番茄SlMAPK6基因克隆及其表达特性分析
岳宁波 李云洲 李玉龙 潘鹏程 潘寅涛 王勇 须文 吴浪 闫见敏


摘要:【目的】克隆番茄促分裂原活化蛋白激酶(MAPK)基因SlMAPK6,并分析其在不同非生物脅迫及信号物质处理下的表达模式,为深入探究SlMAPK6基因在番茄逆境响应中调控机制提供理论参考。【方法】采用同源克隆技术从矮番茄中克隆SlMAPK6基因cDNA全长序列,并进行生物信息学分析,采用实时荧光定量PCR检测矮番茄幼苗在干旱(20% PEG-6000)、盐(300 mmol/L NaCl)及信号物质[0.50 mmol/L水杨酸(SA)和0.10 mmol/L 2,1,3-苯并噻二唑(BTH)]处理下SlMAPK6基因的表达模式。【结果】克隆获得SlMAPK6基因cDNA全长序列为1360 bp,开放阅读框(ORF)为1131 bp,编码376个氨基酸残基,其编码蛋白理论等电点(pI)6.76,不稳定指数44.36,脂肪系数90.00,为不稳定的强脂溶性蛋白,亲水性平均值(GRAVY)-0.396,亲水性氨基酸均匀分布在整个肽链中,故该蛋白为亲水性蛋白;亚细胞定位于细胞核及胞浆质中,无跨膜区,含有36个磷酸化位点,其中丝氨酸(Ser)磷酸位点19个、苏氨酸(Thr)磷酸位点9个。SlMAPK6蛋白二级结构中,α-螺旋占38.03%,延伸链占10.37%,无规则卷曲占51.60%。SlMAPK6蛋白与马铃薯StMAPK4、风铃辣椒CbMAPK4和中华辣椒CcMAPK4蛋白同源性均为99.47%。SlMAPK6和SlMAPK5蛋白的亲缘关系最近,与拟南芥MAPK家族B亚族成员AtMAPK4、AtMPK5、AtMPK11、AtMPK12和AtMPK13蛋白的亲缘关系也较近。经SA、BTH、NaCl和PEG-6000处理后,SlMAPK6基因均出现抑制表达,说明SlMAPK6基因负向调控番茄植株的逆境响应。【结论】SlMAPK6基因可能参与负调控番茄植株的抗病和抗逆胁迫机制。SlMAPK6蛋白通过Ser或Thr磷酸化识别细胞信号的传导功能,在矮番茄逆境胁迫响应中发挥重要作用。
关键词: 番茄;促分裂原活化蛋白激酶6(MAPK6);基因克隆;信号物质;非生物胁迫;表达特性
中图分类号: S641.203.6 文献标志码: A 文章编号:2095-1191(2020)07-1625-09
Abstract:【Objective】 To clone the tomato mitogen-activated protein kinase(MAPK) gene SlMAPK6, to analyze its expression level under different abiotic stresses and signal substances, which provided theoretical reference for exploration of the regulatory mechanism of the SlMAPK6 gene in tomato stress response. 【Method】Using homologous cloning technology to clone the full-length cDNA sequence of SlMAPK6 gene from dwarf tomato, conducting bioinformatics analysis, and real-time fluorescent quantitative PCR was used to detect the expression pattern of SlMAPK6 gene under the drought(20% PEG-6000), salt(300 mmol/L NaCl), signal substances[0.50 mmol/L salicylic acid (SA) and 0.10 mmol/L 2,1,3-benzothiadiazole(BTH)] treatments. 【Result】The full-length cDNA of SlMAPK6 was 1360 bp and contained an open reading frame(ORF) of 1131 bp that encoded 376 amino acids residues, the theoretical isoelectric point(pI) was 6.76, instability index 44.36, and the fat coefficient 90.00,it was an unstable strong fat-soluble protein. The average hydrophilicity(GRAVY) was -0.396, hydrophilic amino acids were evenly distributed in the entire peptide chain, so the protein was a hydrophilic protein, subcellularly located in the nucleus and cytoplasm, without a transmembrane region.It contained 36 phosphorylation sites, including 19 serine(Ser) phosphate sites and 9 threonine(Thr) phosphate sites. In the secondary structure of SlMAPK6 protein, α-helix accounted for 38.03%, extended chain accounted for 10.37%, and random coil accounted for 51.60%. The homology of SlMAPK6 protein with potato StMAPK4, capsicum CbMAPK4 and Chinese capsicum CcMAPK4 were all 99.47%. SlMAPK6 and SlMAPK5 had the closest relationship, and it was also closely related to members of the arabidopsis MAPK family B subfamily: AtMAPK4, AtMPK5, AtMPK11, AtMPK12 and AtMPK13 proteins. After SA, BTH, NaCl and PEG-6000 treatments, the expression of SlMAPK6 gene was suppressed, indicating that SlMAPK6 gene negatively regulated the stress response of tomato plants. 【Conclusion】The SlMAPK6 gene may be involved in negatively regulating the disease resistance and stress resistance of tomato plants. The SlMAPK6 protein recognizes the transduction function of cell signals through Ser or Thr phosphorylation, and plays an important role in the response of dwarf tomato to stress.
