Intraguild predation by polyps of three scyphozoan jellyfi sh: Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum*
TANG Changsheng , SUN Song , , ZHANG Fang ,
1 Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory of Marine Ecology and Environmental Sciences, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
5 Jiaozhou Bay Marine Ecosystem Research Station, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Jellyfi sh blooms occur worldwide and have resulted in serious problems in tourism, fi sheries, coastal industries, and the marine ecosystem. The life cycle of scyphozoan jellyfi sh consists of a pelagic medusa stage and a benthic polyp stage. Success of asexual reproduction of the polyps determines directly the number of medusae; thus, the polyp stage is the key to understanding the population dynamics of medusae. Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum are three scyphozoan jellyfi sh commonly inhabit in Chinese coastal waters. Polyps of A. coerulea are easily visible, while those of N. nomurai and R. esculentum remain yet to be found in the wild. However, distribution of the medusa indicates that the polyps of all three species may occur together. To evaluate the distribution pattern of polyps of the three species and explore intraguild predation by the polyps, we conducted a laboratory experiment that considered the attachment sequence and size relationship of calyx diameter of the polyps. We found that the polyps of A. coerulea preyed on polyps of the other two species in all treatments, except when polyps of R. esculentum were bigger than those of A. coerulea. The polyps of R. esculentum preyed on the polyps of N. nomurai only when polyps of R. esculentum attached fi rst and were bigger than those of N. nomurai. Colonies of N. nomurai polyps were rarely found in the places inhabited by A. coerulea polyps. In addition, A. coerulea polyps are known to inhabit at depths of less than 20 m in coastal sea, thus, we speculate that N. nomurai polyp colonies might occur at depths of more than 20 m. Therefore, our fi nding that polyps of A. coerulea aggressively preyed on polyps of other species may help understand other such systems of jellyfi sh bloom in the world.
Keyword: jellyfi sh bloom; polyps; intraguild predation; nature habitats
1 INTRODUCTION
In recent decades, jellyfi sh blooms are reported frequently from many coastal waters (Purcell et al., 2007; Pauly et al., 2009; Uye, 2014). Massive blooms of the giant jellyfi shNemopilemanomurai(Scyphozoa: Rhizostomea) have been observed in the Bohai Sea, Yellow Sea, and East China Sea every year since 2002, except for 2008, 2010, and 2011 (Dong et al., 2010; Kawahara et al., 2013; Sun et al., 2015b). The moon jellyfi shAureliaauritasp. l. (Linnaeus) is a species of worldwide distribution in inner neritic waters between 70°N and 40°S (Lucas, 2001). The species found in China isA.coerulea(Feng et al., 2017), and it mainly occurs along the tourist coasts and coastal aquaculture regions (Dong et al., 2010). Blooms of these two species have had negative impacts on tourism, fi sheries, coastal industries, and the marine ecosystem (Purcell et al., 2007; Uye, 2008). Blooming is thought to be related to climate change, overfi shing, eutrophication, hypoxia, agriculture, and increasing amounts of anthropogenic hard substrate (Purcell et al., 2007; Purcell, 2012; Sun et al., 2015a). Another species common to China is the edible jellyfi sh,Rhopilemaesculentum(Scyphozoa: Rhizostomea). It is one of the most important fi shery species in China (Dong et al., 2014), and it inhabits a wide range in the Bohai Sea, Yellow Sea, East China Sea, and northern South China Sea (You et al., 2007).
The life cycle of the scyphozoan jellyfi sh consists of a pelagic sexual medusa stage and a benthic asexual polyp stage. Sexual fertilization occurs in the female or in the open seawater (Dong et al., 2008; Schiariti et al., 2012) and fertilized eggs develop into freeswimming planulae, which settle on hard substrate and metamorphose into sessile polyps (Van Walraven et al., 2016). The polyps are perennials (Lucas, 2001), and asexual strobilation of the sessile polyps directly determines the population dynamics of the medusa. Therefore, increasing attention is being paid to the important benthic polyp stage (Kawahara et al., 2006; Hoover and Purcell, 2009; Feng et al., 2015a; Lee et al., 2017). However, the polyps of many species of jellyfi sh have never been found in the wild (Ceh and Riascos, 2017; Feng et al., 2017), and they are known only from experiments using sexual and asexual propagation.
