An enhanced underwater camera apparatus for seabed observation of megabenthic epifauna in the northern Yellow Sea*
YU Nan , SUN Song , , WANG Shiwei LIU Qun ZHANG Guangtao ZHANG Fang SUN Xiaoxia
1 Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 University of Chinese Academy of Sciences, Beijing 100049, China
5 Jiaozhou Bay Marine Ecosystem Research Station, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Seabed photographing has been applied with various underwater camera apparatuses (UCAs) for observations of megabenthic epifauna, which reveals more details than traditional sampling tools do. In this study, we improved a UCA named a towed underwater video-camera system (TUV system) with image processing software for seabed photographing in coastal areas up to 100 m. In May 2017, the TUV system was tested at 4 stations in the Zhangzi Island marine area in the northern Yellow Sea to investigate local megabenthic epifauna, especially brittle stars. At each station, more than 500 good seabed photographs each in area of 0.155 0 m 2 were obtained in just 10 min. Almost all of the epifauna larger than 1 mm could be identifi ed from the photographs, including echinoderms, bivalves, cnidarians, and crustaceans. Three dominant brittle stars ( Ophiopholis mirabilis, Ophiura sarsii vadicola, and Stegophiura sladeni) were spotted, and their abundance, disc diameter, cluster size, and coverage area were calculated and analyzed from the seabed photographs. The results show that the TUV system could be applied in coastal waters of hard sandy bottoms and could be used for quantitative investigations of megabenthic epifauna.
Keyword: seabed photography; brittle stars; underwater camera apparatuses (UCAs); coastal waters
1 INTRODUCTION
Macrobenthic communities are key components of marine ecosystems. Because many macrobenthic organisms are sensitive to the environmental stress and have long life histories, they are important indicators that can be used to assess and evaluate marine ecosystems, especially in coastal waters (Peng et al., 2014; Xu et al., 2016). The use of the seabed photography method for quantitative macrobenthos investigations was originally proposed and applied in the 1930s (Barton, 1935; Harvey, 1939; Johnson, 1939). This method was considered to be more eff ective than traditional methods (corers and trawls) because a large number of seabed images could be obtained for the quantitative analysis of not only species abundance and composition but also the life habits and interspecifi c or intraspecifi c relationships of the macrobenthos (Hecker, 1990; Smith et al., 1993; Piepenburg and Von Juterzenka, 1994; Piepenburg and Schmid, 1996; Bergmann et al., 2011).
The development of seabed photography was accompanied by the development of the underwatercamera apparatuses (UCAs). Two main types of UCAs exist: triggered and towed UCAs. Triggered UCAs are controlled by foot triggers or sonar and deployed when the ship stop or drift with the current. Seabed images are obtained during up and down movements of the UCAs (Ewing et al., 1946; Vevers, 1951; Piepenburg and Von Juterzenka, 1994; Stübing and Piepenburg, 1998; Hughes, 2014). To obtain continuous image of a seabed, towed UCAs are towed at specifi c speeds, and the seabed over a large area is photographed (Huggett, 1987; Gordon et al., 2000; Bluhm, 2001; Fornari and Group, 2003; Bowden et al., 2011).
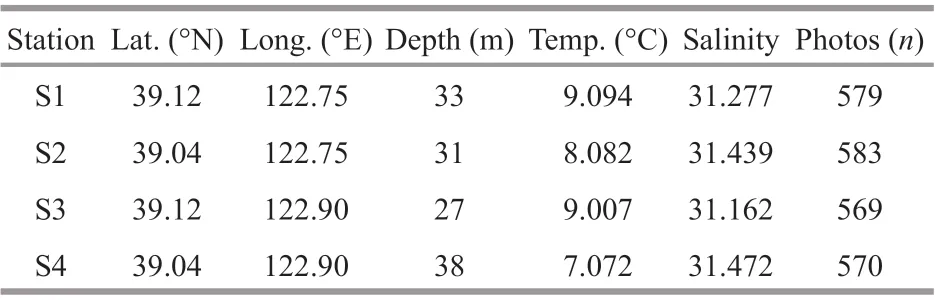
Table 1 Specifi cations of each sampling station
The northern Yellow Sea (NYS) is a semi-closed shallow marginal sea located between the Shandong Peninsula, the Liaodong Peninsula and the Korean Peninsula with an area of approximately 7.1×103km2(Qi et al., 2004). The sea bottom of the NYS is fl at with a depth of less than 70 m, and the sediments are mainly sands and gravels (Wang et al., 2009; Xu et al., 2018). The NYS is one of the most important aquaculture areas in China, especially the Zhangzi Island sea area, which is the largest aquaculture zone for bottom-cultured Japanese scallopsPatinopectenyessoensisand accounts for 46% of the total production ofP.yessoensisin China (Zhang et al., 2008). To evaluate the macrobenthic ecosystem in this sea area, we applied seabed photography for the fi rst time to investigate the megabenthic epifauna.
Benthic sled is a common type of towed UCAs that can be towed over the sea bottom with the use of specifi cally designed sled runners (Uzmann et al., 1977; Theroux, 1984; Hecker, 1990; Nybakken et al., 1998; Ruhl, 2007). However, because of the hard sandy bottom in the NYS, a “cloud” of sediment could be easily caused by the sled runners touching the sediments, thus aff ecting the quality of the photographs. Therefore, to reduce the contact area between the UCA and the sea bottom, we changed the sled runners to wheels and designed a new towed UCA named the towed underwater video-camera system (the TUV system), which could prevent the interference from clouds and increase the fl uency and fl exibility of the system.
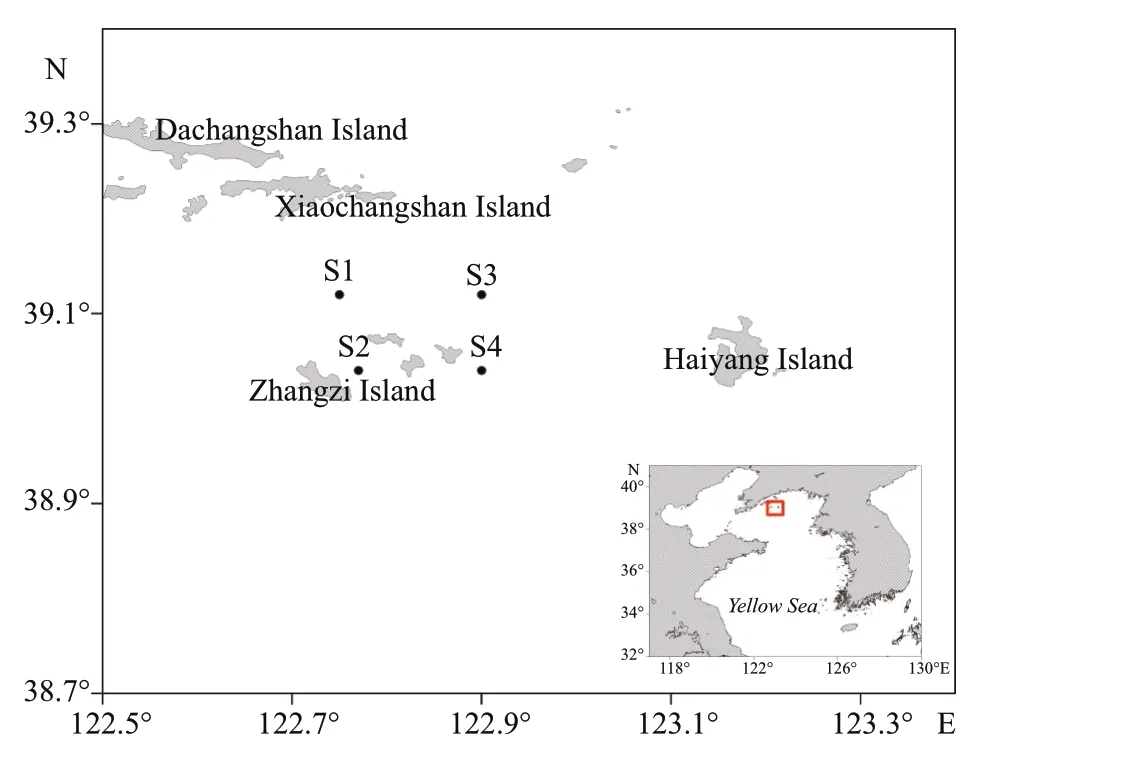
Fig.1 Photograph stations in the Zhangzi Island sea area
In this study, we introduce the TUV system and demonstrate the test results of using this system for investigations of megabenthic epifauna, especially brittle stars, in the Zhangzi Island sea area (the NYS). Almost all epifauna (echinoderms, bivalves, cnidarians, and crustaceans) together with some benthic fi sh and infauna could be identifi ed using this system. Furthermore, in addition to the abundance and disc diameter, the aggregation characteristics of brittle stars could be described by analyzing the coverage rate and the cluster size frequency from the seabed photographs. This study described a new improved UCA that could be practically and extensively applied for seabed photography in coastal waters with hard sandy bottoms. Moreover, the results of this study provided possibilities and prospects for photographic investigations of the benthic ecosystem in the NYS and other similar sea areas.
