancer have focused on its role in watch and wait strategies, with the following findings considered to indicate complete response of the primary tumour after neoadjuvant therapy: Normalization of the rectal wall, with good differentiation between mucosa and muscular layers without significant thickening. The presence of hypointense residual foci is indicative of fibrosis[16,17,51]. De Jonget al[52]conducted a meta-analysis to assess the utility of MRI to detect complete response, reported a pooled accuracy of 75%, sensitivity and specificity of 95% and 31%, and positive and negative predictive values of 83% and 47%, respectively. These findings suggest that MRI may be more useful to rule out complete response rather than to confirm it. In this regard, DWI-MRI is especially promising, as it provides a functional assessment of the tissues and improves the diagnostic accuracy of complete response (defined as the absence of residual hyperintensity)[16,17,51,53-55]. One study found that DWI-MRI increased sensitivity (response prediction) from 50% to 84%[43]; however, the heterogeneous designs of the studies that have evaluated this imaging tool - some of which do not use high resolution imaging - do not allow us to make any definitive conclusions[51,53,56].
The greatest challenge in MRI-based rectal staging is the assessment of regional nodes[17]. In general, MRI is considered to be more efficacious for follow-up staging after neoadjuvant therapy[17,57]. In a meta-analysis carried out by van der Paardtet al[43], the mean sensitivity and specificity rates for determining nodal stage (per patient) were 76.5% and 59.8%, respectively, and 91.7% and 73% per lesion. Only patients with a confirmed lack of nodal involvement should be considered candidates for watch and wait[58]; in this regard, a negative predictive value of 95% has been described in patients with stage ypN0 disease[57]. Based on published data, up to 16% of lymph nodes remain positive after neoadjuvant therapy, even in cases in which the primary tumour shows a complete clinical response[59-61]. Similarly, cases of recurrent nodal disease with apparent negativization have been documented, raising doubts about our ability to ensure all residual nodal disease has been eliminated[62].
A wide range of criteria have been used to define malignant lymph nodes, including size, morphology, and signal intensity, among others factors. However, due to the highly variable results the optimal criteria remain unclear[21,30-32,63]. The utility of morphological criteria after neoadjuvant therapy is limited because negative nodes may show irregular borders or heterogeneity secondary to residual fibrosis or mucinous degeneration[19,64,65]. Nonetheless, in patients treated with radiotherapy, node size decreases in up to 84% of cases; crucially, nodes that remain enlarged are more likely to be malignant[66-68]. Accordingly, a recent consensus statement recommended using nodal size for follow-up assessment after neoadjuvant therapy (with nodes whose short axis diameter is < 5 mm considered benign), given the absence of other reliable criteria[16]. However, several studies have reported the presence of small groups of residual cancerous cells in a significant number of small nodes (up to 3 mm), a finding that limits the sensitivity of this criterion[67,69,70]. Some authors have suggested that these foci could show a late response to treatment, but this hypothesis is unconfirmed and controversial[10,71,72].
Other authors have suggested applying mixed size and morphology criteria, similar to those recommended for primary staging; however, the evidence to support this approach remains insufficient[26,73,74]. Although MRI-DWI improves node localisation, there is no evidence that this imaging modality is more accurate than other approaches in determining malignancy[75,76]. In any case, caution is recommended when trying to establish a possible complete response based solely on MRI data in patients managed with a watch and wait strategy[16,52,77,78]given the less than optimal results obtained to date[55,73,78](Figure 1).
The value of radiomics in assessing rectal cancer by MRI is currently being investigated through the application of tools to perform multifactorial quantitative analysis of digital images[79,80]. Highly promising results have been reported identifying complete response using several different parameters in both T2 and DWI sequences, including changes in the relative signal intensity pre- and post-neoadjuvant therapy, texture analysis, kurtosis, and/or volumetry[47,59,79,81-87]. Some studies have even found that the application of these analyses to pre-treatment staging MRI can predict responders[88,89].
PATIENT SELECTION
One of the most important obstacles in assessing the published findings of watch and wait strategies is the heterogeneity in data quality, mainly due to inadequate staging techniques or insufficient clinical data, which limits our capacity to interpret these findings adequately and to define the clinical characteristics of the patients most likely to benefit from this strategy. It is also difficult to determine the patient profile most likely to achieve a cCR; similarly, it is hard to know the true correlation between clinical and pathological complete response.
Tumour location is an important factor in patient selection, as tumours located in the middle and lower third of the rectum (close to the anal verge) require definitive stoma. Up to 90% of patients who undergo TME develop low anterior resection syndrome (LARS), and 33% and 50% of patients develop, respectively, urinary and sexual dysfunction[3]. Unsurprisingly, these patients generally experience a significant deterioration in QoL. Due to these adverse effects, the main candidates for watch and wait are patients with tumours in these areas of the rectum (in whom TME is indicated) but who successfully achieve a cCR after neoadjuvant therapy, or patients with multiple comorbidities and/or those not considered candidates for surgery. With regard to this latter group, this is considered a different clinical entity and should be excluded from any watch and wait analysis given that surgery is not possible even if indicated.