Considering the overlap of spatial distribution of the medusa stage of the three scyphozoan jellyfi sh species common to Chinese coastal seas, it is possible that the polyps of each species develop near each other. The polyps ofA.auritacan reproduce by budding, fi ssion, or podocysts (Thein et al., 2012), and the polyps ofN.nomuraiandR.esculentumreproduce mainly by podocysts (Dong et al., 2013; Feng et al., 2015b), which produce colonies of millions of individuals that extend the spatial distribution of the polyps. After settlement, the polyps are surprising mobile, using stolons to ‘walk’ around diff erent surfaces or detaching and fl oating to new locations (Hoover and Purcell, 2009). As a result, it is likely that the polyps of diff erent species encounter each other.
The polyps ofAureliasp. l. have been found on natural substrates, such as bare rock, shells, amphipod and polychaete tubes, ascidians, and macroalgae and on artifi cial substrates, such as glass, ceramic, or plastic pieces in the sea (Lucas, 2001). The medusae ofN.nomuraialso breed and form polyps along coastal waters, but the polyps ofN.nomuraihave not yet been found in the fi eld (Toyokawa et al., 2012). However, Feng et al. (2015a) suggested that the Changjiang River estuary and northern Liaodong Bay might be their nursery ground. Confi rming the nursery ground ofN.nomurairemains one of the most important topics of the Chinese National Basic Research Project (Sun et al., 2015a). The polyps ofR.esculentumhave not been found in the fi eld, despite numerous fi eld surveys along Chinese coastal waters (Dong et al., 2013). Why the habitat of polyps of these two species remains unknown is still a puzzle (Feng et al., 2017).
To better understand the dynamics of jellyfi sh blooms, it is essential to understand the intraguild predation relationship of polyps of the most common scyphozoan jellyfi sh species in the Bohai Sea, Yellow Sea, and East China Sea; determine their distribution patterns; and identify possible natural habitats that may host wild polyps ofN.nomuraiandR.esculentum. To our knowledge, no previous studies have focused on the intraguild predation relationship of the polyp stage of these jellyfi sh species at the individual level.
2 MATERIAL AND METHOD
2.1 Material
Mature medusae ofN.nomurai(6 females, 4 males) were captured by hand nets in Jiaozhou Bay in September 2013 and then transferred to a 30-m3pond for fertilization in the laboratory of the Institute of Oceanology, Chinese Academy of Sciences, Qingdao. The rearing temperature and salinity were maintained at 20±0.5°C and 30±0.5, respectively. After planulae were detected, parent medusae were removed and polyethylene corrugated plates were placed in the pond for attachment. Polyps ofN.nomurai(abbreviated to NP hereafter) with two to four tentacles formed on the plates within a week and developed fully to polyps with 16 tentacles one month later. Polyps ofA.coerulea(abbreviated to AP hereafter) were also cultured at the Institute of Oceanology, Chinese Academy of Sciences, Qingdao. Polyethylene corrugated plates were used for attachment of planulae produced by mature medusaeofA.coeruleacollected from nearby Jiaozhou Bay in May 2014. Fully developed polyps ofA.coeruleawere formed after a month at 20±0.5°C and 30±0.5 in sand-fi ltered seawater. Polyps ofR.esculentum(abbreviated to RP hereafter) were bred at Yancheng Jinyang Aquatic Products Breeding Company in Jiangsu, China, in June 2014. Polyps of the three species were fed with newly hatchedArtemianauplii for 1–2 h every 3 d, and freshly sand-fi ltered seawater was used for replacement every 2 d before the experiment. The cultivation temperature and salinity were maintained at 18±0.5°C and 30±0.5, respectively.
