Characteristics of zooplankton community in North Yellow Sea unveiled an indicator species for the Yellow Sea Warm Current in winter: Euchaeta plana*
CHEN Hongju , ZHU Yanzhong , LIU Guangxing ,
1 Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China, Qingdao 266100, China
2 Marine Ecology and Environmental Science Laboratory, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266200, China
3 Research Center of Ecological Environment for Yangtze River Economic Belt, Chinese Research Academy of Environmental Sciences, Beijing 100012, China
Abstract Zooplankton distributions are largely infl uenced by both biotic and abiotic factors in the surrounding environment. Some zooplankton species can be used as bio-indicators for particular currents and water masses to help discover the dynamics of water current in the ocean. In this study, we investigated the distribution of zooplankton in North Yellow Sea (NYS) during winter when the Yellow Sea Warm Current (YSWC) infl uenced that area. Zooplankton communities in the NYS were dominated by temperate and warm-temperate species, such as Calanus sinicus, Paracalanus parvus, Acartia bifi losa, and Sagitta crassa. Two warm-water species, Eucheata plana and Sagitta enfl ata were also present. Cluster analysis grouped NYS zooplankton into three communities, the Shandong Coastal Community (SCC) in the Shandong neritic area, the Yellow Sea Central Community (YSCC) in the central waters of the NYS, and the Liaoning Coastal Community (LCC) in the Liaoning neritic area. Abundances varied signifi cantly among these communities, with an average of 102.2 ind./m 3 in SCC, 179.8 ind./m 3 in YSCC, and 1 244.2 ind./m 3 in LCC. Depth and the bottom (3 m from the sea fl oor) temperature were likely the primary abiotic factors infl uencing zooplankton distributions. The appearance of E. plana, an indicator species for the YSWC path, indicated a signifi cant northwestward trend for the YSWC in 2007.
Keyword: North Yellow Sea; Yellow Sea Warm Current; zooplankton; bio-indicator; community structure
1 INTRODUCTION
Distributions of marine zooplankton are largely infl uenced by abiotic and biotic environmental factors, and these distributions can expand or contract in response to water current variations (Beaugrand, 2005; Hays et al., 2005; Bi et al., 2011). Subtle environmental perturbations can be amplifi ed by nonlinear responses of zooplankton communities, thus zooplankton can be considered indicators of environmental change in some cases (Taylor et al., 2002; Hays et al., 2005). Many zooplankton species can live in a wide range of temperatures and salinities, while some are restricted by specifi c salinity and temperature requirements (Lalli and Parsons, 1993). Such narrowly adapted zooplankton is frequently indicator species and their presence or absence can be used to infer the infl uence of diff erent water masses on a given region (Hooff and Peterson, 2006). Use of zooplankton as bio-indicator provides us valuable information for understanding the transport paths of currents and water masses in the ocean (Hsieh et al., 2004; Hooff and Peterson, 2006).
The Yellow Sea Warm Current (YSWC) originates outside the Yellow Sea (YS), but is one of the most important components of YS circulation, and has attracted wide attention among oceanographic researchers (Le, 1992; Lie et al., 2001; Tang et al., 2001; Wang et al., 2009; Zhao et al., 2011). The YSWC transports warm, saline water from the northeastern East China Sea (ECS) into the YS, and then enters into the inner part of the Bohai Sea (BS) (Guan and Mao, 1982; Su, 1989; Xu et al., 2009; Zhao et al., 2011). The YSWC shows seasonal variability, it is stronger in winter, penetrating well into the YS trough, but becomes much weaker in summer when it is limited to the southern YS trough (Xu et al., 2009). Presence of the YSWC can be tracked based on the horizontal and vertical characteristics of sea temperature and salinity in the North Yellow Sea (NYS) (Bao et al., 2009). Although many researches have studied the YSWC, knowledge about its path and variability in the NYS is still limited (Song et al., 2009).
It is known that the advection of water masses can signifi cantly change the composition of zooplankton communities (Willis et al., 2006). The YSWC brings warm, saline water into the YS, and also transports warm-water species from the northeastern ECS into this region. For example, planktonic foraminifera are good indicators of the YSWC path in the South Yellow Sea (SYS) (Cheng and Cheng, 1962). It has been reported that the occurrence and distributions of several warm-water species, such asEuchaetaplana,E.marina,Sagittaenfl ata,DolilumdenticulatumandAcanthostomellaminutissimain the SYS are related to the presence of the YSWC (Wang et al., 2003, 2013; Chen et al., 2018). Yet, it is still not clear whether these warm-water species can be used as bioindicators for the YSWC in the NYS. In the present study, we characterized the zooplankton community in the NYS based on samples taken during a winter cruise, which covered a large area of the NYS with high spatial resolution. The observed warm-water zooplankton are likely useful bio-indicators for the YSWC in the NYS.
2 MATERIAL AND METHOD
2.1 Sampling procedure
Zooplankton samples were collected from 81 stations in the NYS (36.95°N–39.66°N, 120.95°E–124.02°E) on board R/VDongfanghong2in January 2007 (Fig.1). A ring net (0.8-m diameter, 505-μm mesh) was hauled vertically from the nearbottom (3 m above sea fl oor) to sea surface at a rate of 0.8–1.0 m/s. The net was washed after each tow, and zooplankton samples were preserved in 5% formalin (in seawater) for further analyses.