A significant proportion of the cases included in retrospective watch and wait series are patients who refuse surgery, even though this is not contraindicated. The clinical characteristics of this subset of patients are highly variable, the quality of the data is poor, and there is only limited follow-up data. For these reasons, the highest levels of evidence for watch and wait comes from other patient groups.
Patients with low risk of local recurrence
The standard treatment in patients with early stage LARC without associated poor prognostic factors is surgery without neoadjuvant therapy. If LARC is histopathologically confirmed, no additional treatments are indicated. Surgical resection yields exceptional results in terms of both local and distant control. In this clinical scenario, a watch and wait strategy can only be applied by disregarding existing multidisciplinary protocols, or in the context of a clinical trial. Nevertheless, this approach is increasingly considered a viable option in well-selected patients, especially those who rejected surgery and those with tumours located in the lower third of the rectum[90], knowing that is not yet a standard treatment. At the moment, neoadjuvant treatment-related toxicity is considered to be an important limitation when evaluating watch and wait strategies in patients with low risk of local recurrence as well as the lack of high level of evidence in a clinical scenario where the oncological results of the standard treatment with surgery are excellent.
Patients with a high risk of tumour recurrence

Figure 1 Discrepancies in magnetic resonance sequences in follow-up imaging after neoadjuvant therapy. Magnetic resonance imaging (MRI) of the rectum for initial staging (upper row): High-resolution T2 sequences (A), high b value diffusion-weighted imaging (DWI) (B) and apparent diffusion coefficient (ADC) map (C). Protuberant wall thickening (arrow) with an adjacent enlarged, heterogeneous lymph node (arrow); signs of restricted diffusion are observed in both sequences (hyperintensity in DWI and hypointensity in ADC). MRI after neoadjuvant treatment in the same patient (bottom row) reveals near complete resolution of the main mass on the various imaging sequences. In the affected node, fewer morphological alterations are visible, but with no decrease in size (D) and with signs of restricted diffusion (E and F), suggesting persistent malignancy. No evidence of nodal malignancy is evidenced on the histological analysis of the surgical specimen.
The standard of care in patients with LARC and poor prognostic factors is neoadjuvant therapy followed by TME[91]. These patients have the highest risk of residual tumour persistence after the initial treatment, and treatment intensification is important to achieve a safe surgical plane to ensure complete resection of all cancerous tissue; otherwise, more aggressive interventions-with the associated morbidity, especially in tumours located in the lower rectum-could be necessary[92,93]. Patients who achieve a complete or near-complete clinical response may be excellent candidates for the watch and wait approach, given that LARS is presented in up to 90% of these patients after TME[94]. Nonetheless, the most suitable subgroup for this approach remains unclear because most of the available evidence comes from patients with distal tumours, patients with proximal tumours have been excluded from most clinical trials due to the difficulty of performing digital rectal examination (DRE), which is important to evaluate response and to monitor the course of disease, limiting the possibility of generalizing the use of this strategy in this setting. Regarding surgical treatment, one could argue that salvage surgery might potentially be more challenging than an upfront procedure. In patients previously treated with CRT, salvage surgery has a higher risk of complications[95,96]due to the increased fibrosis in the pelvis and the greater technical difficulty of the surgical procedure, both of which are relevant factors that must be considered as an important limitation in treatment selection for this approach.
On the other hand, long-term outcomes in patients managed with the watch and wait strategy are excellent, with 5-year OS rates ranging from 91% to 96%, as follows: Habr-Gamaet al[97](91%), Martenset al[98](97%), Appeltet al[5](100% at 2 years), and Renehanet al[99](96%). Importantly, this approach does not appear to be associated with worse outcomes in patients who develop locally-recurrent disease during followup[12]. In recent years, some studies have found that patients with locally-recurrent disease present more distant metastases[100,101]. van der Valket al[102]found that OS was lower in patients who achieved a cCR compared to a retrospective cohort with pCR. Notwithstanding those findings, the results must be interpreted in the context of the study limitations: Retrospective study design, differences among patients in clinical characteristics and treatments; lack of MRI assessment in most case, and the moderate correlation between cCR and pCR[103].
TREATMENT DURATION AND INTENSIFICATION
Standard chemoradiotherapy. Dose escalation
Numerous efforts have been made to improve cCR rates by modifying the neoadjuvant therapy scheme to lower the risk of local recurrence in well-selected patients, with promising results[5,58,104]. However, it is difficult to establish a standardized approach due to the diversity of approaches utilized, which include radiation dose escalation - highly conformal external beam radiotherapy (e.g., intensity-modulated radiotherapy; IMRT) or brachytherapy - as well as induction and/or consolidation chemotherapy[5,58,104]. Another approach used in elderly patients who are not candidates for chemotherapy is a short cycle of radiation (25 Gy in 5 sessions) followed by a watch and wait strategy[105].