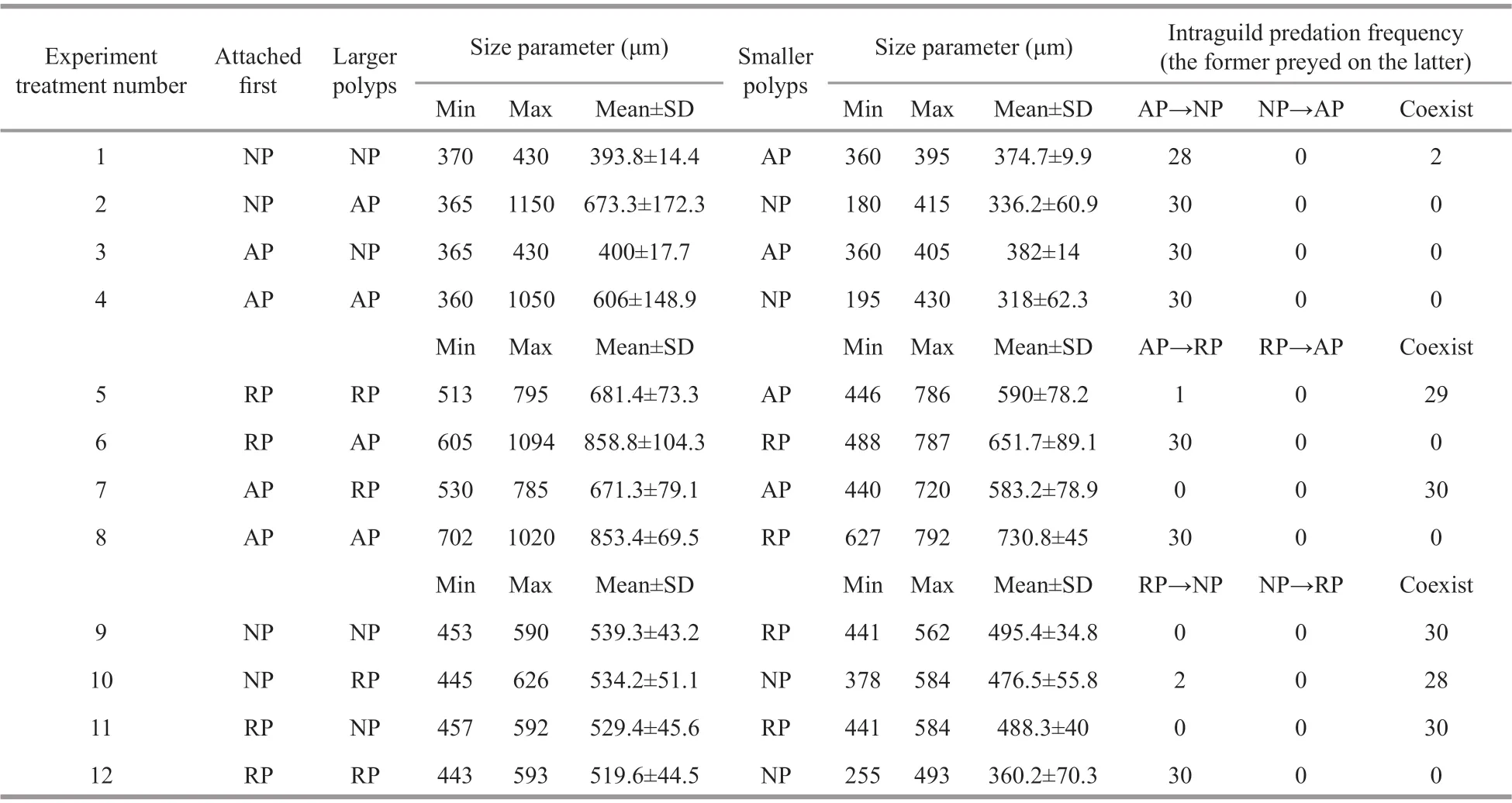
Table 1 Intraguild predation results of all treatments
2.2 Method
In the fi eld, the medusae of the three species become mature at diff erent periods of time (Wang, 2013; Dong et al., 2014; Sun et al., 2015b), indicating that the attachment sequence of the polyps would diff er among diff erent species. After formation, the body size of the polyps also diff ers among diff erent species. To approximately simulate the encounter situation of the polyps of the three species in the fi eld, polyps of each species were allowed to attach fi rst, then they were fed polyps of another species. Additionally, the polyp size of the fi rst species, relative to the second species, was experimentally manipulated.