All zooplankton taxa present in the samples were identifi ed to species level whenever possible, and counted under a stereomicroscope (Leica S8APO, Leica Corporation). The temperature, salinity, and water depth were recorded using a CTD profi ler (Sea-Bird SBE 9, Sea-Bird Electronics, Inc.). Water samples for chlorophylla(chla) analysis were fi ltered through Whatman GF/F fi lters, and the fi lters carrying samples were kept at -20°C until further analysis. Chlafi lters were extracted in 90% acetone, and concentrations determined with a Hitachi F-4500 fl uorescence spectrophotometer.
2.2 Data analysis
Zooplankton data were converted to abundance expressed as individuals per cubic meter (ind./m3) based on the fi ltered water volume (sampling depth × net mouth area). The statistical package Plymouth Routines in Multivariate Ecological Research version 6 (PRIMER v6.1.10, PRIMER-E Ltd.) was used to analyze the data. Biological data were log10(x+1)-transformed, allowing less abundant species to exert some infl uence on the calculation of similarities (Clarke and Warwick, 2001). Hierarchical cluster analyses of similarity between stations were computed on the basis of the Bray-Curtis similarity index. Station groups were arbitrarily identifi ed, and the SIMPROF (similarity profi le) test was applied to determine signifi cant diff erences between the clusters. The one-way ANOSIM (analysis of similarities) test was performed to test for diff erences among station groups (Clarke and Warwick, 2001). Correlations between the biotic and