Key words: tomato; mitogen-activated protein kinase 6(MAPK6);gene cloning; signal substance response; abio-tic stress; expression characters
Foundation item: National Natural Science Foundation of China(31960604); Guizhou Science and Technology Plan Project(QKHPTRC〔2017〕5788-28); Research Project on Talent Introduction of Guizhou University(KDRJHZ〔2017〕50); Laboratory Open Project of Guizhou University(SYSKF2019-55)
0 引言
【研究意義】番茄(Solanum lycopersicum L.)为茄科番茄属一年生或多年生蔬菜作物,是我国重要的园艺蔬菜,其富含维生素、萝卜素及具有抗氧化作用的番茄红素等营养物质,可减缓衰老和预防癌症(Perveen et al.,2015;Petyaev,2016;Crowe-White et al.,2019)。番茄在生长过程中常受到不利生长环境的影响,包括病原微生物侵染、干旱及盐等生物和非生物胁迫,对番茄生产造成严重危害(李翠,2014)。经研究发现,促分裂原活化蛋白激酶(Mitogen-activated protein kinases,MAPK)在植物的生长、发育和胁迫应答中发挥重要作用(Xu and Zhang,2015),因此,克隆番茄MAPK基因并分析其表达特性,深入探究其在番茄响应生物和非生物胁迫中的调控机制,对提高番茄植株抗逆境胁迫能力具有重要意义。【前人研究进展】MAPK在真核生物中普遍存在且高度保守,是一类丝氨酸(Ser)/苏氨酸(Thr)蛋白激酶,能将外源或内源信号分子导入细胞内(Smekalova et al.,2013;Lee et al.,2016)。MAPK级联信号传导由MAPK、MAPK激酶(MAPKK)和MAPKK激酶(MAPKKK)3级信号传导组成(de Zelicourt et al.,2016)。MAPK是末端级联反应信号激酶,通过上游MAPKK和MAPKKK对Ser和Thr残基进行磷酸化来传递信号(Chen et al.,2001;Hame et al.,2006;Rodriguez et al.,2010),从而参与植物的信号转导应答过程(Kalapos et al.,2019)。目前,已有大量的MAPK家族成员从不同植物物种中被鉴定出来,其中拟南芥、水稻、玉米和小麦分别鉴定出20、17、21和15个MAPK蛋白(Lian et al.,2012;Liu et al.,2013;Min and Zhao,2018)。番茄中已鉴定出16个MAPK蛋白(Wang et al.,2018a,2018b)。根据MAPK结构差异,可将其分为A、B、C和D 4个亚族(Mohanta et al.,2015;王洁等,2016),其中,A、B和C亚族含TEY磷酸化位点,D亚族含TDY磷酸化位点。在拟南芥中,A亚族以AtMPK3、AtMPK6和AtMPK10等为代表,主要与环境和激素反应相关,其中,AtMAPK3和AtMPK6在植株发育过程中发挥协同作用,MAPK3突变体和MAPK6突变体表现为胚胎异常,且AtMAPK3和AtMAPK6还参与脱落酸(ABA)、茉莉酸(JA)等激素响应(Ortiz-Masia et al.,2007;Mine et al.,2017);B亚族以AtMPK4、AtMPK5、AtMPK11、AtMPK12和AtMPK13等为代表,主要参与细胞分裂和环境应激反应,其中,AtMPK4还参与调节先天免疫(Ichimura et al.,2000;Petersen et al.,2000;Kosetsu et al.,2011);C亚族以AtMPK1、AtMPK2、AtMPK7和AtMPK14等为代表,主要参与应激反应;D亚族包含AtMPK8、AtMPK9、AtMPK15、AtMPK16、AtMPK17、AtMPK18、AtMPK19和AtMPK20,主要参与逆境胁迫响应和生长发育(孔福苓,2012)。可见,各MAPK亚族均有成员参与非生物和生物应激反应(Wang et al.,2018a)。