2 MATERIAL AND METHOD
2.1 The trial site
The Zhangzi Island sea area is located in the NYS and is ~50 km away from the southern Liaodong Peninsula and aff ected by the Yellow Sea Cold Water Mass and freshwater input by rivers in northern China. The fl at and hard sandy bottom and good natural nutrient conditions in this area make it an ideal aquacultural water for scallops. The Japanese scallopsPatinopectenyessoensisare sowed freely on the bottom layer and collected using bottom trawling nets. The test area was located over 4 stations in the Zhangzi Island sea area in May 2017 (Fig.1; Table 1). Temperature and salinity were monitored by a CTD (AAQ1183-1F, Alec Electronics Co., Ltd.) at each station.
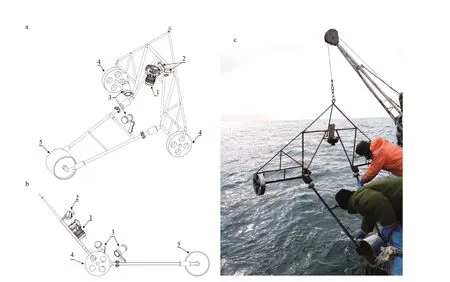
Fig.2 Diagrams and photographs of the towed underwater video-camera system (the TUV system)
2.2 The fi eld procedure
The towed underwater video-camera system (the TUV system) consists of a rolling frame, a main camera (Sony RX100V & Nauticam housing), two surveillance cameras (GoPro HERO 5) and two underwater lights (X-Adventurer M5000) (Fig.2). The weight of the whole system is about 35 kg. The rolling frame is a stainless steel tubular frame with three wheels, including one rear wheel and two side wheels. The rear wheel is 30-cm high and 20-cm wide, and the side wheels are 30-cm high and 5-cm wide. The rear wheel is much heavier than the side wheel to prevent the unstable up and down movements of the frame as it is rolled during towing.
The shot distance of the main camera from the sea fl oor is 47 cm at an angle of 40° from vertical, and the focal length of the camera is fi xed at 24 mm, while the underwater lights are focused on the photographed area. Two surveillance cameras mounted on the upper part of the system are used to observe the running condition of the whole system.
The scale of the photographed area was measured and corrected before operation. Because the shot distance and the focal length were constant, the photographed area was fi xed at 0.155 0 m2. During operation, the TUV system was connected to a wire rope of the winch and was lowered from the drifting ship. When the rear wheel and two side wheels touched the seabed, the system landed on the sea bottom. Then, the wire rope was pulled to the stern and released continually. When the length of the wire rope was approximately two times the water depth, the ship began to move. The system was towed well astern of the ship at a vessel speed of less than 1 knot, and an approximately 10-min video of the seabed was collected. It was necessary to adjust the length of the wire rope and check its tightness in real time to ensure that the system was stable under the speed and inertia of the ship while avoiding stagnation from being trapped by stones and fi shing gear.
After the operation, the system was retracted to the deck, and the video data from the main camera and two surveillance cameras were collected and backed up. Continuous screenshots with a time interval of one-second were collected for each video from the main camera, avoiding repeated images in adjacent photographs. The good photographs were selectedfrom the screenshots of each station while excluding the no-good photographs that were aff ected by sediment, currents, and unsteady towing, which could be observed from the videos of the surveillance cameras.
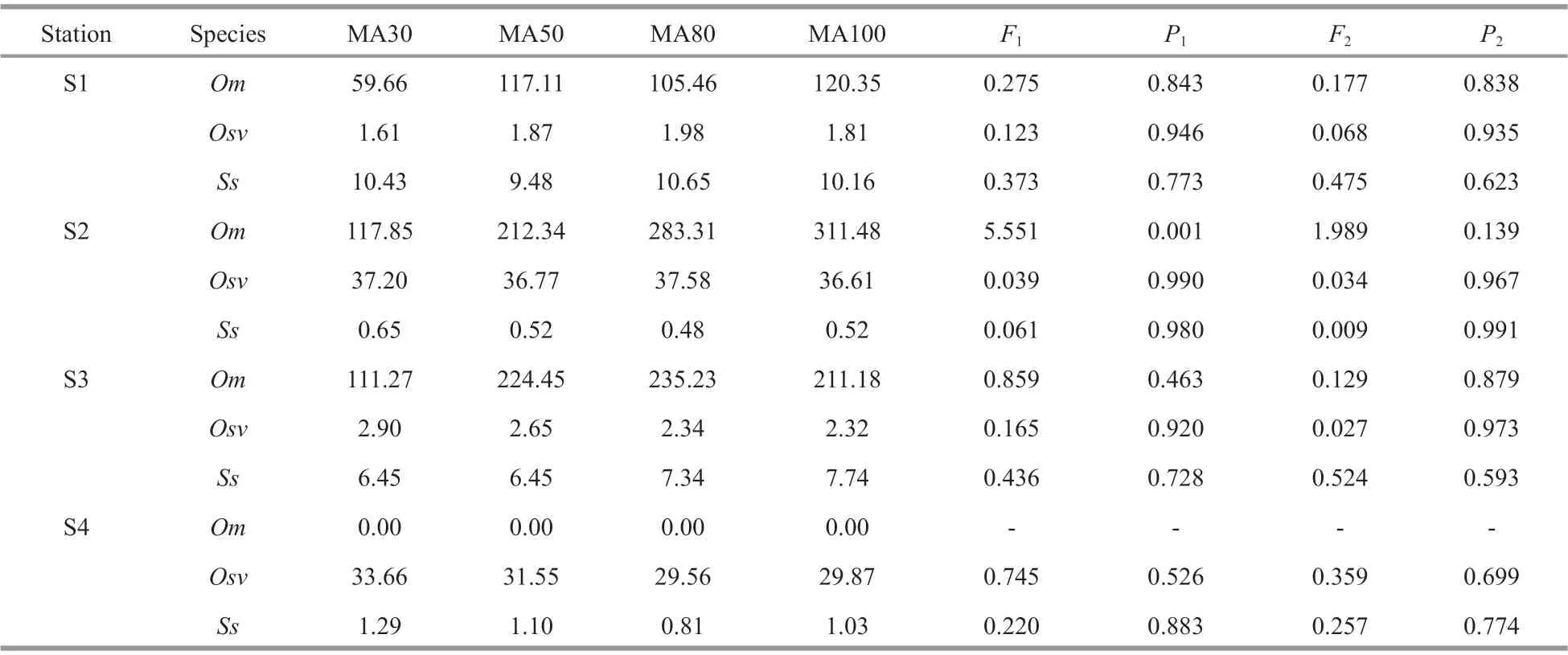
Table 2 The mean abundance (MA) of 3 ophiuroid species counted from 30, 50, 80, and 100 good seabed photographs at each station and the statistics of one-way ANOVA
2.3 Image processing
The megabenthic epifauna with maximum dimensions larger than 1 mm were recorded, and the abundance and biological characteristics of three brittle stars species in the good photographs at each station were calculated and analyzed. The quantities ofOphiurasarsiivadicolaandStegophiurasladenicould be counted directly in each photograph, and the individuals that were partly shown at the edge of the photograph were counted as 0.5. It was diffi cult to count the quantity ofOphiopholismirabilisusing the same method because of the dense aggregations. However, we found that the arms ofO.mirabiliswere relatively clear in the photographs. Therefore, we determined the quantity ofO.mirabilisby counting the number of arms in the photographs and dividing by 5. The area of each photograph was 0.155 0 m2, so the abundance of each ophiuroid species in one photograph was:
Abundance (ind./m2)=quantity (ind.)/0.155 0 m2.