environmental data were determined using the RELATE routine. The BIOENV routine was used to test which environmental variables (depth, surface chla, surface temperature, bottom temperature, surface salinity, and salinity at depth) accounted for patterns in the species data. The relationship between zooplankton abundance and environmental parameters was analyzed by principal component analysis (PCA).
3 RESULT
3.1 Species composition
A total of 39 zooplankton taxa and 19 planktonic larvae were identifi ed (Table 1). Copepods andplanktonic larvae were the dominant organisms. The zooplankton community was dominated by temperate and warm-temperate species, includingCalanussinicus,Paracalanusparvus,Acartiabifi losa, andSagittacrassa. At the same time, two typical warmwater species,EucheataplanaandSagittaenfl atawere recorded.
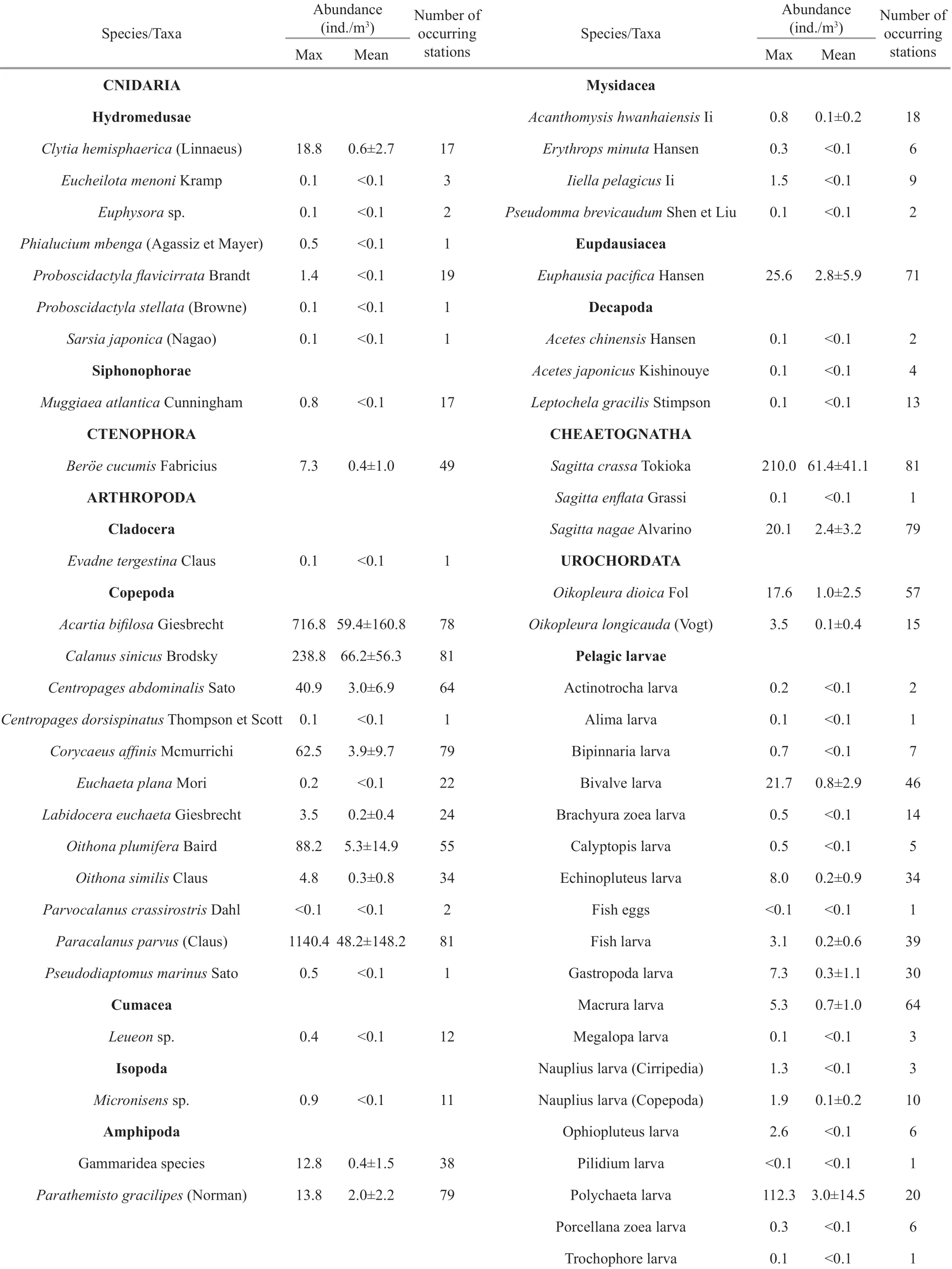
Table 1 The abundance of zooplankton taxa and the number of stations where they appeared
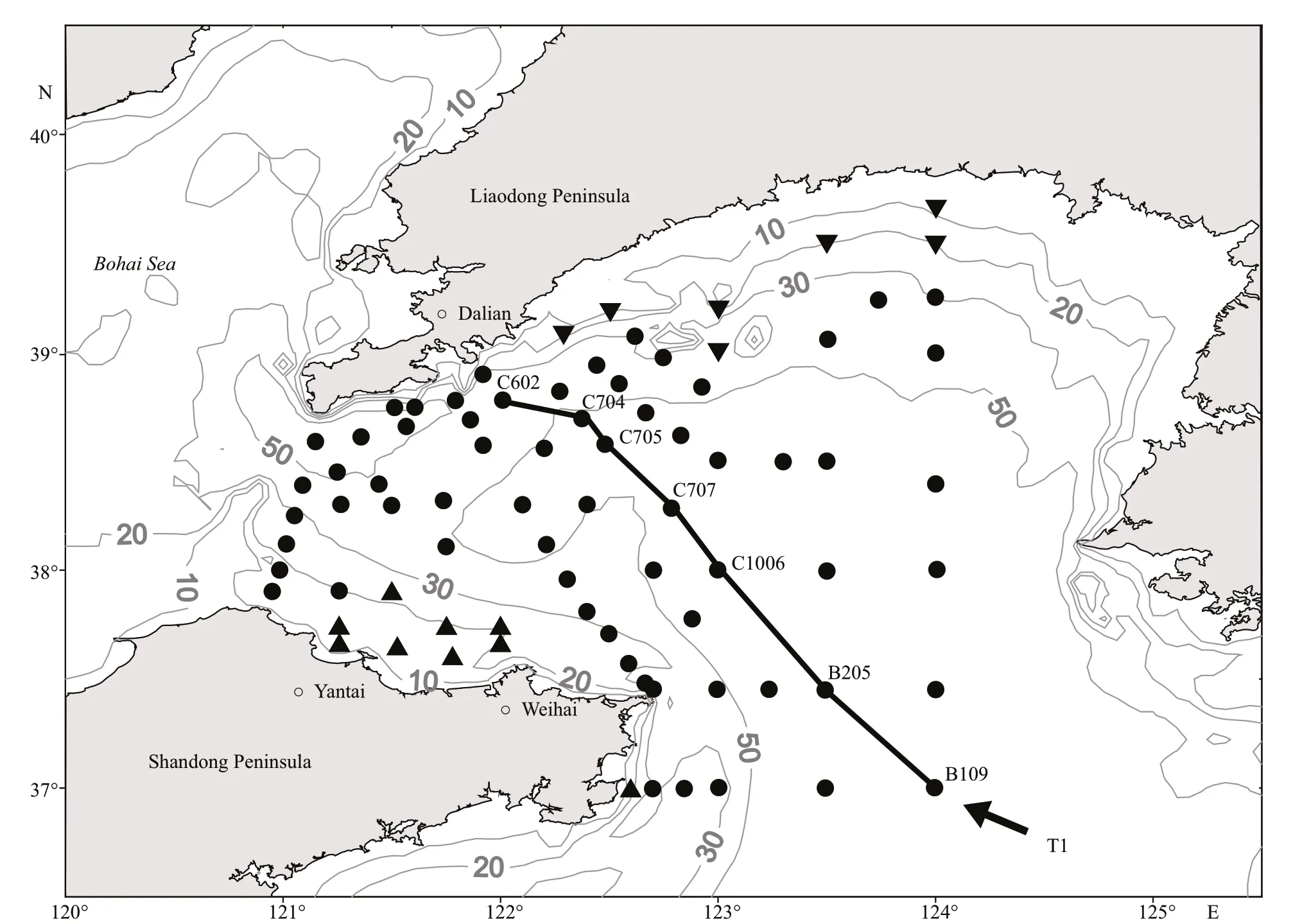
Fig.1 Map of the study area and sampling stations in the NYS, with isobaths of 10, 20, 30, and 50 m
Fig.2 The distribution of winter zooplankton abundances in the North Yellow Sea (ind./m 3)
3.2 Spatial distribution of total zooplankton abundance
The zooplankton abundance of studied stations varied from 35.5 to 2 125.7 ind./m3, with highest values (>500.0 ind./m3) observed along the Liaodong Peninsula inshore region. This was far greater than the abundances observed along the Shandong inshore region and the middle part of the NYS (<100.0 ind./m3) (Fig.2).