【本研究切入点】在番茄MAPK家族中,以A亚族如SlMAPK1、SlMAPK2和SlMAPK3研究相对较多(Singh et al.,2018;Zhang et al.,2018),而B亚族如SlMAPK6(Kong et al.,2012)等研究甚少,其功能尚不清楚,至今鲜见有关SlMAPK6基因克隆及表达的研究报道。【拟解决的关键问题】采用同源克隆技术从矮番茄中克隆SlMAPK6基因cDNA全长序列,并进行生物信息学分析,采用实时荧光定量PCR检测矮番茄幼苗在干旱、盐胁迫及信号物质处理下SlMAPK6基因的表达模式,为深入探究SlMAPK6基因在番茄逆境中的响应调控机制提供理论参考。
1 材料与方法
1. 1 试验材料
以矮番茄为供试材料,引自西北农林科学大学园艺学院,现保存于贵州大学农学院番茄种质资源库。RNA isolater Total RNA Extraction Reagent细胞/组织中总RNA提取试剂盒、HiScript II 1st Strand cDNA Synthesis Kit试剂盒、Taq DNA聚合酶和SYBR Master Mix(2×)购自南京诺维赞生物科技有限公司;水杨酸(SA)、PEG-6000和NaCl购自生工生物工程(上海)股份有限公司;2,1,3-苯并噻二唑(BTH)购自上海源叶生物科技有限公司。
主要仪器设备:人工智能光照培养箱(宁波市科技园区新江南仪器有限公司)、CFX96TM Real-time System荧光定量PCR仪(美国Bio-Rad公司)、T100TM PCR仪(美国Bio-Rad公司)、Tannon3500凝胶成像仪(上海天能科技有限公司)、低温离心机(德国Eppendorf公司)和NanoDrop One微量核酸蛋白浓度测定仪[赛默飞世尔科技(中国)有限公司]。
1. 2 样品胁迫处理
挑选饱满的矮番茄种子,经1.0% NaClO消毒15 min后,用无菌水冲洗残留的NaClO,播种在装有灭菌土壤的营养钵中,置于人工智能光照培养箱进行育苗培养[光照强度240 mol/(m?s),光/暗条件16 h 28 ℃/8 h 18 ℃,相对湿度70%],待苗长至5片真叶时进行非生物胁迫(干旱、盐胁迫)及信号物质处理。其中,干旱和盐胁迫处理,分别采用20% PEG-6000溶液和300 mmol/L NaCl溶液浇灌幼苗根部,以无菌水浇灌的幼苗根部为对照;信号物质处理:分别用0.10 mmol/L BTH和0.50 mmol/L SA喷洒幼苗叶面,以无菌水喷洒幼苗叶面为对照。每处理设3次重复,分别于0、1、3、6、12和24 h采集0.50 g叶片组织,用液氮速凍后,置于-80 ℃保存备用。
1. 3 总RNA提取
采用RNA Isolater Total RNA Extraction Reagent细胞/组织中总RNA提取试剂盒提取矮番茄叶片总RNA,分别用1.0%琼脂糖凝胶电泳和核酸浓度检测仪检测RNA完整性和浓度,采用HiScript II 1st Strand cDNA Synthesis Kit试剂盒以DNA酶处理的1.0 μg总RNA为模板反转录合成cDNA,-80 ℃保存备用。
1. 4 基因克隆
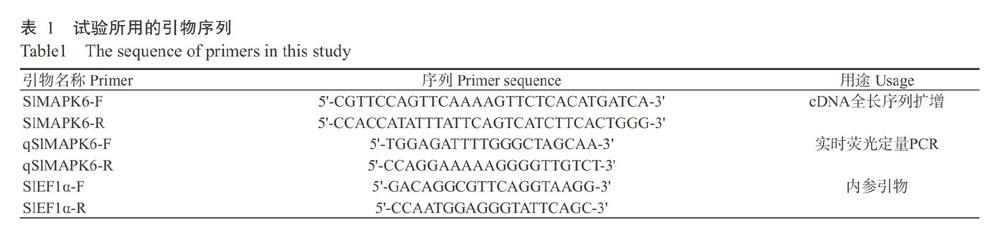
根据Sol Genomics Network网站公布的SlMAPK6基因序列(登录号Solyc05G049970),利用Primer Premier 5.0设计其全长序列扩增引物SlMAPK6-F和SlMAPK6-R(表1),委托生工生物工程(上海)股份有限公司合成。以cDNA为模板,用Taq DNA聚合酶克隆SlMAPK6基因cDNA全长序列。反应体系20.0 μL:2×Taq PCR Master Mix 10.0 μL,10 μmol/L正、反向引物各1.0 μL,cDNA模板2.0 μL,ddH2O补足至20.0 μL。扩增程序:94 ℃预变性2 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 10 min,进行35个循环;72 ℃延伸10 min。PCR产物送往北京诺赛基因组研究中心有限公司测序。
1. 5 生物信息学分析