Fully developed polyps with 16 tentacles were used in the experiment. For each case, the polyps of one species were detached carefully from the root of the pedal disc with a dissecting needle under a dissecting microscope. Ten undamaged polyps were transferred to one well of a six-well cell culture plate containing 15-mL of freshly fi ltered seawater. After 2-d of acclimation without food in an incubator (18°C), all polyps were able to reattach. Ten polyps of another species, also detached carefully from the settle plates, then were collected into a capillary pipette and released within the tentacles of each polyp of the former species. The calyx diameter of all polyps was measured with the ocular micrometer, and the predation reactions of each polyp and its fi nal state after 5 d were observed. Because the calyxes of the polyps were not uniformly circular, the approximate diameter was calculated by the average of maximum and minimum diameters. Twelve situations were tested, with three replicates containing 30 pairs of polyps in total per situation (Table 1). For every replicate, the predation percentage was recorded as follows:
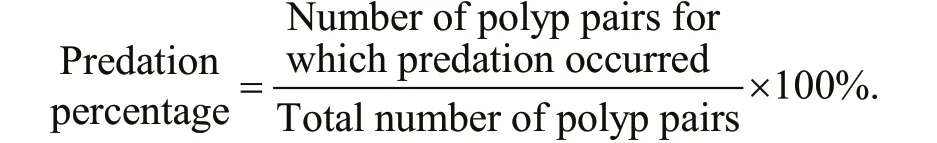
The experiment was conducted in a temperature controlled room (18±0.5°C). The seawater in the culture plates was cautiously replaced with freshly fi ltered seawater of the same temperature and salinity daily. The experiment began on September 23, 2014 and lasted for 50 d.
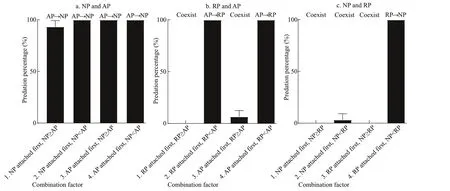
Fig.1 Mean predation percentage of polyps (mean±SD) for two attachment sequences and two size relationships of calyx diameter

Table 2 Summary of variables not in the results of the binary logistic regression equation
2.3 Overview of the sea area
The sea surface temperature and average salinity of the Bohai Sea, Yellow Sea, and East China Sea ranges from -1.5 to 29.7°C and 30 to 34, respectively. The major ocean currents include the Kuroshio, Yellow Sea Warm Current, Yellow Sea Cold Water Mass, Bohai Sea circulation, and longshore current. The area is characterized by a monsoon climate, which mainly includes a warm temperate monsoon and a subtropical monsoon climate. The most remarkable feature of the topography is the wide stretch of continental shelf, which tilts from northwest to southeast (Su, 2005). The seafl oor mainly consist of argillaceous sediment in the nearshore area and arenaceous sediment in the central and off shore areas (Liu, 1992).
2.4 Statistical analysis
Binary logistic regression using SPSS 23.0 was used to test the following three null hypotheses, and GraphPad Prism 8 was used to plot the results:
H01:whether or not AP preyed on NP was not related to attachment sequence and size relationship;
H02: whether or not AP preyed on RP was not related to attachment sequence and size relationship;
H03: whether or not RP preyed on NP was not related to attachment sequence and size relationship.
3 RESULT
Neither asexual reproduction nor cyst formation was observed in any treatment during 5 d under the given experimental condition. Polyps ofA.coeruleapreyed on polyps ofN.nomuraiin all treatments containing this pair (Fig.1a) and digested them into white fl occulent residues, some of which were released from theA.coeruleamouth, within 24 h. The calyx diameter of polyps ofN.nomuraiandA.coeruleawere 180–430 μm and 360–1 150 μm, respectively. AP predation on NP did not diff er signifi cantly with attachment sequence or size relationship (Table 2); thus,H01was not refused.
Polyps ofA.coeruleapreyed on polyps ofR.esculentumwhen polyps ofA.coeruleawere larger than polyps ofR.esculentumand digested them into white fl occulent residues within 24 h, regardless of which polyp species attached fi rst. However, the two species could coexist when the polyps ofA.coeruleawere smaller than the polyps ofR.esculentumfor more than 5 d (Fig.1b). The calyx diameter of polyps ofR.esculentumandA.coeruleawere 488–795 μm and 440–1 094 μm, respectively. AP predation on RP diff ered depending on calyx size (Table 2), and therefore,H02was rejected. Further analysis of the variables revealed that the predation reaction diff erence was caused by the calyx size relationship of RP and AP (Table 2).