In 2004, Habr-Gamaet al[8]reported the first results of the watch and wait strategy in 71 patients with LARC who achieved a cCR after standard neoadjuvant therapy (50.4 Gy to the pelvic volume plus fluoropyrimidine-based chemotherapy), with a local recurrence rate of only 2.8%. However, subsequent studies were unable to replicate those results, with reported local recurrence rates ranging from 5% to 60%[104]. This variability is likely due to patient selection bias; for example, Habr-Gamaet al[8]only evaluated patients who showed no evidence of recurrent disease 12 mo after neoadjuvant therapy.
With regard to radiation dose escalation, Appeltet al[5]conducted a prospective, observational study in patients with tumours located ≤ 6 cm from the anal verge (stage T2-3,N0-N1) received high dose radiotherapy (50 Gy) to the pelvic volume (1.6 Gy/session), 30 Gy (2 Gy/session) to the tumour, and a brachytherapy boost (5 Gy). The patients also received concomitant oral tegafur [300 mg/(m2·d)]. At six weeks, response was assessed with CT, MRI, endoscopy, and four biopsies from the initial tumour site (previously ink-marked). Although Maaset al[58]were only able to include 11% of their patients in the watch and wait strategy after standard treatment, 78% of patients achieved a cCR (35% stage T2N0). The local recurrence rate at 12 months was 15.5%, with 69% of patients presenting good anal sphincter function; grade 3 diarrhea was observed in 8%. In terms of long terms toxicity, the main adverse effect was grade 3 rectal bleeding, affecting 7% of the patients, a finding that led the authors to reconsider the application of the brachytherapy boost.
In another study, Habr-Gamaet al[106]found that dose escalation (54 Gy plus six cycles of type 5-FU-LV chemotherapy) resulted in better cCR rates (57%). However, the 2-year local recurrence rate was 27%, probably due to the disease stage (T2N0); in these patients the standard treatment was surgery, which has a higher probability of achieving a cCR after high dose CRT. In this regard, this CRT scheme proposed by Habr-Gamaet al[107]should be performed in a clinical trial. In another study, the same authors retrospectively compared dose-intensified CRT to conventional treatment. At 5 years, patients in the experimental arm presented a significantly higher cCR rate (67%vs30%;P= 0.001). However, there were no differences in surgery-free survival among the patients who achieved a cCR. By contrast, a study[108]based on data from the National Cancer Database found no benefit to radiation dose escalation, although it is worth noting that most of the patients in that study who received higher doses were older, had more comorbidities, and were more likely to be medically inoperable.
A wide range of neoadjuvant therapies have been described in the studies that have evaluated watch and wait strategies. In general, the reported cCR rates are high, especially in patients who receive intensified neoadjuvant therapy, although treatment-related toxicity is also higher[7-11,106]. Habr Gamaet al[106]retrospectively evaluated patients with stage cT2N0 tumours located < 7 cm from the anal verge, reporting a cCR rate of 56.6% with standard treatment versus 85.7% in the dose escalated (54 Gy) group (P< 0.001), with a 5 years surgery-free survival rate of 78%[106].
Contact X-ray brachytherapy - High-dose rate brachytherapy
Brachytherapy can also be used to escalate radiation doses. The value of this technique is that it permits local application of a higher dose directly to the tumour, thus preserving the surrounding healthy tissue. The brachytherapy dose is delivered either by contact X-ray applicators (CXB) or with endorectal or perineal intraluminal applicators, using high-dose rate brachytherapy (HDR-BT). Sun Myintet al[109]evaluated inoperable patients (stage cT2-T3) treated with dose-escalated CRT (45 Gy at 1.8 Gy/fr) plus a 90 Gy boost with CXB (30 Gy/fr to the rectal surface), finding a cCR of 63.8% in patients with residual tumour < 3 cm. The local recurrence rate at 2.5 years was 11.3%. Gérardet al[110]treated patients with stage cT2-T3 rectal cancer with 50 Gy CRT (2Gy/fr) plus a 90 Gy boost of CXB (except for tumours < 3.5 cm, in which CXB was performed before radiotherapy), reporting a cCR rate of 86% and a local recurrence rate at 3 years of 10%. In both series, the most common toxicity was grade 1-2 proctitis, with grade 3 proctitis described in 0-9% of cases. Garantet al[111]evaluated dose escalation with HDR-BT in patients with inoperable stage cT2-T3 rectal cancer, finding a cCR rate of 86.6% in patients who received radiotherapy alone (40 Gy; 2.5 Gy/fr) plus HDR-BT (3 fractions of 10 Gy). The 3-year local control rate was 67.1%, with a local recurrence rate of 21.9%. The most common adverse effect was rectal toxicity, with nearly all patients experiencing grade 1-3 proctitis, and 12.8%-13% developing grade 3 proctitis. Urogenital and cutaneous toxicities were also observed in this group, but not in those who underwent CXB. A retrospective study performed in the United Kingdom by Smithet al[112]evaluated radiation dose escalation in 14 patients, who were treated with CXB or HDR-BT. In that study, a complete or partial clinical response was observed in 79% of cases, with colostomy-free survival of 93%.