3.3 Multivariate analysis
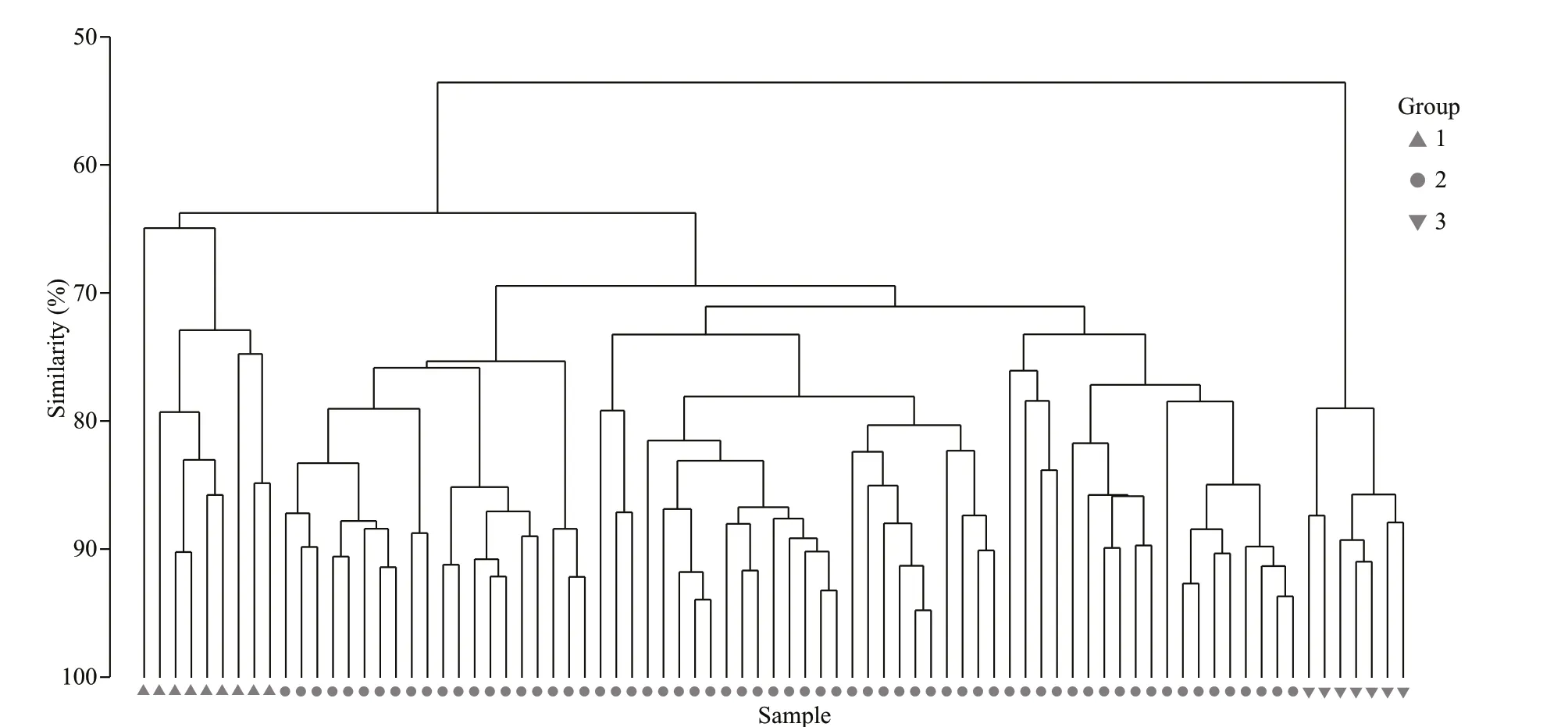
Fig.3 Cluster analysis of the zooplankton community structure
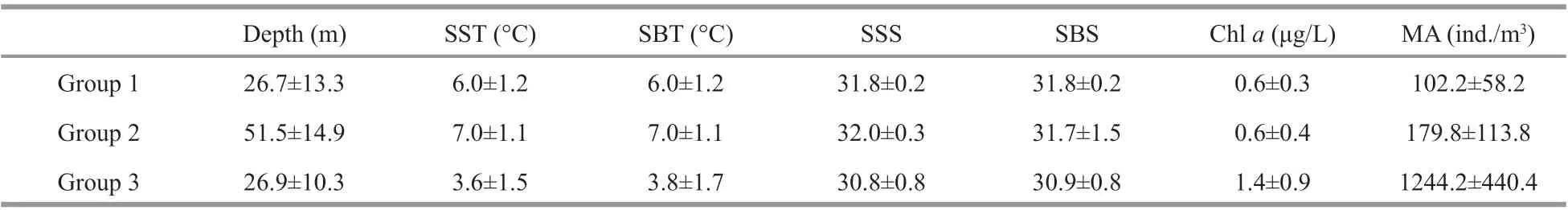
Table 2 Environmental parameters and the average abundance of zooplankton in diff erent groups
The hierarchical cluster analyses divided 81 stations into 3 groups, containing 9, 65, and 7 stations, respectively (Fig.3). The SIMPROF test confi rmed the signifi cance of this grouping (P<0.01). The ANOSIM pairwise test showed that the species compositions at Group 1 and Group 2 stations were signifi cantly diff erent (R=0.596,P<0.01), as were diff erences between Group 1 and Group 3 stations (R=0.835,P<0.01), and Group 2 and Group 3 stations (R=0.835,P<0.01). Each group showed strong geographic integrity of the included stations (Fig.1). Group 1 occupied the off shore area of the Shandong Peninsula, and was characterized byS.crassa,C.sinicus,P.parvusandCentropagesabdominalis; Group 2 was located in the middle region of the NYS and was characterized byS.nagae,A.bifi losa,Euphausiapacifi caandParathemistogracilipes, in addition to Group 1 representative species; Group 3 was located along the Liaodong Peninsula and contained dominant species similar to Group 1, but with signifi cantly higher total abundances. The average depth, temperature and salinity of the water in stations belonging to Group 2 were greater than those in Groups 1 and 3 (Table 2). Environmental factors (excepting bottom salinity) were signifi cantly diff erent among groups (One-way ANOVA,P<0.01).