The polyps ofR.esculentumpreyed on polyps ofN.nomuraionly when RP attached fi rst and the calyx diameter of RP was larger than that of NP. Under these conditions, RP digested NP into white fl occulent residues within 24 h. When these condition were not present, polyps of the two species could coexist for more than 5 d (Fig.1c). The calyx diameter of polyps ofN.nomuraiandR.esculentumwere 255–592 μm and 441–626 μm, respectively. RP predation on NP diff ered depending on conditions (Table 2), thusH03was rejected. Further analysis of the variables revealed that the predation reaction diff erence was caused by both the attachment sequence and the calyx size relationship of NP and AP (Table 2).
4 DISCUSSION
In this study, the polyps ofA.coeruleapreyed on the polyps ofN.nomurai. When AP attached fi rst, NP were captured by the tentacles of AP and placed in the mouth of the polyps within a few hours. When NP attached fi rst, polyps of both species exhibited the capture reaction of the tentacles, but ultimately AP were able to turn upside down, swallow NP, and digest them into white fl occulent residues within 24 h. The calyx diameter size relationship of AP and NP did not signifi cantly aff ect the predation reaction. This fi nding is not unique among cnidarians, as Kaliszewicz (2013) concluded that larger size did not guarantee competitive superiority among hydras, which are also sessile sit-and-wait predators. Our data showed that the newly formed polyps ofN.nomuraiwere preyed upon by the polyps ofA.coerulea, even when theA.coeruleapolyps were present for only a short time. Even when the colony of polyps ofN.nomuraiformed fi rst, the newly formedA.coeruleapolyps preyed on them and took over the living space. These results indicate that colonies ofN.nomuraipolyps could not survive in locations inhabited byA.coerulea.
The polyps ofA.coeruleaare distributed widely in coastal waters (Malej et al., 2012). Our scuba diving group foundA.coeruleapolyps along the coast of Shandong Province (Qingdao), Liaoning Province (Dalian), and Hebei Province (Qinghuangdao), and this distribution might explain why it is so diffi cult to fi ndN.nomuraipolyps in the fi eld. Russell (1970) reported thatA.auritapolyps were not found deeper than 20 m, and a variety of in situ experiments carried out at depths between 0.3 m and 25 m reported the same fi nding (Brewer, 1978; Hernroth and Gröndahl, 1985; Keen, 1987; Feng et al., 2017). Relative to the medusae ofA.aurita, which occur mainly in nearshore waters (Dong et al., 2012; Wan and Zhang, 2012) and are rare in deep waters (Zhang et al., 2012),N.nomuraiis a deep sea species that shows diurnal vertical movement and migration between depths of over 100 m and the surface (Honda et al., 2009). Consequently, we speculate that the polyps ofN.nomuraimight be located in their natural habitat at a depth >20 m in Chinese coastal waters or in locations where polyps ofA.coeruleaare not present. Similarly, Ceh and Riascos (2017) proposed that the polyps of the scyphozoan jellyfi shChrysaoraplocamiai, whose natural habitat remains unknown, might be located up to 20-m deep based on the results of planulae settlement preference for substrate color.
The results of our experiment showed that polyps ofA.coeruleapreyed on polyps ofR.esculentumwhen the calyx diameter size of the former was larger than that of the latter. However, AP could not prey on RP when the calyx diameter of AP was smaller than that of RP; slight damage to the tentacles of RP was detected, but both polyp types survived and coexisted for more than 5 d. Similarly, Li et al. (2012) and Chi et al. (2013) reported that AP and RP could coexist for 24 h. Our results suggest that the ability of AP to prey on RP is determined by the calyx size relationship between the two polyp types, but overall AP is competitively superior to RP, even though colonies of the two species can coexist to some extent.
Polyps ofR.esculentumandN.nomuraicoexisted for more than 5 d in most cases, except whenR.esculentumpolyps attached fi rst and when the calyx diameter was larger than that ofN.nomurai. This result indicates that the polyps ofR.esculentumare slightly more competitive than those ofN.nomurai. It might be attributed to the same class and order (Scyphozoan: Rhizostomea) they shared and similar morphological characteristics (Liu et al., 2009).