Short-course radiotherapy
Rupinskiet al[113]evaluated neoadjuvant short-course radiotherapy (SCRT) in a small series (n= 30) of older patients (> age 70) who received 5 sessions of radiotherapy at 5 Gy/session. Of these patients, 20% achieved a cCR and were kept under observation. Of the 30 patients, three were stage T2N0 and three T3N0. Tumour regrowth was observed in 16.6% of patients. The authors concluded that watch and wait is feasible after SCRT without associated chemotherapy[113].
The available evidence suggests that, due to technological advances in EBRT techniques, radiation doses can be safely elevated to increase the cCR rate and the number of patients eligible for conservative strategies. Most of the studies published to date have included a high percentage of patients with early-stage disease. Given that we still lack data from randomized controlled trials, dose escalation cannot yet be considered a standard approach. Although the addition of a brachytherapy boost has been shown to improve cCR rates, prospective studies are needed to better define the role of brachytherapy in organ preservation strategies. Similarly, consensus-based guidelines are needed to define and describe the main technical aspects of endorectal brachytherapy (e.g., technique, dose, point of prescription, volume delimitation, and constraints). Such studies would also help to better determine which patients would truly benefit from this approach.
Consolidation chemotherapy and induction chemotherapy
Optimization of chemotherapy schemes and agents could improve the cCR rate, although these chemotherapy regimens are normally reserved for patients with poor prognostic factors. Various chemotherapy schemes are available, such as induction chemotherapy (ICT) and consolidation chemotherapy (CCT), including active regimens that include a combination of agents, However, due to the heterogeneity of the available studies, no firm conclusions can be drawn at present.
CONSOLIDATION CHEMOTHERAPY
The pCR rate can be increased by extending the interval between neoadjuvant CRT and surgery (without additional treatment), but this strategy also increases the risk of distant progression. The addition of chemotherapy during this time period could prevent distant spread and help to downstage the primary tumour.
García-Aguilaret al[6]conducted a non-randomized, multicenter study to evaluate 256 patients with stage 2 or 3 rectal cancer. One arm received standard chemoradiation followed by surgery 6-8 wk later, with a pCR of 18%. In the others arm, CCT was added to the treatment protocol to extend the interval between CRT and surgery, leading to a significant increase in the pCR rate, as follows: 25% for a 12-wk interval (two cycles of mFOLFOX6), 30% for a 16-wk interval (four cycles of mFOLFOX6), and 38% for a 20-wk interval (six cycles of mFOLFOX6) (P= 0.004). However, it is not clear the extent to which these differences are attributable to patient selection bias and/or the delay in evaluating treatment response, rather than to the direct effects of treatment.
CCT after SCRT is an interesting therapeutic strategy that has been explored in other studies[114-116]. In a phase 3 clinical trial in Poland[115], this approach improved 3-year OS outcomes versus standard treatment (73%vs65%,P= 0.046), with less acute toxicity. The ongoing RAPIDO study[116], which is currently comparing SCRT followed by 6 cycles of CAPOX to long-cycle CRT with capecitabine, will better define the role of consolidation chemotherapy as a standard of care in these patients. Nevertheless, it is worth emphasizing that Habr-Gamaet al[97,107,117]have previously reported good results using CCT as part of a treatment intensification strategy followed by watch and wait.
INDUCTION CHEMOTHERAPY
Administration of all chemotherapy treatments prior to CRT [total neoadjuvant therapy (TNT)] may increase adherence, an approach which has been investigated in several studies. The Spanish Group of Rectal Cancer[118,119]randomized 108 patients with LARC to receive either concurrent CRT with CAPOX followed by surgery plus postoperative adjuvant chemotherapy (4 cycles of CAPOX), or induction chemotherapy (4 cycles of CAPOX) followed by the same treatment combination used in the other arm (i.e., CRT followed by surgery). Treatment adherence was higher in the ICT arm, with a lower proportion of patients developing severe (grade 3-4) chemotherapy-related adverse effects. Between-group differences in pCR (13%vs14%) were not clinically significant.
Other studies-including the EXPERT, EXPERT-C[120], AVACROSS[121]trials-have reported higher R0 resection rates with ICT, although without any improvement in pCR. In the EXPERT-C and AVACROSS studies, there was no benefit to adding targeted therapies to induction chemotherapy in this clinical scenario.
Given the limited available evidence, it is not possible to reach definitive conclusions regarding which of the two treatment options (CCTvsICT) has better adherence, nor which approach induces greater primary tumour regression.