将测序结果提交至NCBI数据库进行BLAST比对,以获得SlMAPK6蛋白的同源氨基酸序列。采用ProtParam对SlMAPK6蛋白进行理化性质分析;分别利用PSIPRED和SWISS-MOODEL预测SlMAPK6蛋白二、三级结构;采用Softberry-Protcomp进行SlMAPK6蛋白亚细胞定位;采用TMHMM预测SlMAPK6蛋白跨膜区;采用NetPhos 3.1预测SlMAPK6蛋白磷酸化位点;通过ProtScale预测SlMAPK6蛋白亲/疏水性;使用DNAMAN进行氨基酸序列多重比对。基于SlMAPK6蛋白、番茄MAPK家族成员和拟南芥MAPK家族成员的氨基酸序列,用MEGA 7.0的邻接法(Neighbor-joining,NJ)构建系统发育进化树,Booststrap参数为1000。
1. 6 实时荧光定量PCR检测
根据扩增获得的SlMAPK6基因序列设计定量引物qSlMAPK6-F和qSlMAPK6-R(表1)。以矮番茄叶片cDNA为模板,采用CFX96TM Real-time System荧光定量PCR仪检测SlMAPK6基因在干旱、盐胁迫及信号物质处理下的相对表达量。反应体系20.0 μL:SYBR Master Mix(2×) 10.0 μL,10 μmol/L正、反向引物各0.8 μL,cDNA模板4.0 μL,DEPC H2O补足至20.0 μL。每个样品设3个重复。扩增程序:95 ℃预变性10 min;95 ℃ 15 s,60 ℃ 15 s,进行40个循环;95 ℃ 10 s,65 ℃ 10 s,95 ℃ 5 s。以SlEF1α基因为内参,其引物见表1。
1. 7 统计分析
使用Excel 2010整理处理数据,采用2-△△Ct法计算目的基因的相对表达量(Livak and Schmittegen,2001),并以Origin 2017制图。
2 结果与分析
2. 1 SlMAPK6基因克隆结果
以矮番茄叶片cDNA为模板进行PCR扩增,获得1条长度约1300 bp的条带(图1),与预期结果相符。测序结果显示,该片段全长1360 bp,开放阅读框(ORF)为1131 bp,编码376个氨基酸残基。
2. 2 SlMAPK6蛋白氨基酸序列同源性分析结果
根据克隆获得的SlMAPK6基因核苷酸序列推导其编码蛋白(SlMAPK6)的氨基酸序列,通过NCBI数据库的BLAST获得其同源蛋白氨基酸序列,经同源性分析发现,SlMAPK6蛋白与马铃薯(Solanum tuberosum)StMAPK4、风铃辣椒(Capsicum baccatum)CbMAPK4和中华辣椒(C. chinense)CcMAPK4蛋白的同源性均为99.47%。对这4种同源蛋白进行氨基酸序列多重比对分析,结果(图2)显示,SlMAPK6蛋白仅第149和311位氨基酸与StMAPK4蛋白存在差异,但与CbMAPK4和CcMAPK4蛋白无差异;CbMAPK4蛋白仅第43和226位氨基酸与CcMAPK4蛋白存在差异,但CcMAPK4蛋白第43位氨基酸与StMAPK4和SlMAPK6无差异;StMAPK4蛋白第138位氨基酸与其他3种蛋白均存在差异。综上所述,SlMAPK6蛋白与马铃薯、风铃辣椒和中华辣椒MAPK4蛋白的同源性极高,序列高度保守。
2. 3 SlMAPK6蛋白理化性质预测结果
SlMAPK6蛋白由376个氨基酸组成,分子量43.09 kD,总原子数6040,理论等电点(pI)6.76,带负电荷氨基酸残基总数[天冬氨酸(Asp)+谷氨酸(Glu)]47个,带正电荷氨基酸残基总数[精氨酸(Arg)+赖氨酸(Lys)]45个,不稳定指数44.36,为不稳定蛋白;脂肪系数为90.00,为强脂溶性蛋白。SlMAPK6蛋白亲水性平均值(GRAVY)-0.396,多肽链第114位的Lys为最低分值(-2.578),亲水性最强;第234位亮氨酸(Leu)为最高分值(2.111),疏水性最强,从整体来看,亲水性氨基酸均匀分布在整个肽链中,故该蛋白为亲水性蛋白(图3)。Softberry-Protcomp亚细胞定位预测结果显示,SlMAPK6蛋白可能存在于细胞核及胞浆质中。TMHMM预测结果显示,SlMAPK6蛋白无跨膜区(图4)。
MAPK作为末端级联反应信号激酶,磷酸化位点预测分析有助于了解其在细胞信号传导过程中的作用。NetPhos 3.1预测结果显示,SlMAPK6蛋白含有36个磷酸化位点,其中,Ser磷酸位点19个,Thr磷酸位点9个(图5)。
2. 4 SlMAPK6蛋白二、三级结构预测结果