The polyps ofA.coeruleaaggressively outcompeted polyps of other species, and this pattern might be true in other coastal areas of the world, especially in areas where blooms of diff erent species of jellyfi sh co-occur. Intraguild predation by the polyps might be useful for the study of searching for polyps of other species in other areas as well.
5 CONCLUSION
In this study,A.coeruleaandR.esculentumpolyps were able to coexist to some extents, and the same was true forR.esculentumandN.nomuraipolyps. However, it was almost impossible forN.nomuraipolyps to settle and form colonies in locations inhabited byA.coerulea. Considering the wide distribution of polyps ofA.coeruleaalong Chinese coastal seas at a depth of less than 20 m, we propose thatN.nomuraipolyps might be located in the natural habitat at a depth of more than 20 m. Future studies should search for the polyps ofN.nomuraiat greater depths and confi rm the location of nursery grounds. The results of this study off er valuable insights into competition between polyps of diff erent co-occurring species of jellyfi sh that likely will be applicable to other locales.
6 DATA AVAILABILITY STATEMENT
All data generated and/or analyzed during this study are available upon request by contacting with the corresponding author.
References
Brewer R H. 1978. Larval settlement behavior in the jellyfi shAureliaaurita(Linnaeus) (Scyphozoa: Semaeostomeae).Estuaries, 1(2): 120.
Ceh J, Riascos J M. 2017. Cryptic life stages in scyphozoan jellyfi sh: larval settlement preferences of the South American sea nettleChrysaoraplocamia.JournalofExperimentalMarineBiologyandEcology, 490: 52-55.
Chi X P, You K, Ma C H, Yuan Y H, Chen S Q, Yang Y F, Liu X T. 2013. Preliminary study on the competition in the larvae stage betweenAureliaauritaandRhopilemaesculentumduring a short term.ActaOceanologicaSinica, 35(6): 140-146. (in Chinese with English abstract)
Dong J, Jiang L X, Sun M, Wang B, Li Y L, Tan K F, Chai Y, Sun S. 2013. Research on the Biological of Large Jellyfi sh in the Bohai Sea and Northern Yellow Sea. Ocean Press, Beijing. (in Chinese)
Dong J, Jiang L X, Tan K F, Liu H Y, Purcell J E, Li P J, Ye C C. 2008. Stock enhancement of the edible jellyfi sh (RhopilemaesculentumKishinouye) in Liaodong Bay, China: a review.Hydrobiologia, 616(1): 113-118.
Dong Z J, Liu D Y, Keesing J K. 2010. Jellyfi sh blooms in China: dominant species, causes and consequences.MarinePollutionBulletin, 60(7): 954-963.
Dong Z J, Liu D Y, Keesing J K. 2014. Contrasting trends in populations ofRhopilemaesculentumandAureliaauritain Chinese waters.In: Pitt P A, Lucas C H eds. Jellyfi sh Blooms. Springer, Dordrecht. p.207-218.
Dong Z J, Liu D Y, Wang Y J, Di B P, Song X K, Shi Y J. 2012. A report on a moon jellyfi shAureliaauritabloom in Sishili Bay, northern Yellow Sea of China in 2009.AquaticEcosystemHealth&Management, 15(2): 161-167.
Feng S, Wang S W, Sun S, Zhang F, Uye Si. 2017. Strobilation of three scyphozoans (Aureliacoelurea,NemopilemanomuraiandRhopilemaesculentum) in the fi eld at Jiaozhou Bay, China.MarineEcologyProgressSeries, 591: 141-153.
Feng S, Wang S W, Zhang G T, Sun S, Zhang F. 2017. Selective suppression ofinsituproliferation of scyphozoan polyps by biofouling.MarinePollutionBulletin, 114(2): 1 046-1 056.
Feng S, Zhang F, Sun S, Wang S W, Li C L. 2015a. Eff ects of duration at low temperature on asexual reproduction in polyps of the scyphozoanNemopilemanomurai(Scyphozoa: Rhizostomeae).Hydrobiologia, 754(1): 97-111.