TIMING OF ASSESSMENT
Several strategies have been shown to improve cCR rates. The simplest-but not least important-approach is to extend the time between completion of neoadjuvant therapy and reassessment. Several retrospective studies in patients with LARC have shown that extending the interval between CRT and surgery increases tumour regression and improves pCR rates[122-124]. The optimal time interval is 8 wk, as studies show that this yields the best pCR outcomes[125,126]. Reassessment before 8 wk is not recommended, as the results could be interpreted as a false incomplete res-ponse[97,107,117].
In the studies conducted to date to evaluate the watch and wait strategy[5,58,97-99,117,127-132], cCR has been assessed at various time points, ranging from 4 to 20 wk after completion of neoadjuvant therapy (Table 1). Consequently, the optimal time to assess cCR remains undefined.
Given these findings, it appears that assessment of treatment response to determine the cCR should be performed sometime around week 8 after completion of CRT. However, this criterion may need to be adjusted according to the patient's initial tumour stage, since more advanced tumours require a longer time interval to reach a cCR. Nonetheless, the initial reassessment should not be excessively delayed given the importance of early determination of poor response to neoadjuvant therapy to avoid delaying surgery unnecessarily.
FOLLOW-UP PROTOCOLS
The watch and wait strategy in rectal cancer has several important drawbacks, including the lack of a consensus-based definition of treatment response and followup protocols, as well as the poor reliability of the current predictors of response.
In patients managed with a watch and wait strategy, the main recommendation given by specialised centres is close monitoring through frequent follow-up visits. However, these recommendations are probably not practical in routine clinical practice at most centres[133]. In general, the initial assessment of treatment response should be performed 6-10 wk after completion of neoadjuvant therapy, with intensive surveillance during the first two years and longer follow-up intervals thereafter[58,107,123,127,129,134].
In the absence of prospective controlled trials, at present is not possible to provide well-defined, evidence-based guidelines on the optimal follow-up protocols to improve prognosis[12,105]. While endoscopy is the main tool for follow-up evaluation, the use of MRI is increasing. MRI findings should correlate with the combined findings of DRE and endoscopy, the combination that offers the best diagnostic accuracy for the evaluation of complete response[16,59,135]and for initial disease staging[16,61,136]. Most protocols also recommend determination of CEA levels after neoadjuvant therapy since normalization (< 5 ng/dL) of this biomarker in patients with elevated levels prior to treatment appears to predict treatment response[137-139].