According to the BIOENV routine, the best fi t was obtained from the combination of water depth, SST, and SSS (Spearman’s rank correlationρ=0.571), and the best fi ts for single environmental variables were obtained for water depth (ρ=0.561) and SSS (ρ=0.457).
3.4 Distributions of warm-water species
Two warm-water species,E.plana(adult and copepodite) andS.enfl atawere recorded in this survey.S.enfl atawas only present at one station (<0.1 ind./m3), whileE.plana, appeared at 22 stations, with an average abundance of 0.1 ind./m3(Table 1, Fig.4). The highest abundance ofE.planawas found in station B109 (0.23 ind./m3). Station C602 (38.78°N, 122.00°E) was the northwest limit ofE.planadistribution (0.04 ind./m3). A signifi cantly decreasing gradient ofE.planaabundance was detected from the southeast (B109) to the northwest (C602).
Water temperature and salinity showed no signifi cant diff erences between sea surface and bottom at all stations, indicating vertical mixing of the water column during the survey, however, temperature and salinity decreased in a NW direction from 8.3°C and 32.4 at B109 to 4.6°C and 31.8 at C602. This pattern was consistent with the observed variation of warm-water species along transect T1 (Fig.5).

Fig.4 Horizontal distribution of warm-water species abundance (ind./m 3)
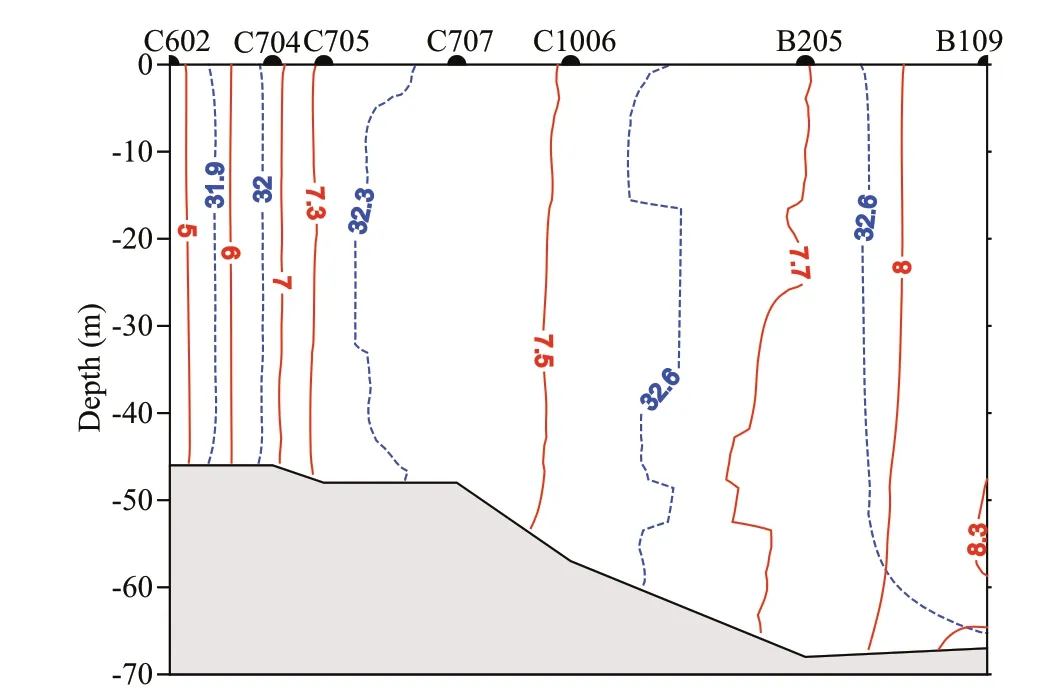
Fig.5 Vertical isotherms (red solid line, °C) and isohalines (blue dotted line) along transect T1
Factor loading analysis on the relationship between environmental factors and the presence ofE.planashowed that the fi rst two principal components could explain 72.9% of the variation (Fig.6). No linear relationship betweenE.planaand environmental variables was found.