To determine the number of good photographs selected for processing at each station, we randomly selected 30, 50, 80, and 100 good photographs at each station to calculate the abundance of the three brittle stars and examined the mean abundance of these 4 groups of photographs for each species in software SPSS using one-way ANOVA. The statistical results showed that there was a signifi cant diff erence (F3,256= 5.551,P=0.001<0.01) in the mean abundance ofO.mirabilisat S2, and the mean abundance of the group of 30 photographs was signifi cantly less than that of the other groups. Thus, we removed the mean abundance of the group of 30 photographs and compared the remaining three groups. The results showed that the diff erence disappeared (Table 2). Therefore, 50 good seabed photographs were randomly selected to calculate the abundances of the three brittle stars as well as for the analyses of biological characteristics.
To describe the biological characteristics of the three brittle stars, the coverage areas and cluster sizes ofO.mirabilisand the disc diameters ofO.sarsiivadicolaandS.sladeniwere calculated using image processing software (Shewei 1.0), which was designed and corrected to measure the actual area and length in the photographs from the TUV system (Fig.3). The coverage area ofO.mirabiliswas the area of the seafl oor that was occupied by the organism. In the software, all clusters ofO.mirabilisin one photograph were circled by linking the ends of the outer arms to obtain the coverage area, then, the coverage rate was calculated (coverage area/0.155 0) (Fig.3a). Meanwhile, the sizes of the complete clusters ofO.mirabilisin the photographs were measured separately in the software to help establish the frequencies of the ranges of cluster sizes (<0.02 m2, 0.02–0.04 m2, 0.04–0.06 m2, 0.06–0.08 m2, 0.08–0.12 m2, >0.12 m2) at the dominant stations. The clusters that all exceeded the range of the photograph were calculated as >0.12 m2. The disc diameters ofO.sarsiivadicola, andS.sladeniwere measured from the base of one arm to the opposite interradius in this software (Fig.3b–d).
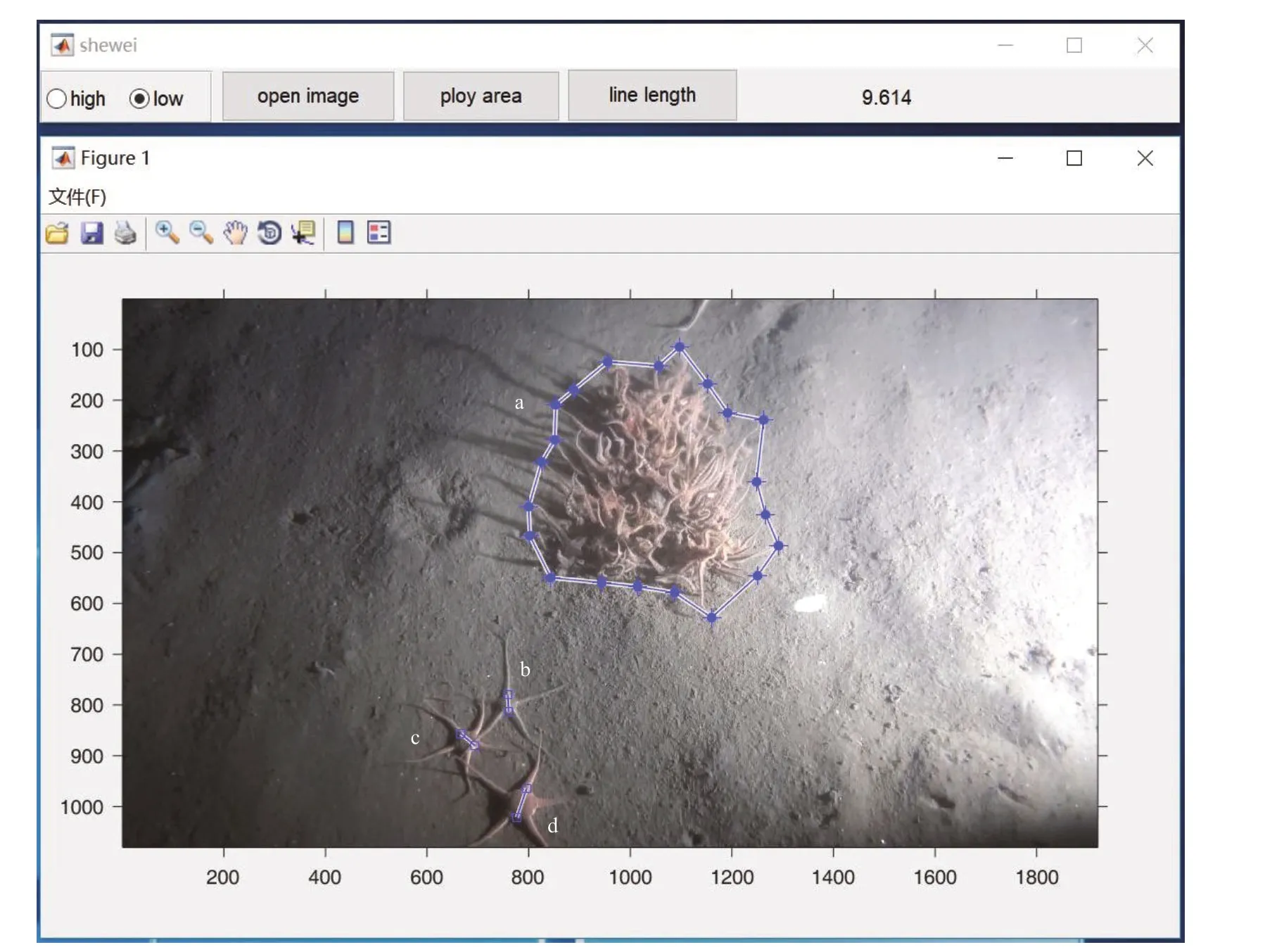
Fig.3 The image processing software for calculating the coverage areas and the cluster sizes of Ophiopholis mirabilis and disc diameters of Ophiura sarsii vadicola and Stegophiura sladeni
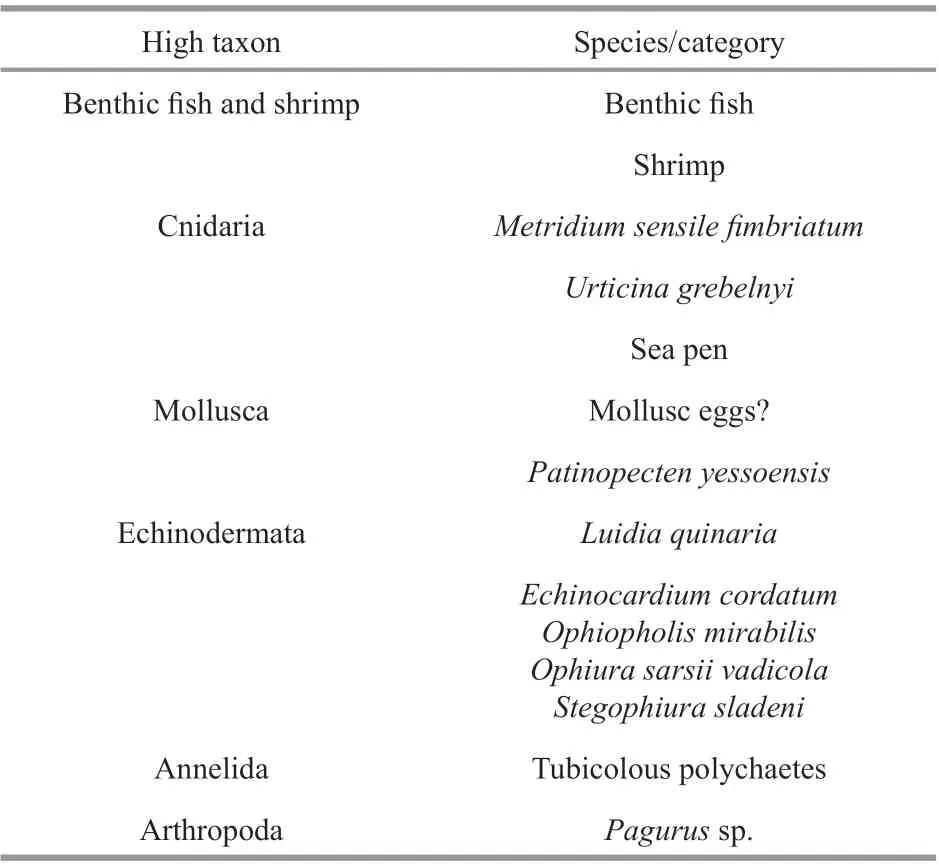
Table 3 The categories of macrobenthos recorded in the stations
2.4 Statistical analyses
The signifi cance of the diff erences in coverage rates and cluster sizes ofO.mirabilisamong stations were tested with the One-way ANOVA, followed by a LSD post hoc test for multiple comparison. The diff erence between disc diameters ofO.sarsiivadicola, andS.sladeniwas tested with Student’st-test for independent samples. Statistical analyses were performed with IBM SPSS Statistics (v. 23.0) software.