The following endoscopic findings were first defined by Habr-Gamaet al[140]as predictors of response: Complete elimination of the rectal tumour, replaced by a flat, regular, whitish scar, with telangiectatic vessels on its surface. These findings have been shown to have a high negative predictive value[141]. Other endoscopic findings, such as the presence of ulcerations, mucous irregularities, nodules, stenosis, or persistence of rectal masses indicate incomplete response. Nonetheless, none of these findings are reliable predictors of response, as measured by sensitivity and (especially)specificity[8,142-144]. In other words, these signs of remission are not always present in patients with a pCR, only presenting in 25% to 77% of cases, depending on the series[104,140,145,146]. Similarly, certain mucous abnormalities, particularly flat, regular ulcerations, are common in patients with complete remission[8,141,144]. In case of uncertainty, a second early reassessment, performed 6-12 wk after treatment, could be justified to identify tumours that are likely to respond eventually[147]. The persistence of large, anfractuous masses or ulcers indicates - to a high degree of certainty - a lack of response. (Figure 2 and Figure 3)
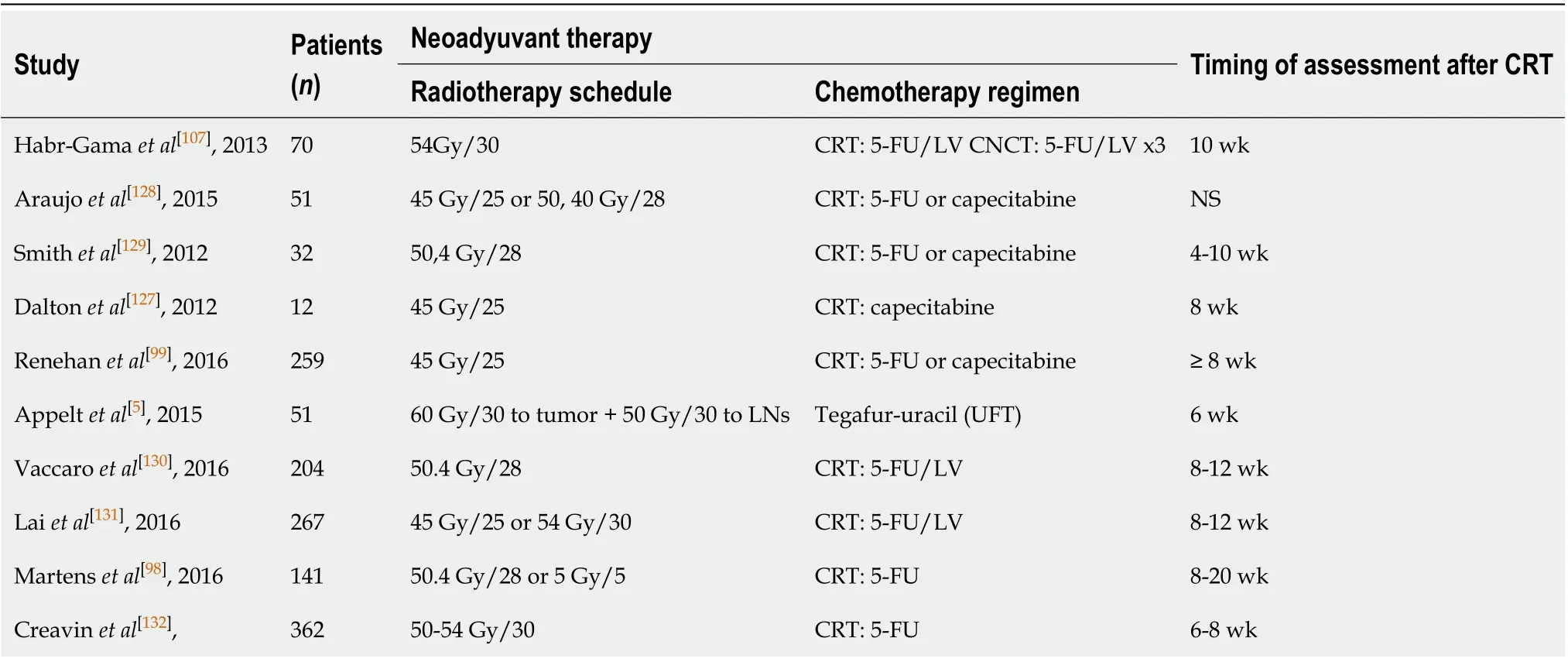
Table 1 Time between completion of neoadjuvant therapy and first reassessment in watch and wait clinical studies
The utility of performing additional biopsies is highly controversial, as biopsies do not appear to be superior to optical diagnosis by the endoscopist[141]. Moreover, biopsy has such a high false negative rate that it is impossible to reliably rule out the presence of residual disease, nor can biopsy examination be used to determine the degree of invasiveness[8,142,143]. Therefore, despite the widespread use of this procedure, its use cannot be recommended[133]. Similarly, endorectal ultrasound has not demonstrated sufficient diagnostic accuracy to provide any real utility in follow-up, despite the fact that it is routinely used in experienced centres[133,148-155].
OUTCOMES AND MANAGEMENT OF TUMOUR REGROWTH
Some authors have investigated alternative strategies to reduce the morbidity and mortality associated with conventional treatment, especially in tumours located in the lower third of the rectum. One such strategy is transanal resection before or after neoadjuvant therapy, mainly in cases with cT2 disease[156,157]. Other strategies include local resection of cT2 tumours followed by CRT, an approach that yields excellent results, as evidenced by the study carried out by the American College of Surgeons Oncology Group (ACOSOG Z6041). That study included 72 patients, finding 3-year disease-free survival (DFS) and OS rates of 87% and 96%, respectively, at a median follow-up of 4.2 years[156].
Conventional treatment (neoadjuvant therapy followed by TME) has been compared to local resection in several randomized trials, including the trial performed by Lezocheet al[157], as well as the GRECCAR (2017)[158]and Dutch CARTS study (2018)[159]. None of those trials found any significant between-group differences in DFS. In the Lezoche trial, the DFS rates were 89% and 94%, respectively, for local resectionvsTME (P= 0.609). It is worth noting, however, that 36% of the patients in the local resection arm later required TME, which increased treatment-related morbidity. As a result, there were no clear benefits for local resection compared to standard treatment. These findings were later confirmed in the GRECCAR and CART studies[158,159].

Figure 2 Clinical incomplete response. A: Endoscopic evaluation after 9 wk of chemoradiotherapy completion, detecting a small, but irregular, residual ulcer. B: Regrowth is more evident 12 wk later, as a deep, irregular and necrotic ulcer.