4 DISCUSSION
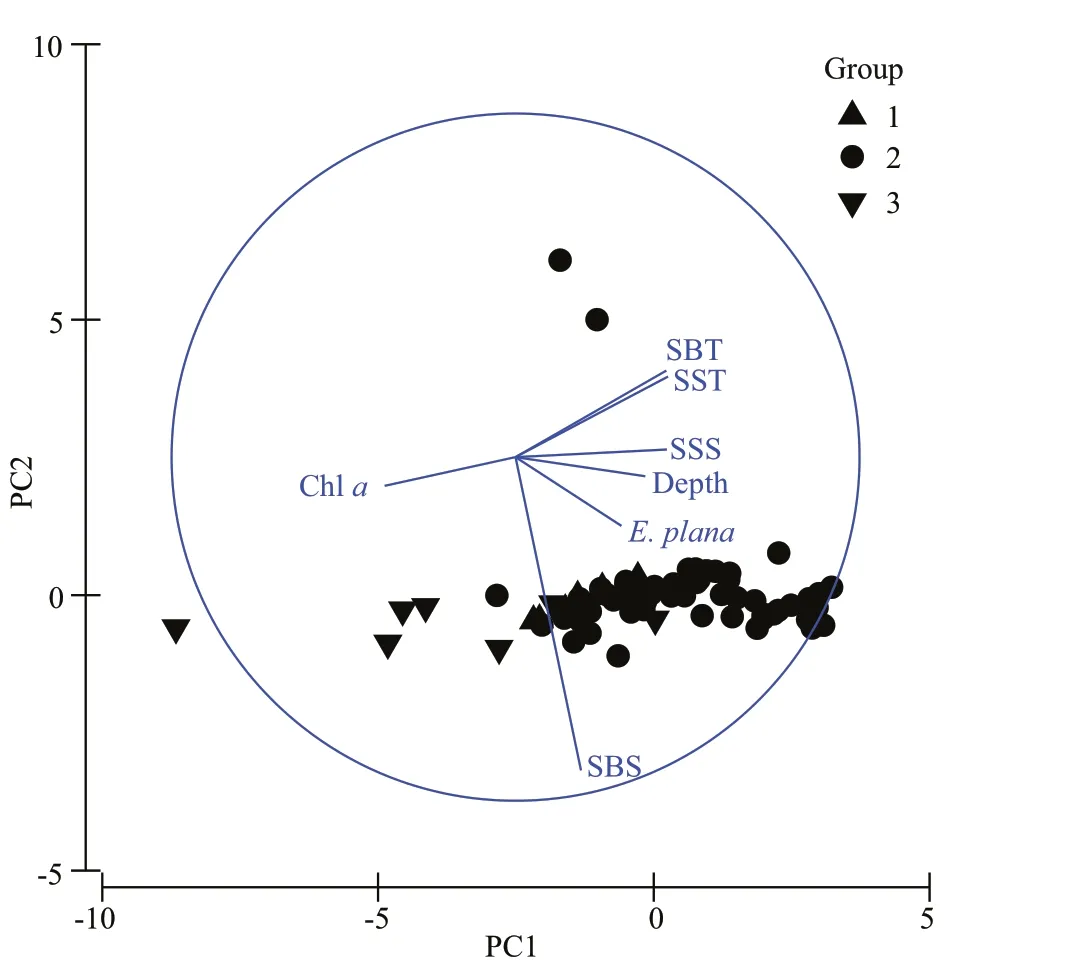
Fig.6 PCA scatter diagram of the warm-water species Euchaeta plana and environmental factors
Based on the species composition and abundance of zooplankton in the NYS during this cruise, 81 stations were divided into 3 groups by the hierarchical cluster analysis, including the Shandong Coastal Community (SCC, corresponding to Group 1), the Yellow Sea Central Community (YSCC, corresponding to Group 2) and the Liaoning Coastal Community (LCC, corresponding to Group 3). The SCC was located inshore of 20 m isobaths off the Shandong coast, which was greatly infl uenced by low-salinity water from the BS, and this group was characterized by temperate, eurythermal, low-salinity species. The YSCC experienced the highest temperature, highest salinity and greatest water depth of all three communities. The LCC was located inshore of the 30 m isobaths off the Liaoning coast. This community was controlled by the Liaonan Coastal Current, the lowest temperature and salinity water mass in this region (Fig.4). All three communities, derived from station ordination, had their own environmental characteristics (Table 2). During winter, comprehensive mixing was evident as temperature and salinity showed a little variation in horizontal or vertical direction. Consequently, zooplankton communities in the study area were very similar during that season (>50%, Fig.3).
There was no signifi cant variation between zooplankton communities assessed here and during the 1950s, and community structure was also similar to the previous description (Cheng, 1965). Cheng (1965) grouped zooplankton in the NYS into three communities, each occupying a specifi c sea area (Shandong coast, Liaoning coast, and the central part of NYS, respectively), with its characteristic and dominant species. The present result is similar to Cheng (1965), with the same community distribution pattern and dominant species in each community. In recent years, typical warm-water zooplankton have been recorded in the NYS with high abundance in autumn (Yang et al., 2012; Franco et al., 2014), an observation not previously documented. Yang et al. (2012) hypothesized that climate change was responsible for the northward expansion of warmwater species. While our results suggest that certain warn-water species likely function well as indicators for the YSWC, further, reiterative fi eld investigations are needed to elucidate the long-term variability of warm-water zooplankton in the NYS.