3 RESULT
3.1 Megabenthic epifauna in the seabed photographs

Fig.4 Macrobenthos in the seabed photographs

Fig.5 Three ophiuroid species
The numbers of good seabed photographs obtained at each station using the TUV system were 579, 583, 569, and 570 (Table 1). The categories of macrobenthos recorded from the seabed photographs were listed in Table 3. Almost all epifauna larger than 1 mm could be identifi ed in the seabed photographs, including echinoderms, bivalves, cnidarians, and crustaceans (Fig.4c–j & i). In addition, benthic fi sh and some of the infauna that were partly exposed on the seafl oor, such as the tubicolous polychaetes, were also found (Fig.4a, b & k). From these seabed photographs, three dominant brittle stars,Ophiopholismirabilis,Ophiurasarsiivadicola, andStegophiurasladeni, were identifi ed (Fig.5).
3.2 Abundance and distribution of the dominant ophiuroid species
As the most abundant ophiuroid species, the mean abundance ofO.mirabiliswas 138.448±18.01 ind./m2(Fig.6). The mean abundance of the second most dominant species,O.sarsiivadicola, was 18.21± 1.43 ind./m2(Fig.6). Lastly, the mean abundance ofS.sladeniwas 4.39±0.51 ind./m2(Fig.6).
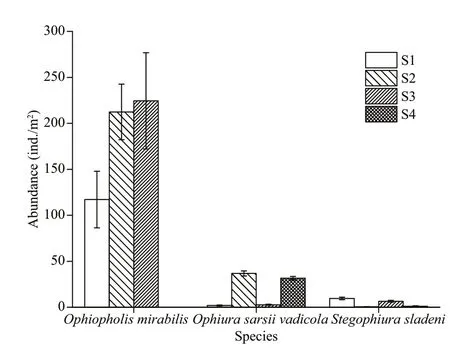
Fig.6 Abundances (±SE) of Ophiopholis mirabilis, Ophiura sarsii vadicola, and Stegophiura sladeni at four stations in the Zhangzi Island sea area
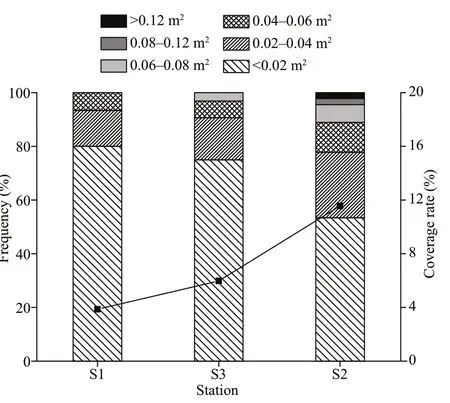
Fig.7 Cluster size-range frequency distribution (left axis) and coverage rate (right axis) of Ophiopholis mirabilis at the dominant stations
The distribution areas of the three ophiuroid species were diff erent.O.mirabiliswas distributed in S1, S2, and S3, but not in S4 (Fig.6).O.sarsiivadicolawas mainly distributed in S2 and S4, whileS.sladeniwas mainly distributed in S1 and S3 (Fig.6).
3.3 Biological characteristics of the dominant ophiuroid species
The coverage rates ofO. mirabiliswere 3.86%, 11.56%, 5.98% and 0% at S1, S2, S3 and S4, respectively (Fig.7). There was signifi cant diff erence in coverage rates among stations (F3,199=27.865,P<0.01), and the coverage rate at S2 was the largest (LSD,P<0.01). We counted 92 complete clusters, and 84.78% of the clusters were in the <0.02 m2and 0.02–0.004 m2groups, showing thatO.mirabilisin this area mostly gathered in small clusters (Fig.7). There was signifi cant diff erence in cluster sizes ofO.mirabilisamong its dominant stations (F2,91=3.164,P<0.05). We arranged the cluster size-range frequency following the coverage rate and found that more large-clusters ofO.mirabilisoccurred when the coverage rate was higher.