Feng S, Zhang G T, Sun S, Zhang F, Wang S W, Liu M T. 2015b. Eff ects of temperature regime and food supply on asexual reproduction inCyaneanozakiiandNemopilemanomurai.Hydrobiologia, 754(1): 201-214.
Hernroth L, Gröndahl F. 1985. On the biology ofAureliaaurita(L.) 3. Predation byCoryphellaverrucosa(gastropoda, opisthobranchia), a major factor regulating the development ofAureliapopulations in the Gullmar Fjord, western Sweden.Ophelia, 24(1): 37-45.
Honda N, Watanabe T, Matsushita Y. 2009. Swimming depths of the giant jellyfi shNemopilemanomuraiinvestigated using pop-up archival transmitting tags and ultrasonic pingers.FisheriesScience, 75(4): 947-956.
Hoover R A, Purcell J E. 2009. Substrate preferences of scyphozoanAurelialabiatapolyps among common dockbuilding materials.Hydrobiologia, 616(1): 259-267.
Kaliszewicz A. 2013. Is larger better in sit-and-wait predators? Competitive superiority inHydra.Hydrobiologia, 714(1): 105-114.
Kawahara M, Ohtsu K, Uye S I. 2013. Bloom or non-bloom in the giant jellyfi shNemopilemanomurai(Scyphozoa: Rhizostomeae): roles of dormant podocysts.JournalofPlanktonResearch, 35(1): 213-217.
Kawahara M, Uye S I, Ohtsu K, Iizumi H. 2006. Unusual population explosion of the giant jellyfi shNemopilemanomurai(Scyphozoa: Rhizostomeae) in East Asian waters.MarineEcologyProgressSeries, 307: 161-173.
Keen S L. 1987. Recruitment ofAureliaaurita(Cnidaria: Scyphozoa) larvae is position-dependent, and independent of conspecifi c density, within a settling surface.MarineEcologyProgressSeries, 38: 151-160.
Lee H E, Han C H, Kim B, Yoon W D. 2017. Eff ects of temperature and salinity on the asexual reproduction ofNemopilemanomurai(Scyphozoa: Rhizostomeae).OceanScienceJournal, 52(4): 573-579.
Li Z F, Liu C S, Zhuang Z M, Zou A G, Chen S Q, Yan J P, Liu C L. 2012. The mutual predatory relationship of jellyfi shes in diff erent life stages betweenAureliasp.1 andRhopilemaesculenta.OceanologiaetLimnologiaSinica, 43(3): 539-544. (in Chinese with English abstract)
Liu C Y, Wang W B, Dong J, Wang Y Q, Zhou H, Sun M, Lu Z C, Yu X G. 2009. The life history ofRhopilemahispidumand comparison of morphological characteristics among some scyphistomae.ProgressinFisherySciences, 30(4): 102-107. (in Chinese with English abstract)
Liu X Q. 1992. Map of the newest bottom-material types of china off shore continental shelf.MarineGeology&QuaternaryGeology, 12(4): 11-20. (in Chinese with English abstract)
Lucas C H. 2001. Reproduction and life history strategies of the common jellyfi sh,Aureliaaurita, in relation to its ambient environment.Hydrobiologia, 451(1-3): 229-246.
Malej A, Kogovšek T, Ramšak A, Catenacci L. 2012. Blooms and population dynamics of moon jellyfi sh in the northern Adriatic.CahiersdeBiologieMarine, 53(3): 337-342
Pauly D, Graham W, Libralato S, Morissette L, Palomares M L D. 2009. Jellyfi sh in ecosystems, online databases, and ecosystem models.Hydrobiologia, 616(1): 67-85.
Purcell J E, Uye S I, Lo W T. 2007. Anthropogenic causes of jellyfi sh blooms and their direct consequences for humans: a review.MarineEcologyProgressSeries, 350: 153-174.
Purcell J E. 2012. Jellyfi sh and ctenophore blooms coincide with human proliferations and environmental perturbations.AnnualReviewofMarineScience, 4: 209-235.
Russell F S. 1970. The Medusae of the British Isles II. Pelagic Scyphozoa, with a Supplement to the First Volume of Hydromedusae. Cambridge University Press, New York. 284p.