It is well known that current advection can infl uence the community composition and distribution of zooplankton (Basedow et al., 2004; Willis et al., 2006), therefore, the continuous distribution of indicator species observed here indicates the path and scope of the YSWC. Warm-water species have been recorded in the SYS during winter in diff erent years. Thirty-nine warm-water species were recorded in the SYS in winter of 2001 (Wang et al., 2003), and 11 warm-water species were recorded during winter in 2009 (Wang et al., 2013). The horizontal distribution pattern of warm-water species in SYS is thought to be infl uenced by the YSWC fl ow along the YS trough, and the northern limit of YSWC penetration was previously inferred to be around 35°N–36°N (Wang et al., 2013). Previously, warm-water species richness in the NYS was observed to be much lower than in the SYS overall. The northern limit of warm water species did not reach the NYS during winter 1959, however, two warm-water species,DoliolumdenticulatumandS.enfl atawere recorded in the NYS in the winter of 2009 (Yang et al., 2012), but no indicator species were reported.
In the present investigation, two warm-water species,E.planaandS.enfl ata, were recorded in the NYS.S.enfl atawas recorded at one station only, so it cannot be considered as an indicator due to infrequent occurrence.E.planais a typical warm-water species, generally distributed in the ECS and Pacifi c Ocean off Japan (Chen and Zhang, 1965). The YSWC is the only physical process known to transport warm and saline water from off shore into the NYS (Tang et al., 2001), so the existence of warm-water species could indicate the infl uence of the YSWC.E.planawas recorded in 22 stations, and its spatial distribution was highly consistent with the current path of the YSWC that enters the NYS at 124.5°E and fl ows in a northwest direction (Bao et al., 2009; Zhao et al., 2011). These previous observations, along with our current data set, suggest thatE.planais an indicator species for the YSWC. The YSWC crossed the center of the NYS and a high-salinity tongue extended along transect T1, as previously observed (Bao et al., 2009). Along T1, the abundance ofE.planadecreased gradually from the southeast (B109) to the northwest (C602). Since the YSWC trailed off gradually at its northwestern most extent due to mixing with coastal water and infl uences of the coastal climate (Su, 1989), the distribution ofE.planarefl ected the current path of the YSWC within the NYS. The data provided evidence thatE.planamay be considered an indicator species for the current path of YSWC in the NYS. The infl uence of YSWC was limited in YSCC, and the coastal communities SCC and LCC were unaff ected.
The YSWC is considered a compensatory current under strong north wind conditions (Zhao et al., 2011), and reached the NYS only weakly during our study. The comparatively warmer YSWC changed gradually in its northward journey—temperature and salinity decreased. However, the YSWC cannot be accurately recognized by just temperature and salinity. Therefore,E.planamay be a good indicator for identifying the presence and track of the YSWC. This paper is only a case study conducted in January 2007. Because the strength and path of the YSWC experience signifi cant seasonal and interannual variation (Lie et al., 2001), and because other complex factors may aff ect zooplankton distributions, further observations are required to fi rmly establishE.planaor other taxa as indicator species to the YSWC.
5 CONCLUSION
The zooplankton communities in the NYS were dominated by temperate and warm-temperate species, such asC.sinicus,P.parvus,A.bifi losaandS.crassa. Two warm-water species,E.planaandS.enfl atawere also recorded as well. Three zooplankton communities, i.e., the Shandong Coastal Community (SCC), the Yellow Sea Central Community (YSCC), and the Liaoning Coastal Community (LCC) were distinguished by cluster analysis. The appearance ofE.planaindicates a signifi cant northwestward trend of YSWC. The infl uence of the YSWC was limited in the YSCC, and coastal communities in the SCC and LCC were unaff ected.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENT
We thank Prof. LI Fuhua from Institute of Oceanology, Chinese Academy of Sciences, Dr. ZHANG Huan from the University of Connecticut and Dr. ZHUANG Yunyun from Ocean University of China for helping to revise the manuscript. We are grateful to the captain and crew of R/VDongfanghong2, and also to Mr. XU Donghui and LIN Jian for their help on board. Thanks to Prof. LI Zhengyan from Ocean University of China for providing chladata. The authors would also like to thank the anonymous reviewers for their corrections and recommendations in improving the manuscript.
References
Bao X W, Li N, Yao Z G, Wu D X. 2009. Seasonal variation characteristics of temperature and salinity of the North Yellow Sea.PeriodicalofOceanUniversityofChina, 39(4): 553-562. (in Chinese with English abstract)
Basedow S L, Eiane K, Tverberg V, Spindler M. 2004. Advection of zooplankton in an Arctic fjord (Kongsfjorden, Svalbard).Estuarine,CoastalandShelfScience, 60(1): 113-124.
Beaugrand G. 2005. Monitoring pelagic ecosystems using plankton indicators.ICESJournalofMarineScience, 62(3): 333-338.
Bi H S, Peterson W T, Strub P T. 2011. Transport and coastal zooplankton communities in the northern California current system.GeophysicalResearchLetters, 38(12): L12607.
Chen Q C, Zhang S Z. 1965. The planktonic copepods of the Yellow Sea and the East China Sea: I. Calanoida.StudiaMarinaSinica, 7: 20-131. (in Chinese with English abstract)
Chen X, Li H B, Zhao Y, Zhao L, Dong Y, Zhang W C, Xiao T. 2018. Distribution of diff erent biogeographical tintinnids in Yellow Sea and Bohai Sea.JournalofOceanUniversityofChina, 1 7(2): 371-384.