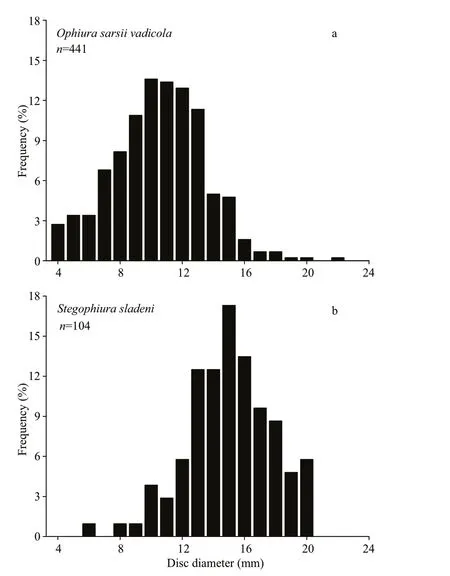
Fig.8 Size (disc diameter) frequencies of Ophiura sarsii vadicola (a) ( n=441) and Stegophiura sladeni (b) ( n=104) at four stations in the Zhangzi Island sea area
In theO.sarsiivadicolapopulation, the disc diameter frequency showed a peak between 7 mm and 15 mm with a proportion of 86.85% of the 441 measured discs (Fig.8a). In theS.sladenipopulation, the disc diameter frequency showed a peak between 12 mm and 20 mm with a proportion of 90.38% of the 104 measured discs (Fig.8b). The disc diameters ofO.sarsiivadicolawere smaller than that ofS.sladeni(t=-14.28,P<0.01).
4 DISCUSSION
4.1 The seabed photography method
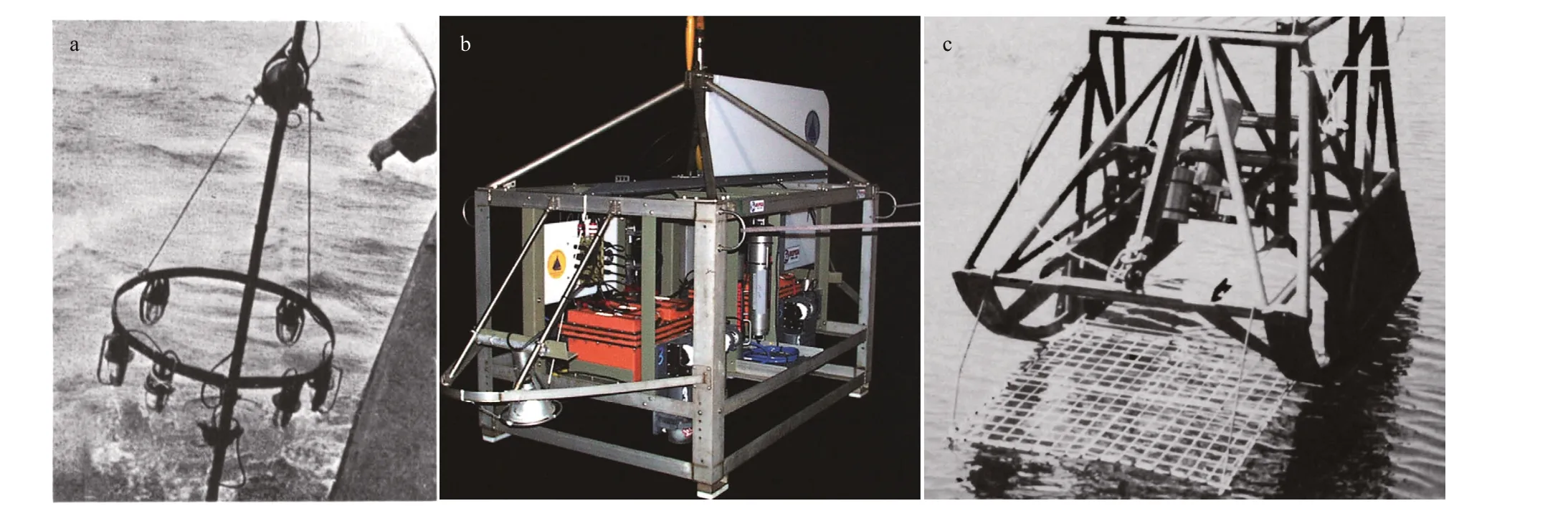
Fig.9 Examples of underwater camera apparatuses (UCAs) for seabed photographic investigations of megabenthic epifauna
For a long time, vision has been one of the most direct senses used by human beings to explore the mysteries of the ocean. In 1893, Boutan took the fi rst known underwater photograph by diving with a camera, which was considered the pioneering work of seabed photography (Boutan, 1893). Because of the patchy distribution of benthos, the seabed photography method was considered more eff ective than traditional methods (corers and trawls) for the investigation of the megabenthic epifauna by means of diff erent UCAs (Hecker, 1990; Smith et al., 1993; Piepenburg and Von Juterzenka, 1994; Piepenburg and Schmid, 1996; Bergmann et al., 2011). The original triggered UCA developed by Ewing and Vevers was controlled by a foot trigger (Ewing et al., 1946; Vevers, 1951, 1952) (Fig.9a). When the foot trigger touches the seafl oor, the entire UCA is triggered to work, and seabed photographs are obtained via the up and down movements of the UCA, such as “the photo probe” applied in the Arctic areas and the “bed-hop camera system” applied in western Scotland (Piepenburg and Von Juterzenka, 1994; Piepenburg and Schmid, 1996, 1997; Stübing and Piepenburg, 1998; Hughes, 2014) (Table 4). To determine the precise photographed area and reduce the occurrence of clouds caused by the contact between the foot trigger and the seafl oor, acoustic-controlled UCAs triggered by sonar were designed by using precision graphic recorder (PGR), precision depth recorder (PDR) or distance monitoring system (DMS) (Johnson et al., 1956; Edgerton and Cousteau, 1959; Hersey, 1959; Backus, 1966; Edgerton and Udintsev, 1973; Ohta, 1976; 1984; Fujita et al., 1987; Fujita and Ohta, 1988, 1989). For acoustic-controlled UCAs, an acoustic pulse generator or a pinger is mounted on the camera frame and regularly emits sound signals upwards and downwards. The time interval between the upward signal (the direct ping) and the downward signal (the bottom echo) is received and recorded by a PGR, PDR, or DMS to locate the position of the underwater camera.