SlMAPK6蛋白二级结构预测结果(图6)显示,α-螺旋占38.03%,延伸链占10.37%,无规则卷曲占51.60%,说明α-螺旋和无规则卷曲是SlMAPK6蛋白的主要结构部件。SlMAPK6蛋白三级结构预测结果(图7)与二级结构预测结果基本相符。图6和图7均显示,SlMAPK6蛋白中无规则卷曲的占比最高。由于酶的功能部位常位于无规则卷曲中,可能是SlMAPK6在MAPK级联反应中发挥功能的主要原因。
2. 5 系统发育进化树分析结果
由图8可知,SlMAPK6蛋白与SlMAPK5蛋白的亲缘关系最近,与拟南芥MAPK家族成员AtMAPK4、AtMPK5、AtMPK11、AtMPK12和AtMPK13蛋白等亲缘关系也较近。据前人报道,AtMAPK4蛋白在免疫调节中起负反馈调节(Ichimura et al.,2000),故推测SlMAPK6蛋白在番茄的免疫调节中发挥相似功能。
2. 6 SlMAPK6基因在非生物胁迫及信号物质处理下的表达模式
对矮番茄幼苗进行干旱(PEG-6000)、盐(NaCl)及信号物质(SA和BTH)处理,并采用实时荧光定量PCR检测不同处理下SlMAPK6基因的表达模式。由图9-A和图9-B可知,SA处理后1和3 h,SlMAPK6基因表达量均较对照显著升高(P<0.05,下同);BTH处理后3 h,SlMAPK6基因表达量较对照显著升高,但SA和BTH处理后6~24 h,SlMAPK6基因的表达趋势相似,均为抑制表达。由图9-C可知,NaCl处理后,SlMAPK6基因表达较对照整体上无明显变化,僅在处理后1和12 h与对照存在显著差异,但处理后1 h为诱导表达,处理后12 h为抑制表达。由图9-D可知,PEG-6000处理后,SlMAPK6基因的整体表达趋势为抑制表达,仅在处理后6 h为诱导表达。综上所述,经SA、BTH、NaCl和PEG-6000处理后,SlMAPK6基因均出现抑制表达,表明SlMAPK6基因负向调控番茄植株的逆境胁迫响应。
3 讨论
MAPK级联信号参与调控植物的多种生命活动,如生长增殖、分化、运动和凋亡及抗病、抗逆防御反应(Abdukhi,2014)。MAPK处于MAPK级联信号系统的下游,在整个信号传导中发挥关键作用。番茄生长过程中常遭遇多种逆境胁迫,严重影响其生长发育而导致大幅减产,因此研究MAPK级联信号相关基因在番茄逆境中的调控功能尤为重要。目前,已从番茄中鉴定出16个MAPK家族成员,其中,A亚族的SlMAPK1、SlMAPK2和SlMAPK3基因研究较多,研究结果显示,三者参与调控番茄植株的抗病、抗逆防御反应(Li et al.,2017a,2017b;Wang et al.,2018b;杨杨等,2018)。经研究证实,SlMAPK1和SlMAPK2蛋白与拟南芥AtMAPK6蛋白同源,而SlMAPK3蛋白与拟南芥AtMAPK3蛋白同源(孔福苓,2012)。AtMAPK3和AtMAPK6蛋白参与调控拟南芥多种生物与非生物胁迫(Beckers et al.,2009;Yu et al.,2010;Meng et al.,2013),即上述研究相互印证结论的准确性。迄今,有关B亚族成员的研究报道极少,仅证实SlMAPK6属于B亚族成员(Kong et al.,2012),而鲜见有关SlMAPK6基因克隆及表达的研究报道。本研究通过同源克隆技术获得SlMAPK6基因序列,并对其进行同源性分析,结果显示,SlMAPK6蛋白与拟南芥AtMAPK4蛋白高度同源,推测SlMAPK6蛋白与拟南芥AtMAPK4蛋白功能相似。前人研究表明,AtMAPK4基因负调控拟南芥的自然免疫系统(Ichimura et al.,2000;Petersen et al.,2000;Kosetsu et al.,2011),故推测SlMAPK6基因负调控番茄植株的自然免疫系统。
本研究通过生物信息学分析发现,SlMAPK6是一个亲水性蛋白,存在于细胞核及胞浆质中,含有36个磷酸化位点,其中Ser磷酸位点19个,Thr磷酸位点9个。经前人研究证实,MAPK级联反应的磷酸化过程中丝氨酸和苏氨酸发挥重要功能(Min and Zhao,2018),故推测SlMAPK6通过Ser磷酸位点和Thr磷酸位点激活下游蛋白发挥作用,后续可通过Western blotting技术进行验证。