Cheng T C, Cheng S Y. 1962. On the ecology of the planktonic Foraminifera of the Yellow Sea and the East China Sea.OceanologiaetLimnologiaSinica, 4(1-2): 60-85. (in Chinese with English abstract)
Cheng T C. 1965. The structure of zooplankton communities and its seasonal variation in the Yellow Sea and in the western East China Sea.OceanologiaetLimnologiaSinica, 7(3): 199-204. (in Chinese with English abstract)
Clarke K R, Warwick R M. 2001. Changes in Marine Communities: An Approach to Statistical Analysis and Interpretation. Primer-E Ltd., Plymouth.
Franco P, Chen H J, Liu G X. 2014. Distribution and abundance of pelagic tunicates in the North Yellow Sea.JournalofOceanUniversityofChina, 13(5): 782-790.
Guan B X, Mao H L. 1982. A note on circulation of the East China Sea.ChineseJournalofOceanologyandLimnology, 1(1): 5-16.
Hays G C, Richardson A J, Robinson C. 2005. Climate change and marine plankton.TrendsinEcology&Evolution, 20(6): 337-344.
Hooff R C, Peterson W T. 2006. Copepod biodiversity as an indicator of changes in ocean and climate conditions of the northern California current ecosystem.LimnologyandOceanography, 51(6): 2 607-2 620.
Hsieh C H, Chiu T S, Shih C T. 2004. Copepod diversity and composition as indicators of intrusion of the Kuroshio branch current into the northern Taiwan Strait in spring 2000.ZoologicalStudies, 43: 393-403.
Lalli C M, Parsons T R. 1993. Biological Oceanography: An Introduction. Oxford: Elsevier Butterworth- Heinemann. 314p.
Le K T. 1992. The origin of the Yellow Sea Warm Current in winter.ActaOceanologicaSinica, 14(2): 9-19. (in Chinese)
Lie H J, Cho C H, Lee J H, Lee S, Tang Y X, Zou E M. 2001. Does the Yellow Sea Warm Current really exist as a persistent mean fl ow?JournalofGeophysicalResearch:Oceans, 106(C10): 22 199-22 210.
Song X, Lin X P, Wang Y. 2009. The variability of the Yellow Sea Warm Current axis in winter and its possible reason.PeriodicalofOceanUniversityofChina, 39(S1): 259-266. (in Chinese with English abstract)
Su Y S. 1989. The analysis of geographical environment and circulation in the Yellow Sea and East China Sea.JournalofOceanUniversityofQingdao, 19(1): 145-158. (in Chinese with English abstract)
Tang Y X, Zou E M, Lie H J. 2001. On the origin and path of the Huanghai Warm Current during winter and early spring.ActaOceanologicaSinica, 23(1): 1-12. (in Chinese with English abstract)
Taylor A H, Allen J I, Clark P A. 2002. Extraction of a weak climatic signal by an ecosystem.Nature, 416(6881): 629-632.
Wang H W, Yu F, Lv L G, Diao X Y, Guo J S. 2009. Characteristics of spatial and interannual variation in the Yellow Sea Warm Current area in winter.AdvancesinMarineScience, 27(2): 140-148. (in Chinese with English abstract)
Wang L, Li C L, Yu F. 2013. Zooplankton community structure in the south Yellow Sea in winter and indication of the Yellow Sea warm current.OceanologiaetLimnologiaSinica, 44(4): 853-859. (in Chinese with English abstract)
Wang R, Gao S W, Wang K, Zuo T. 2003. Zooplankton indication of the Yellow Sea Warm Current in winter.JournalofFisheriesofChina, 27(S1): 39-48. (in Chinese with English abstract)
Willis K, Cottier F, Kwasniewski S, Wold A, Falk-Petersen S. 2006. The infl uence of advection on zooplankton community composition in an Arctic fjord (Kongsfjorden, Svalbard).JournalofMarineSystems, 61(1-2): 39-54.
Xu L L, Wu D X, Lin X P, Ma C. 2009. The study of the Yellow Sea Warm Current and its seasonal variability.JournalofHydrodynamics, 21(2): 159-165.
Yang Q, Wang Z L, Fan J F, Shao K S, Li H J. 2012. Zooplankton diversity and its variation in the Northern Yellow Sea in the autumn and winter of 1959, 1982 and 2009.ActaEcologicaSinica, 32(21): 6 747-6 754. (in Chinese with English abstract)
Zhao S, Yu F, Diao X Y, Si G C. 2011. The path and mechanism of the Yellow Sea Warm Current.MarineSciences, 35(11): 73-80. (in Chinese with English abstract)
 Journal of Oceanology and Limnology2020年6期
Journal of Oceanology and Limnology2020年6期
- Journal of Oceanology and Limnology的其它文章
- Eff ects of vitamin C defi ciency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discu s hannai Ino*
- Leptolaimus holovachovi sp. nov. (Nematoda) from Shenzhen Mangrove Nature Reserve, Shenzhen, South China*
- Profi le and development of microsatellite primers for Acanthogobius ommaturus based on high-throughput sequencing technology*
- Digging out molecular markers associated with low salinity tolerance of Nannochloropsis oceanica through bulked mutant analysis*
- An enhanced underwater camera apparatus for seabed observation of megabenthic epifauna in the northern Yellow Sea*
- Intraguild predation by polyps of three scyphozoan jellyfi sh: Nemopilema nomurai, Aurelia coerulea, and Rhopilema esculentum*