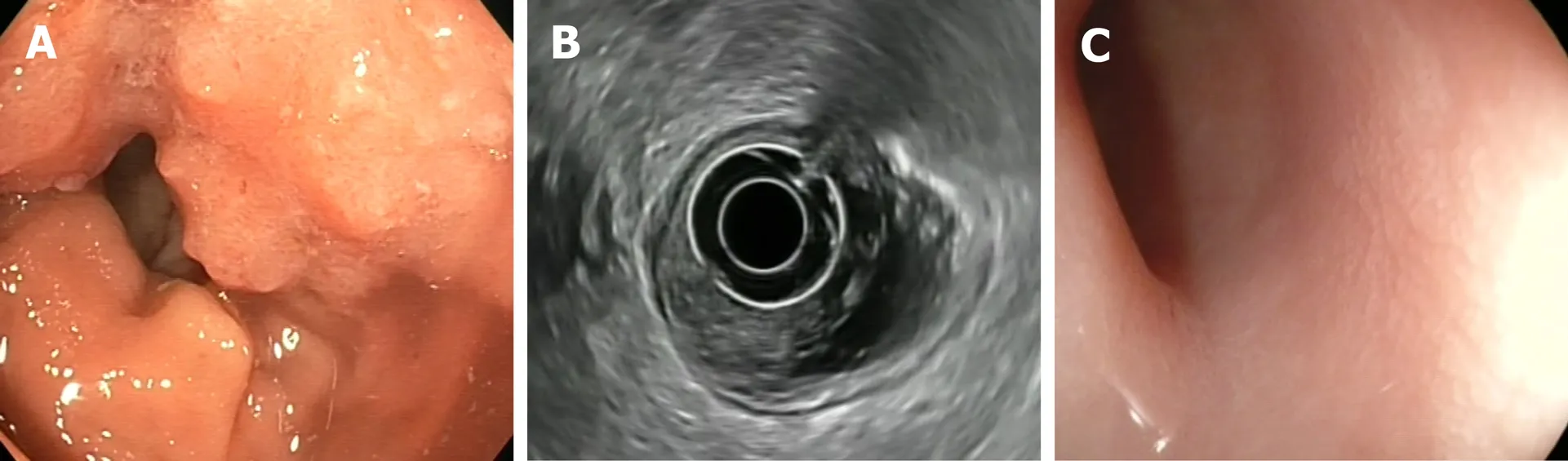
Figure 3 Clinical complete response. A: Endoscopic view of a rectal tumor prior to the neoadjuvant chemoradiotherapy; B: Endoscopic ultrasound with radial probe, showing that the tumor (T) is located within the mucosa, submucosa and muscular layers (uT2N0); C: Flat scar 10 wk after treatment completion: An endoscopic response feature.
In the management of tumour regrowth with the watch and wait strategy, the main difficulty in attempting to draw firm conclusions from the current evidence base is that most of the available studies are retrospective, often comprised of small, highly heterogeneous samples with wide variety in the characteristics of the patients, the tumour types, and even treatment regimens. Approximately 30% of patients who achieve a cCR after neoadjuvant therapy experience local regrowth[105], especially in the first two years. At some point during follow-up, most of these patients will be candidates for salvage surgery, either local excision, low anterior resection, or abdominoperineal excision. Although some authors currently favour local resection[160], TME remains the treatment of choice after local regrowth[107]; however, in 2%-3% of these patients, salvage therapy may not be feasible due to an unresectable local invasion, concomitant non-curative systemic recurrence, or the presence of significant medical comorbidities[161]. Surgery for local regrowth is known as “salvage surgery” or “regrowth deferred surgery”.
In the OnCoRe project[104], 88% of patients with non-metastatic local regrowths were salvaged, a slightly higher rate than reported by Konget al[162](83.8%) and Smithet al[163](85%), and well above the 68.4% rate described by Onet al[164]and the 69% rate reported in the International Watch and Wait Database[107]. Moreover, the salvage rate in the OnCoRe study were close to those described by Chadiet al[165](89%) and by the Habr-Gama group (90%)[161](Table 2).
According to Smithet al[163], treatment outcomes (OS and DFS) in patients who undergo salvage surgery are comparable to those achieved in patients who undergo conventional surgery. That said, most of the reported survival outcomes are based on only 3 years of follow-up. Nasiret al[160]presented similar short-term results. In the longer term, the Habr-Gama group reported a 5-year OS of 63.3% in patients who underwent salvage surgery[166], substantially less than the 85% reported in the International Watch and Wait Database[107]. Onet al[164]found no significant differences in survival rates between salvage and upfront surgery (92.3%vs92.9%, respectively) (Table 2).
Deferred surgery for local regrowth has shown promising short-term oncological and surgical results. However, the risk of distant metastases in patients managed with the watch and wait strategy remains undefined and this will need to be assessed through randomized controlled trials. The emergence of local regrowth in a patient managed with the watch and wait strategy should not be considered equivalent to local recurrence in a patient treated with radical surgery or transanal excision[103,111].Local recurrence after surgery indicates a failure of definitive therapy; consequently, the potential for successful salvage is low, with only 20%-30% of patients with locallyrecurrent rectal cancer ultimately undergoing a potentially-curative R0 resection[167].

Table 2 Tumor regrowth and salvage surgery in watch and wait clinical studies
QoL
QoL is a crucial aspect when considering the treatment strategy in patients with LARC. QoL is particularly relevant for sphincter preservation. Studies have shown a clear improvement in QoL in patients managed with a watch and wait approach versus surgical patients with a postoperative pCR, with a lower Wexner incontinence score (0.8vs3.5) (P= 0.182) and defecation frequency (1.8 times/dvs2.8 times/d) (P= 0.323)[58].