Schiariti A, Christiansen E, Morandini A C, Da Silveira F L, Giberto D A, Mianzan H W. 2012. Reproductive biology ofLychnorhizalucerna(Cnidaria: Scyphozoa: Rhizostomeae): Individual traits related to sexual reproduction.MarineBiologyResearch, 8(3): 255-264.
Su J L. 2005. Off shore Hydrology of China. Ocean Press, Beijing. (in Chinese)
Sun S, Sun X X, Jenkinson I R. 2015a. Preface: giant jellyfi sh blooms in Chinese waters.Hydrobiologia, 754(1): 1-11.
Sun S, Zhang F, Li C L, Wang S W, Wang M X, Tao Z C, Wang Y T, Zhang G T. 2015b. Breeding places, population dynamics, and distribution of the giant jellyfi shNemopilemanomurai(Scyphozoa: Rhizostomeae) in the Yellow Sea and the East China Sea.Hydrobiologia, 754(1): 59-74.
Thein H, Ikeda H, Uye S I. 2012. The potential role of podocysts in perpetuation of the common jellyfi shAureliaauritas.l. (Cnidaria: Scyphozoa) in anthropogenically perturbed coastal waters.Hydrobiologia, 690(1): 157-167.
Toyokawa M, Shibata M, Cheng J H, Li H Y, Ling J Z, Lin N, Liu Z L, Zhang Y. 2012. First record of wild ephyrae of the giant jellyfi shNemopilemanomurai.FisheriesScience, 78(6): 1 213-1 218.
Uye S I. 2008. Blooms of the giant jellyfi shNemopilemanomurai: a threat to the fi sheries sustainability of the East Asian Marginal Seas.PlanktonandBenthosResearch, 3(S1): 125-131.
Uye S I. 2014. The giant jellyfi shNemopilemanomuraiin East Asian Marginal Seas.In: Pitt P A, Lucas C H eds. Jellyfi sh Blooms. Springer, Dordrecht. p.185-205.
Van Walraven L, Driessen F, Van Bleijswijk J, Bol A, Luttikhuizen P C, Coolen J W P, Bos O G, Gittenberger A, Schrieken N, Langenberg V T, Van Der Veer H W. 2016. Where are the polyps? Molecular identifi cation, distribution and population diff erentiation ofAureliaauritajellyfi sh polyps in the southern North Sea area.MarineBiology, 163(8): 172.
Wan A Y, Zhang G T. 2012. Annual occurrence of moon jellyfi shAureliasp.1 in the Jiaozhou Bay and its impacts on zooplankton community.OceanologiaetLimnologiaSinica, 43(3): 494-501. (in Chinese with English abstract)
Wang Y T, Zheng S, Sun S, Zhang F. 2015. Eff ect of temperature and food type on asexual reproduction inAureliasp.1 polyps.Hydrobiologia, 754(1): 169-178.
Wang, Y T. 2013. Study on the Key Process of Life Cycle ofAureliacoerulea, Institute of Oceanology, Chinese Academy of Sciences, 9-10. (in Chinese)
You K, Ma C H, Gao H W, Li F Q, Zhang M Z, Qiu Y T, Wang B. 2007. Research on the jellyfi sh (RhopilemaesculentumKishinouye) and associated aquaculture techniques in China: current status.AquacultureInternational, 15(6): 479-488.
Zhang F, Sun S, Jin X S, Li C L. 2012. Associations of large jellyfi sh distributions with temperature and salinity in the Yellow Sea and East China Sea.Hydrobiologia, 690(1): 81-96.
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Leptolaimus holovachovi sp. nov. (Nematoda) from Shenzhen Mangrove Nature Reserve, Shenzhen, South China*
- Profi le and development of microsatellite primers for Acanthogobius ommaturus based on high-throughput sequencing technology*
- Digging out molecular markers associated with low salinity tolerance of Nannochloropsis oceanica through bulked mutant analysis*
- An enhanced underwater camera apparatus for seabed observation of megabenthic epifauna in the northern Yellow Sea*
- Characteristics of zooplankton community in North Yellow Sea unveiled an indicator species for the Yellow Sea Warm Current in winter: Euchaeta plana*