本研究对矮番茄幼苗分别进行干旱(PEG-6000)、盐(NaCl)及信号物质(SA和BTH)处理,结果发现SlMAPK6蛋白参与番茄植株的多种非生物胁迫及信号物质响应。BTH是SA类似物,属于人工合成的植物诱抗剂(于力等,2013),在SA和BTH的诱导下,SlMAPK6基因的表达趋势整体相似,但又存在差异,暗示SlMAPK6基因参与SA和BTH诱导抗逆境响应的作用机制存在差异。前人研究结果显示,BTH能诱导植物抵抗多种逆境(Ramasamy et al.,2015;Frackowiak et al.,2019),而SA是植物体内普遍存在的内源信号分子,在植物的多种逆境信号中发挥重要作用(Rivas-San and Plasencia,2011;Oracz and Karpinski,2016;Li et al.,2017b;Wu et al.,2019),暗示SA和BTH诱导的SlMAPK6通路参与调控植物的抗逆响应。经NaCl处理后,SlMAPK6基因表达较对照整体上无明显变化,仅在处理后1和12 h与对照存在显著差异,但处理后1 h为诱导表达,处理后12 h为抑制表达,其原因可能是NaCl对植物的渗透胁迫需要时间,处理后12 h基因下调表达可能是植物对高浓度渗透胁迫的反馈调节;同样,PE-G6000处理6 h为诱导表达,其他时间点均为抑制表达,推测处理后6 h基因高效表达是植株对PEG-6000模拟干旱的逆境反馈调节。结合生物信息学分析结果,推测SlMAPK6基因可能受SA和BTH信号调节参与番茄的抗逆向应,且在番茄植株非生物逆境胁迫中发挥负调控作用。
4 结论
SlMAPK6基因可能参与负调控番茄植株的抗病和抗逆胁迫机制。SlMAPK6蛋白可能通过Ser或Thr磷酸化识别细胞信号的传导功能,在矮番茄逆境胁迫响应中发挥重要作用。
参考文献:
孔福苓. 2012. 番茄促分裂原活化蛋白激酶(SlMAPK)家族基因的分离及表达特征分析[D]. 杭州:浙江大学. [Kong F L. 2012. Genome-wide identification and expression profiles analysis of mitogen-activated protein kinase family genes in Solanum lycopersicum[D]. Hangzhou:Zhejiang University.]
李翠. 2014.番茄SpMPKs基因响应非生物胁迫的功能分析[D]. 杨凌:西北农林科技大学. [Li C. 2014. The function of SpMPKs on tomato tolerance to abiotic stresses[D]. Yangling:Northwest A & F University.]
王洁,王燕,潘长田,何艳军,刘雪,卢钢. 2016. 番茄SlMAPK9-2基因分离及表达分析[J]. 核农学报,30(8):1480-1490. [Wang J,Wang Y,Pan C T,He Y J,Liu X,Lu G. 2016. Cloning and expression analysis of SlMAPK9-2 in Solanum lycopersicum L[J]. Journal of Nuclear Agricultural Sciences,30(8):1480-1490.]
杨杨,刘灿,郑鄢燕,生吉萍,申琳. 2018. SlMAPK1/2/3和SlACS2参与NO对抗病酶CHI和PAL的诱导[J]. 中国食品学报,18(7):18-25. [Yang Y,Liu C,Zheng Y R,Sheng J P,Shen L. 2018. SlMAPK1/2/3 and SlACS2 involved in NO-induced activity of phenylalnine ammonialyase and chitinase[J]. Journal of Chinese Institute of Food Science and Technology,18(7):18-25.]
于力,孫锦,郭世荣,阎君,朱为民. 2013. 苯并噻二唑诱导番茄对番茄黄化曲叶病毒病的抗性[J]. 江苏农业学报, 29(1):71-75. [Yu L,Sun J,Guo S R,Yan J,Zhu W M. 2013. Resistance to tomato yellow leaf curl virus in tomato induced by benzothiadiazole[J]. Jiangsu Journal of Agricultural Sciences,29(1):71-75.]