Generally, triggered UCAs are deployed when the ship is at a station or slowly drifting to obtain in situ seabed images at depths of 10–2 000 m (Table 4). To obtain continuous image of the seabed, the towed UCAs were developed that could be towed by a ship at a certain speed and photograph the seabed over a large area (i.e. 1–10 km transects), and consist of towed off -bottom UCAs and towed on-bottom UCAs. The towed off -bottom UCAs are towed above the seabed at a constant height (generally 2–5 m and sometimes up to 10 m) at a speed of less than 2 knots. The height above the seabed is monitored in real time by an acoustic system or a laser positioning system. These UCAs can take up to 1 000 photographs per run, thus providing continuous coverage of the seabed over lengths of several kilometers and widths of 2–8 m, such as the wide-area survey photography system (WASP), towed digital camera and multi-rock coring system (TowCam) (Fig.9b), TowCam fi sh, ocean fl oor observation system (OFOS) and Deep Towed Imaging System (DTIS), which were applied at depths of 200–5 000 m (Huggett, 1987; Bluhm, 1993, 2001; Gordon et al., 2000; Fornari and Group, 2003; Jones et al., 2007; Fodrie et al., 2009; Bergmann et al., 2011; Bowden et al., 2011; Rybakova et al., 2013). However, because of the large spatial scales of operation, the seabed images taken by off -bottom UCAs often have low resolution, and only animals with a maximum dimension of 2 cm or bigger can be precisely identifi ed (Gordon et al., 2000; Jones et al.,2007; Fodrie et al., 2009; Bowden et al., 2011). Towed on-bottom UCAs are called “camera sleds” or “benthic sleds”. These systems can be towed on the sea bottom using specifi cally designed sled runners (Uzmann et al., 1977; Theroux, 1984; Hecker, 1990; Nybakken et al., 1998; Ruhl, 2007) (Fig.9c). The height of the camera above the seabed is generally within 1 m so that the epifauna can be observed and identifi ed accurately in the photographs (Table 4). Therefore, towed on-bottom UCAs are more useful for investigations of certain species in a fl at area.
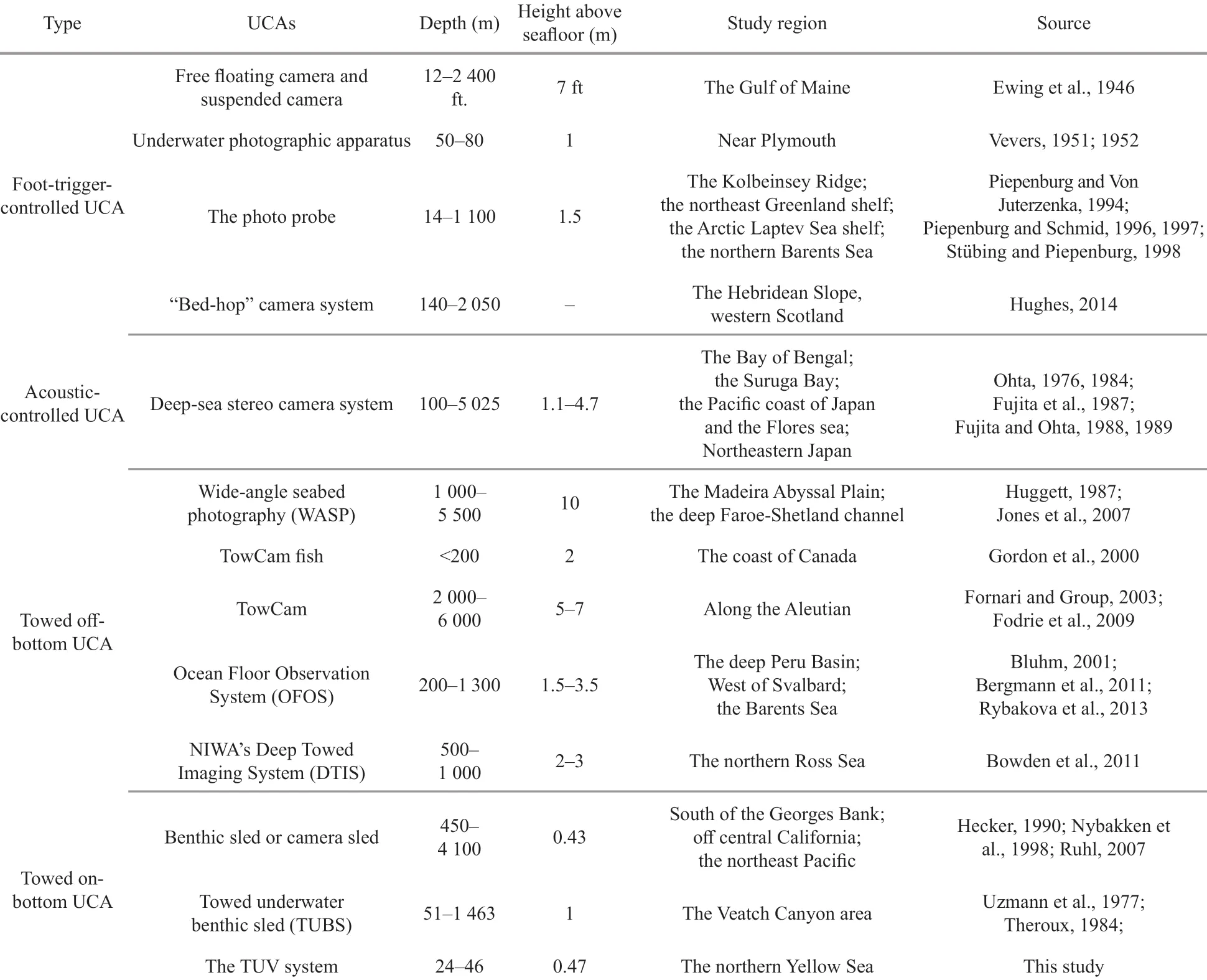
Table 4 Published underwater camera apparatuses (UCAs) for seabed photographic investigation of megabenthic epifauna
4.2 The TUV system used in this study
The TUV system in this study is categorized as a towed on-bottom UCA. For seabed photography, “clouds” are a major factor that interferes with the quality of photographs. These clouds are mainly caused when the underwater apparatus touches the sediment, and then muddy sand is stirred up via the fl ow of the current. To reduce the infl uence of clouds, we replaced the sled runners with wheels, which made this system more suitable for seabed with hard sandy sediments. In our design, the camera was always in front of the wheels because there was a 130° angle between the camera frame and the wheel frame. As long as the system was towed forward, the clouds caused by the wheels remained behind the system and did not enter the photographed area. However, we also found that once the system stalled, the clouds immediately fi lled the photographed area. Therefore, it was essential to keep the system running uniformly over the seafl oor during operations. This required cooperation between the speed of the ship and the wire rope of the winch, which can be practiced and summarized before job performing in diff erent areas and on diff erent ships. Certainly, calm weather and good topography were also important for the operation of the system as well as other UCAs.
The height of the main camera of the TUV system above the seafl oor is 47 cm, which is lower than that of most UCAs (Table 4). This height was determined by the transparency of the coastal waters and the stability of the TUV system. At this height, almost all of the epifauna larger than 1 mm could be observed, and their biological characteristics could be accurately described with this system, such as the disc diameters and cluster sizes of the brittle stars that were analyzed in this study. However, because of the limitations of the photographed area, the clusters ofO.mirabilislarger than 0.12 m2were diffi cult to precisely measure. Thus, in the future work, the photographed area should be increased to better analyze the biological characteristics of benthic organisms.