Renehanet al[99]compared 3-year colostomy-free survival (CFS) rates in patients who had achieved a cCR with the watch and wait strategy versus a control group who underwent surgical resection after failing to achieve a cCR. The CFS was significantly higher in the watch and wait group (74%vs47%; hazard ratio, 0.445;P< 0.0001), with a 26% absolute difference at 3-years in the percentage of patients without a permanent colostomy. Another study found a high sphincter preservation rate at one year (72%), with no faecal incontinence in 69% of patients at 2 years, and a median Wexner score of 0 (IQR, 0-0) at all timepoints[5].
The comparative QoL study by Hupkenset al[168]merits mention due to the better outcomes in the watch and wait arm on physical and emotional function (36-item short form) and better physical function, and functional and cognitive capacity outcomes on the European Organization for the Cancer Research and Treatment questionnaire (QLQ-C30).
FUTURE PERSPECTIVES
Despite the substantial increase in recent years in the number of published studies on the watch and wait approach-a direct result of the growing interest in this strategy, together with an increase in follow-up data-several aspects surrounding the optimal management of patients with LARC. There is a clear need to determine which patients would most benefit from the watch and wait approach, as this would permit us to individualize treatment in accordance with individual risk profiles.
Multiple clinical trials (Table 3) are current underway to evaluate different strategies to improve complete clinical response rates. One such strategy is radiotherapy dose escalation, an approach that is supported by the findings of prospective multicenter studies in patients with early stage rectal cancer (NCT00952926 and NCT02438839), demonstrating high organ-preservation rates[5]. That said, we still do not know whether the excellent results reported in those studies are more attributable to the tumour stage or to the higher radiation doses. Intensification of chemotherapy is also being assessed, as exemplified by the phase 3 randomized trial underway at the Memorial Sloan Kettering Cancer Center (MSKCC) (NCT02008656)[169]. In that trial, indication chemotherapy is compared to consolidation chemotherapy in patients with a cCR, offering them the option of non-surgical management with organ preservation.The results will provide crucial data on the risk of distant metastases in patients selected for watch and wait who receive intensified systemic treatment.

Table 3 Selected ongoing clinicals trials in patients with rectal cancer in a watch-and-wait program
Patients with multiple comorbidities are routinely excluded from clinical trials. Consequently, virtually all of the available data on these patients come from retrospective or non-randomized studies. Accordingly, these data must be interpreted cautiously given the potential for bias, as these patients are often dissuaded from surgery and directed towards watch and wait. As a consequence, OS outcomes in these patients tend to be worse than would otherwise occur if comparisons were made between similar groups with comparable clinical characteristics.
Alternative approaches are currently being explored in an effort to reduce the morbidity and mortality associated with TME for LARC. The TAU-TEM (NCT01308190)[170]and STAR-TREC trials (NCT02945566)[171]are both evaluating the viability of less aggressive surgical approaches in these patients. The results of these trials are expected to provide data comparing this alternative surgical approach to standard treatment and watch and wait.
In the absence of randomized clinical trials, the International Watch and Wait Database (IWATCH-AND-WAITD), created in 2014 (http://watch-and-waitw.iwatchand-waitd.org), has the largest number of patients managed with a watch and wait strategy[107]. That database includes both retrospective and prospective data and the evidence base for watch and wait will increase substantially when long-term outcomes in these patients become available.
CONCLUSION
There are clear short-term advantages-mainly reduced morbidity and better quality of life-to omitting surgery in patients with locally-advanced rectal cancer who have successfully achieved a complete clinical response after neoadjuvant therapy. In this clinical scenario, numerous studies have been conducted to date. However, many questions remain, including: (1) The optimal intensity and duration of clinical, radiological, and pathological follow-up; (2) Whether neoadjuvant therapy should be intensified based on the initial clinical stage; and (3) The need to identify strategies to reliably diagnose the greatest number of patients with cCR.
Based on the current data, the watch and wait strategy appears to be safe option in patients with LARC who have achieved a cCR after neoadjuvant therapy and who either present a high surgical risk or refuse surgical treatment. However, data from prospective multicentre studies are needed to confirm the non-inferiority of this approach in terms of cancer control versus standard treatment before this strategy can be more widely offered.
 World Journal of Gastroenterology2020年29期
World Journal of Gastroenterology2020年29期
- World Journal of Gastroenterology的其它文章
- Endoscopic management of gastrointestinal leaks and fistulae: What option do we have?
- Evaluation of intrahepatic manifestation and distant extrahepatic disease in alveolar echinococcosis
- Multivariate predictive model for asymptomatic spontaneous bacterial peritonitis in patients with liver cirrhosis
- Clinicopathological characteristics and surgical outcomes of sarcomatoid hepatocellular carcinoma
- Patients' perspectives on smoking and inflammatory bowel disease: An online survey in collaboration with European Federation of Crohn's and Ulcerative Colitis Associations
- Dysregulation of microRNA in cholangiocarcinoma identified through a meta-analysis of microRNA profiling