Abdukhi N. 2014. Functional analysis of MAP kinase pathways in plant defense responses in Solanum lycopersicum[D]. Ottawa:Carleton University.
Beckers G J M,Jaskiewicz M,Liu Y D,Underwood W R,He S Y,Zhang S Q,Conrath U. 2009. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana[J]. The Plant Cell,21(3):944-953.
Chen Z,Gibson T B,Robinson F,Silvestro L,Pearson G, Xu B E,Wright A,Vanderbilt C,Cobb M H. 2001. MAP kinases[J]. Chemical Reviews,101(8):2449-2476.
Crowe-White K M,Phillips T A,Ellis A C. 2019. Lycopene and cognitive function[J]. Journal of Nutritional Science.doi:10.1017/jns.2019.16.
de Zelicourt A,Colcombet J,Hirt H. 2016. The role of MAPK modules and ABA during abiotic stress signaling[J]. Trends in Plant Science,21(8):677-685.
Frackowiak P,Pospieszny H,Smiglak M,Obrepalska-Steplowska A. 2019. Assessment of the efficacy and mode of action of Benzo(1,2,3)-thiadiazole-7-carbothioic acid s-methyl ester(BTH) and its derivatives in plant protection against viral disease[J]. International Journal of Molecular Scien-ces,20(7):1-27.
Hame L P,Nicole M C,Sritubtim S,Morency M J,Ellis M,Ehlting J,Beaudoin N,Barbazuk B,Klessig D,Lee J,Martin G,Mundy J,Ohashi Y,Scheel D,Sheen J,Xing T,Zhang S Q,Seguin A,Brian E,Ellis B E. 2006. Ancient signals:Comparative genomics of plant MAPK and MAPKK gene families[J]. Trends in Plant Science,11(4):1360-1385.
Ichimura K,Mizoguchi T,Yoshida R,Yuasa T,Shinozaki K. 2000. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6[J]. The Plant Journal,24(5):655-665.
Kalapos B,Hlavová M,Nádai T V,Galiba G, Bi?ová K,Dóczi R. 2019. Early evolution of the mitogen-activated protein kinase family in the plant kingdom[J]. Scientific Reports,9:1-14.
Kong F L,Wang J,Cheng L,Liu S Y,Wu J,Peng Z,Lu G. 2012. Genome-wide analysis of the mitogen-activated protein kinase gene family in Solanum lycopersicum[J]. Gene,499(1):108-120.
Kosetsu K,Matsunaga S,Nakagami H,Colcombet J,Sasabe M,Soyano T,Takahashi Y,HirtH,Machida Y. 2011. The MAP kinase MPK4 is required for cytokinesis in Arabidopsis thaliana[J]. The Plant Cell,22(11):3778-3790.
Lee Y, Kim Y J,Kim M H,Kwak J M. 2016. MAPK cascades in guard cell signal transduction[J]. Frontiers in Plant Science. doi:10.3389/fpls.2016.00080.
Li Y Z,Qin L,Zhao J J,Muhammad T,Cao H H,Li H L,Zhang Y,Liang Y. 2017a. SlMAPK3 enhances tolerance to tomato yellow leaf curl virus(TYLCV) by regulating salicylic acid and jasmonic acid signaling in tomato(Solanum lycopersicum)[J]. PLoS One,12(2):e0172466.
Li Z,Yu J J,Peng Y,Huang B R. 2017b. Metabolic pathways regulated by abscisic acid,salicylic acid and γ-aminobutyric acid in association with improved drought tolerance in creeping bentgrass(Agrostis stolonifera)[J]. Physiologia Plantarum,159(1):42-58.
Lian W W,Tang Y M,Gao S Q,Zhang Z,Zhao X,Zhao C P. 2012. Phylogenetic analysis and expression patterns of the MAPK gene family in wheat(Triticum aestivum L.)[J]. Agricultural Sciences in China,11(8):1227-1235.
Liu Y K,Zhang D,Wang L,Li D Q. 2013. Genome-wide analysis of mitogen-activated protein kinase gene family in maize[J]. Plant Molecular Biology Reporter,31(6):1446-1460.
Livak K J,Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△Ct method[J]. Methods,25(4):402-408.
Meng X Z,Xu J,He Y X,Yang K Y,Mordorski B,Liu Y D,Zhang S Q. 2013. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance[J]. The Plant Cell,25(3):1126-1142.
Min J, Zhao Q C. 2018. Comparative analysis of plant MKK gene family reveals novel expansion mechanism of the members and sheds new light on functional conservation[J]. BMC Genomics,19(1):407-424.