The accuracy of the UCAs usually depends on the precise location of the camera above the sea bottom. However, even with good shipboard controls and advanced measurement techniques, it was not always possible for all UCAs to maintain absolutely constant shot distance and altitude of the camera above the bottom. For example, for the TowCam applied in the Aleutian margin, only photographs taken at 3–5 m above the bottom were used for analysis (Fodrie et al., 2009). There were some contact delays in the foottriggered-controlled UCA when it was deployed in the soft sediments (Edgerton and Udintsev, 1973). In addition, for the Acoustic-controlled UCA, the stepwise output of the pinger was a limited factor on the accuracy of the shot distance measurement because there was no distance information recorded in the silent period of the pinger (Ohta, 1976). In the TUV system, due to the sandy sediments and unstable towing, the shot distance was mainly aff ected by the sinking and lifting of the rear wheel and side wheels. Therefore, after each operation, we would carefully check the videos from the surveillance cameras to analyze the condition of the system underwater. All good seabed photographs used for analysis were chosen when the wheels of the system were completely exposed on the seafl oor without serious sinking or lifting (no more than 3 cm).
For image analysis, some photo processing systems, programs, and databases were designed and developed to help with image correction, automated species identifi cation, and abundance calculation. For post-voyage analysis of DTIS, Ocean Floor Observation Protocol (OFOP) software was applied to run and correct video transects with precise spatial and temporal information (Bowden et al., 2011). For images acquired via benthic sleds, a perspective grid system was used to digitize the relative location and length of each individual, and the DISTANCE computer program was used to calculate the abundance of dominant megafauna (Wakefi eld and Genin, 1987; Ruhl, 2007). To accelerate and ease the time-consuming of manual image analysis, the BIIGLE web 2.0 database was used to label and recognize diff erent biota and habitats from images obtained by the Ocean Floor Observation System (OFOS) (Bergmann et al., 2011). Because the aggregative and patchy distribution is an important characteristic of benthos, in this study, we developed measurement software to quantify the living units of brittle stars, such as the disc diameter ofOphiurasarsiivadicolaandStegophiurasladeniand the cluster size ofOphiopholismirabilis, to help determine the patchy or small-scale distribution characteristics of benthic organisms.
5 CONCLUSION
The TUV system is a towed on-bottom UCA that can be applied in coastal waters. The test results from the Zhangzi Island sea area showed that this system could be used for photographic investigations of megabenthic epifauna, including evaluations of the composition, abundance, and population distribution characteristics. Further research is needed to focus on improving the photographed structure to obtain largescale seabed images to help analyze the aggregation and patchy distribution of the benthos. Meanwhile, a more intelligent and automated image-processing platform should be established to improve the effi ciency and quality of image processing. Additionally, it is necessary to apply the TUV system in other coastal waters to help establish the seabed photographic methods for research of benthic communities.
6 DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We thank the captain and the crew of R/V#19Liao-Chang-Yufor their help during the expedition to the Zhangzi Island sea area, and the support from Zhangzidao group.
References
Backus R H. 1966. The “Pinger” as an aid in deep trawling.ICESJournalofMarineScience, 30(2): 270-277, https://doi.org/10.1093/icesjms/30.2.270.
Barton O. 1935. Five hundred fathorms deep.NaturalHistoryMagazine, 35: 144-145.
Bergmann M, Langwald N, Ontrup J, Soltwedel T, Schewe I, Klages M, Nattkemper T W. 2011. Megafaunal assemblages from two shelf stations west of Svalbard.MarineBiologyResearch, 7(6): 525-539, https://doi.org/10.1080/17451000.2010.535834.
Bluhm H. 1993. Eff ects of deepsea mining for manganese nodules on the abyssal megabenthic community.In: Off shore Technology Conference. Off shore Technology Conference, Houston, Texas. https://doi.org/10.4043/ 7134-MS.
Bluhm H. 2001. Re-establishment of an abyssal megabenthic community after experimental physical disturbance of the seafl oor.DeepSeaResearchPartII:TopicalStudiesinOceanography, 48(17-18): 3 841-3 868, https://doi.org/ 10.1016/S0967-0645(01)00070-4.
Boutan L. 1893. Memoire sur la photographie sous-marine.ArchivesofZoologicalExperimentalGeneticsNotesReview, 21: 281-324.
Bowden D A, Schiaparelli S, Clark M R, Rickard G J. 2011. A lost world? Archaic crinoid-dominated assemblages on an Antarctic seamount.DeepSeaResearchPartII:TopicalStudiesinOceanography, 58(1-2): 119-127, https://doi.org/10.1016/j.dsr2.2010.09.006.
Edgerton H E, Cousteau J Y. 1959. Underwater camera positioning by sonar.ReviewofScientifi cInstruments, 30(12): 1 125-1 126, https://doi.org/10.1063/1.1716461.
Edgerton H E, Udintsev G. 1973. Rift valley observations by camera and pinger.DeepSeaResearchandOceanographicAbstracts, 20(7): 669-670, https://doi.org/10.1016/0011-7471(73)90035-1.
Ewing M, Vine A, Worzel J L. 1946. Photography of the ocean bottom.JournaloftheOpticalSocietyofAmerica, 36(6): 307-307, https://doi.org/10.1364/JOSA.36.000307.
Fodrie F J, Levin L A, Rathburn A E. 2009. High densities and depth-associated changes of epibenthic megafauna along the Aleutian margin from 2000-4200 m.JournaloftheMarineBiologicalAssociationoftheUnitedKingdom, 89(8): 1 517-1 527, https://doi.org/10.1017/S0025315409000903.
Fornari D J, Group T C. 2003. A new deep-sea towed digital camera and multi-rock coring system.Eos,TransactionsAmericanGeophysicalUnion, 84(8): 69-73, https://doi.org/10.1029/2003EO080001.
Fujita T, Ohta S, Oji T. 1987. Photographic observations of the stalked crinoidMetacrinusrotunduscarpenter in Suruga Bay, central Japan.JournaloftheOceanographicalSocietyofJapan, 43(6): 333-343, https://doi.org/10.1007/BF02109286.
Fujita T, Ohta S. 1988. Photographic observations of the life style of a deep-sea ophiuroidAsteronyxloveni(Echinodermata).DeepSeaResearchPartA.OceanographicResearchPapers, 35(12): 2 029-2 043, https://doi.org/10.1016/0198-0149(88)90123-9.
Fujita T, Ohta S. 1989. Spatial structure within a dense bed of the brittle starOphiurasarsi(Ophiuroidea: Echinodermata) in the bathyal zone off otsuchi, Northeastern Japan.JournalofOceanography, 45(5): 289-300, https://doi.org/10.1007/BF02123483.
Gordon D C, Kenchington E L R, Gilkinson K D, Mckeown D L, Steeves G, Chin-Yee M, Vass W P, Bentham K, Boudreau P R. 2000. Canadian imaging and sampling technology for studying marine benthic habitat and biological communities.In: Proceedings of the ICES 2000 Annual Science Conference. Bruges, Belgium.
Harvey E N. 1939. Deep-sea photography.Science, 90(2330): 187, https://doi.org/10.1126/science.90.2330.187.
Hecker B. 1990. Variation in megafaunal assemblages on the continental margin south of New England.DeepSeaResearchPartA.OceanographicResearchPapers, 37(1): 37-57, https://doi.org/10.1016/0198-0149(90)90028-T.
Hersey J B. 1959. Acoustically monitored bottom coring.DeepSeaResearch, 6(2): 170-172, https://doi.org/10.1016/0146-6313(59)90068-1.
Huggett Q J. 1987. Mapping of hemipelagic versus turbiditic muds by feeding traces observed in deep-sea photographs.GeologicalSociety,London,SpecialPublications, 31(1): 105-112, https://doi.org/10.1144/GSL.SP.1987.031.01.09.
Hughes D J. 2014. Benthic habitat and megafaunal zonation across the Hebridean Slope, western Scotland, analysed from archived seabed photographs.JournaloftheMarineBiologicalAssociationoftheUnitedKingdom, 94(4): 643-658, https://doi.org/10.1017/S0025315413001896.
Johnson E R. 1939. Under sea cinematography.JournaloftheSmpte-SocietyofMotionPictureandTelevisionEngineers, 3-17.
Johnson H R, Backus R H, Hersey J B, Owen D M. 1956. Suspended echo-sounder and camera studies of midwater sound scatterers.DeepSeaResearch, 3(4): 266-272, https://doi.org/10.1016/0146-6313(56)90016-8.
Jones D O B, Bett B J, Tyler P A. 2007. Megabenthic ecology of the deep Faroe–Shetland channel: a photographic study.DeepSeaResearchPartI:OceanographicResearchPapers, 54(7): 1 111-1 128, https://doi.org/10. 1016/j.dsr.2007.04.001.
Nybakken J, Craig S, Smith-Beasley L, Moreno G, Summers A, Weetman L. 1998. Distribution density and relative abundance of benthic invertebrate megafauna from three sites at the base of the continental slope off central California as determined by camera sled and beam trawl.DeepSeaResearchPartII:TopicalStudiesinOceanography, 45(8-9): 1 753-1 780, https://doi.org/10. 1016/S0967-0645(98)80016-7.
Ohta S. 1976. A precise and continuous monitoring system of the distance between the near-bottom instruments and the sea fl oor.JournaloftheOceanographicalSocietyofJapan, 32(2): 65-73. https://doi.org/10.1007/BF02107373.
Ohta S. 1984. Star-shaped feeding traces produced by echiuran worms on the deep-sea fl oor of the Bay of Bengal.DeepSeaResearchPartA.OceanographicResearchPapers, 31(12): 1 415-1 432, https://doi.org/10.1016/0198-0149(84)90080-3.
Peng S Y, Li X Z, Wang H F, Zhang B L. 2014. Macrobenthic community structure and species composition in the Yellow Sea and East China Sea in jellyfi sh bloom.ChineseJournalofOceanologyandLimnology, 32(3): 576-594, https://doi.org/10.1007/s00343-014-3068-8.
Piepenburg D, Schmid M K. 1996. Distribution, abundance, biomass, and mineralization potential of the epibenthic megafauna of the Northeast Greenland shelf.MarineBiology, 125(2): 321-332, https://doi.org/10.1007/BF00346313.
Piepenburg D, Schmid M K. 1997. A photographic survey of the epibenthic megafauna of the Arctic Laptev Sea shelf: distribution, abundance, and estimates of biomass and organic carbon demand.MarineEcologyProgress, 147(1-3): 63-75, https://doi.org/10.3354/meps147063.
Piepenburg D, Von Juterzenka K. 1994. Abundance, biomass and spatial distribution pattern of brittle stars (Echinodermata: Ophiuroidea) on the Kolbeinsey Ridge north of Iceland.PolarBiology, 14(3): 185-194, https://doi.org/10.1007/BF00240523.
Qi J, Li F Y, Song J M, Gao S, Wang G Z, Cheng P. 2004. Sedimentation rate and fl ux of the north Yellow sea.MarineGeology&QuaternaryGeology, 24(2): 9-14. (in Chinese with English abstract)
Ruhl H A. 2007. Abundance and size distribution dynamics of abyssal epibenthic megafauna in the northeast Pacifi c.Ecology, 88(5): 1 250-1 262, https://doi.org/10.1890/06-0890.
Rybakova E, Galkin S, Bergmann M, Soltwedel T, Gebruk A. 2013. Density and distribution of megafauna at the Håkon Mosby Mud Volcano (the Barents Sea) based on image analysis.Biogeosciences, 10(5): 3 359-3 374, https://doi.org/10.5194/bg-10-3359-2013.
Smith K L Jr, Kaufmann R S, Wakefi eld W W. 1993. Mobile megafaunal activity monitored with a time-lapse camera in the abyssal North Pacifi c.DeepSeaResearchPartI:OceanographicResearchPapers, 40(11-12): 2 307-2 324, https://doi.org/10.1016/0967-0637(93)90106-D.
Stübing D, Piepenburg D. 1998. Occurrence of the benthic trachymedusaPtychogastriapolarisAllman, 1878 (Cnidaria: Hydrozoa) off Northeast Greenland and in the northern Barents Sea.PolarBiology, 19(3): 193-197, https://doi.org/10.1007/s003000050234.
Theroux R B. 1984. Photographic systems utilized in the study of sea-bottom populations.In: Underwater Photography Scientifi c and Engineering Applications. Van Nostrand Reinhold Company, New York. p.69-94.
Uzmann J R, Cooper R A, Theroux R B, Wigley R L. 1977. Synoptic comparison of three sampling techniques for estimating abundance and distribution of selected megafauna: submersible vs camera sled vs otter trawl.MarineFisheriesReview, 39(12): 11-19.
Vevers H G. 1951. Photography of the sea fl oor.JournaloftheMarineBiologicalAssociationoftheUnitedKingdom, 30(1): 101-112, https://doi.org/10.1017/S0025315400012601.
Vevers H G. 1952. A photographic survey of certain areas of sea fl oor near plymouth.JournaloftheMarineBiologicalAssociationoftheUnitedKingdom, 31(2): 215-221, https://doi.org/10.1017/S0025315400052942.
Wakefi eld W W, Genin A. 1987. The use of a Canadian (perspective) grid in deep-sea photography.DeepSeaResearchPartA.OceanographicResearchPapers, 34(3): 469-478, https://doi.org/10.1016/0198-0149(87)90148-8.
Wang W, Li A C, Xu F J, Huang P, Li Y. 2009. Distribution of surface sediments and sedimentary environment in the North Yellow Sea.OceanologiaetLimnologiaSinica, 40(5): 525-531. (in Chinese with English abstract)
Xu Y H, Pan S M, Gao J H, Hou X L, Ma Y F, Hao Y P. 2018. Sedimentary record of plutonium in the North Yellow Sea and the response to catchment environmental changes of infl ow rivers.Chemosphere, 207: 130-138, https://doi.org/10.1016/j.chemosphere.2018.05.082.
Xu Y, Sui J X, Yang M, Sun Y, Li X Z, Wang H F, Zhang B L. 2016. Variation in the macrofaunal community over large temporal and spatial scales in the southern Yellow Sea.JournalofMarineSystems, 173: 9-20, https://doi.org/10. 1016/j.jmarsys.2016.11.006.
Zhang J H, Fang J G, Wang S H. 2008. Carrying capacity forPatinopectenyessoensisin Zhang Zidao Island, China.JournalofFisheriesofChina, 32(2): 236-241. (in Chinese with English abstract)
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Leptolaimus holovachovi sp. nov. (Nematoda) from Shenzhen Mangrove Nature Reserve, Shenzhen, South China*
- Profi le and development of microsatellite primers for Acanthogobius ommaturus based on high-throughput sequencing technology*
- Digging out molecular markers associated with low salinity tolerance of Nannochloropsis oceanica through bulked mutant analysis*
- Characteristics of zooplankton community in North Yellow Sea unveiled an indicator species for the Yellow Sea Warm Current in winter: Euchaeta plana*
- Intraguild predation by polyps of three scyphozoan jellyfi sh: Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum*