Mine A,Berens M L,Nobori T,Anver S,Fukumoto K,Winkelmuller T M,Takeda A,Becker D,Tsuda K. 2017. Pathogen exploitation of an abscisic acid- and jasmonate-inducible MAPK phosphatase and its interception by Arabidopsis immunity[J]. Proceedings of the National Aca-demy of Sciences of the United States of America,114(28):7456-7461.
Mohanta T K,Arora P K,Mohanta N,Parida P,Bae H. 2015. Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants[J]. BMC Genomics,16(1):58-77.
Oracz K,Karpinski S. 2016. Phytohormones signaling pathways and ROS involvement in seed germination[J]. Frontiers in Plant Science,7:864. doi:10.3389/fpls.2016.00864.
Ortiz-Masia D,Perez-Amador M A,Carbonell J,Marcote M J. 2007. Diverse stress signals activate the C1 subgroup MAP kinases of Arabidopsis[J]. FEBS Letters,581(9):1834-1840.
Perveen R,Suleria H A R,Anjum F M,Butt M S,Pasha I,Ahmad S. 2015. Tomato(Solanum lycopersicum) carote-noids and lycopenes chemistry; metabolism,absorption,nutrition,and allied health claims—A comprehensive review[J]. Critical Reviews in Food Sicence and Nutrition,55(7):919-929.
Petersen M,Brodersen P,Naested H,Andreasson E,Lindhart U,Johansen B,Nielsen H B,Lacy M,Austin M J,Parker J E,Sharma S B,Klessig D F,Martienssen R,Mattsson O,Jensen A B,Mundy J. 2000. Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance[J]. Cell,103(7):1111-1120.
Petyaev I M. 2016. Lycopene deficiency in ageing and cardiovascular disease[J]. Oxidative Medicine and Cellular Longevity. doi:10.1155/2016/3218605.
Ramasamy A D,Bokshi A I,Phan-Thien K,Mcconchie R M. 2015. Seed treatment with benzothiadiazole induces resistance against powdery mildew disease caused by sphaerotheca fuliginea and increases the activities of pathogenesis-related enzymes in cucumber plants[J]. Journal of Horticultural Science & Biotechnology,90(1):63-70.
Rivas-San V M,Plasencia J. 2011. Salicylic acid beyond defence:Its role in plant growth and development[J]. Journal of Experimental Botany,62(10):3321-3338.
Rodriguez M C S,Petersen M,Mundy J. 2010. Mitogen-activated protein kinase signaling in plants[J]. Annual Review of Plant Biology,61:621-649. doi:10.1146/annure-varplant-042809-112252.
Singh A,Nath O,Singh S,Kumar S,Singh I K. 2018. Genome-wide identification of the MAPK gene family in chickpea and expression analysis during development and stress response[J]. Plant Gene,13:25-35. doi:10.1016/j.plgene.2017.12.001.
Smekalova V,Doskocilova A,Komis G,Samaj J. 2013. Crosstalk between secondary messengers,hormones and MAPK modules during abiotic stress signalling in plants[J]. Biotechnology Advances,32(1):2-11.
Wang H,Gong M,Guo J Y,Xin H,Gao Y,Chao Liu C,Dai D Q,Tang L Z. 2018. Genome-wide identification of Jatropha curcas MAPK,MAPKK,and MAPKKK gene fa-milies and their expression profile under cold stress[J]. Scientific Reports,8(1):16163. doi:10.1038/s41598-018-34614-1.
Wang L,Zhao R R,Li R,Yu W Q,Yang M J,Sheng J P,Shen L. 2018. Enhanced drought tolerance in tomato plants by overexpression of SlMAPK1[J]. Plant Cell Ti-ssue and Organ Culture,133(1):27-38.
Wu Z J,Han S M,Zhou H D,Tuang Z H,Wang Y Z, Jin Y, Shi H Z, Yang W N. 2019. Cold stress activates disease resistance in Arabidopsis thaliana through a salicylic acid dependent pathway[J]. Plant Cell and Environment,42(9):2645-2663.
Xu J,Zhang S Q. 2015. Mitogen-activated protein kinase cascades in signaling plant growth and development[J]. Trends in Plant Science,20(1):56-64.
Yu L J,Nie J N,Cao C Y,Jin Y K,Yan M,Wang F Z,Liu J,Xiao Y,Liang Y H,Zhang W H. 2010. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana[J]. New Phytologist,188(3):762-773.
Zhang S J,Wang L,Zhao R R,Yu W Q,Li R,Li Y J,Sheng J P,Shen L. 2018. Knockout of SlMAPK3 reduced di-sease resistance to botrytis cinerea in tomato plants[J]. Journal of Agricultural and Food Chemistry,66(34):8949-8956.
(責任编辑 陈 燕)

