Study of the AQP4 expression in traumatic brain edema and multimodal MRI imaging
Li Ai, Hai-Xia Chen, Jiang-Jun Qin, Hong Lu✉
1.The Seventh People's Hospital of Chongqing
2. Sanya Central Hospital(The Third People's Hospital of Hainan)
Keywords:Aquaporin Brain edema Magnetic resonance imaging Brain trauma
ABSTRACT The aquaporin-4 (AQP4) is a highly selective membrane protein. It is important for the body to maintain the water balance between internal and external environment of cells, the studies have found that the abnormal expression of AQP4 is related to the occurrence of many diseases. The cerebral edema is the most common and serious complication of brain trauma, and its pathogenesis is closely related to AQP4. The development of multimodal magnetic resonance imaging (M-MRI) could been provided imaging basis for accurate diagnosis of traumatic brain edema. In recent years, the correlation between AQP4 and M-MRI has become a hotspot of research. This paper reviews the research progress on the correlation between AQP4 expression in traumatic cerebral edema and M-MRI.
The aquaporin-4 (AQP4) is a protein that transports intracellular and extracellular water molecules. It plays an important role in regulating water metabolism and maintaining water balance. The occurrence of many diseases in human body is related to the abnormal expression of AQP4 on cell membrane[1,2]. The incidence of traumatic brain disease is increasing year by year, and the brain edema is the most serious complication of brain trauma. In recent years, the studies have found that the occurrence, development and elimination of traumatic brain edema are closely related to AQP4 [3,4]. The multimodal magnetic resonance imaging technology can more comprehensively and accurately evaluate the course of traumatic brain injury. In particular, the AQP4 magnetic resonance molecular imaging can quantitatively evaluate the structural changes within the brain tissue, and can noninvasively and quantitatively evaluate the spatiotemporal expression of AQP4 [5].
1 Overview of AQP4
1.1 Structure and distribution of AQP4
The aquaporins (AQPs) are proteins that transport intracellular and extracellular water molecules. Peter agre discovered the first AQP1[6] in 1987. Up to now, the 13 subtypes (AQP0-AQP12) have been found in human body[7]. Through the structural analysis of AQPs, it is found that these protein channels have common "hourglass" structural feature[8] (Figure 1). The AQP4 is composed of six-helix polypeptide chains. Each AQP4 protein monomer is transmembrane for six times and contains five rings. Both the N-terminal and C-terminal are located in the cell. And the outside of the cell is the hydrophilic A, C, E rings, there are hydrophobic B and D rings in the cell (Figure 2 and Figure 3). The B and E rings play a decisive role in the transport of water molecules. Because of their special amino acid sequences, if their structures are changed, then the transport of water molecules is blocked [8].
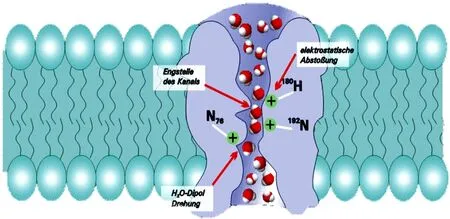
Figure 1 AQP4 “hourglass” model (from Lan YL, 2017)
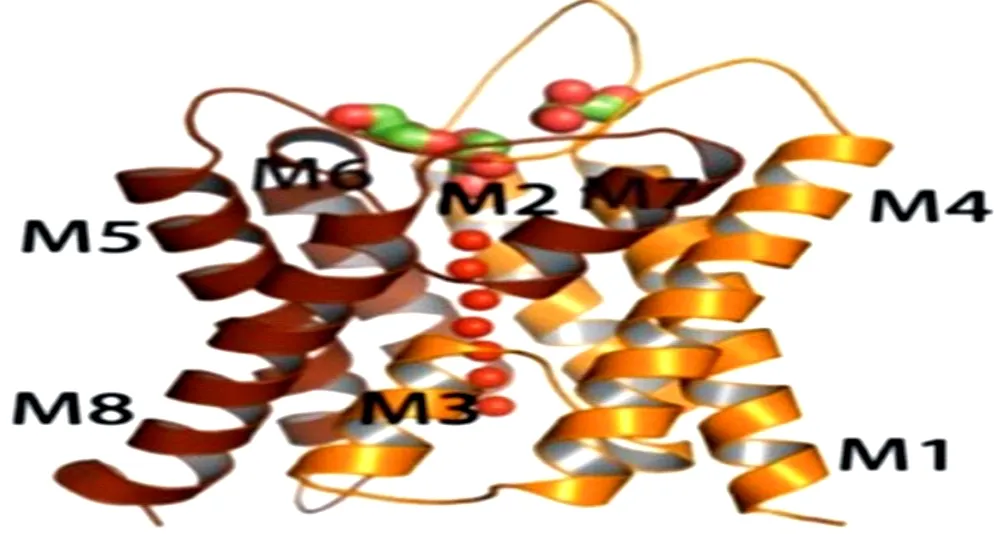
Figure 2 Crystal structure of AQP4
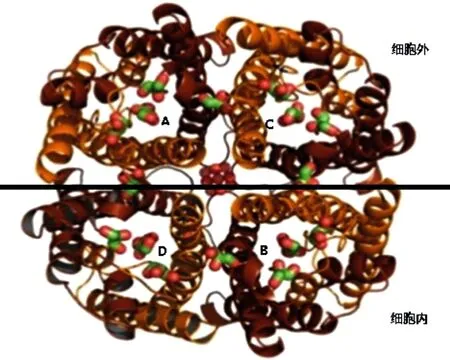
Figure 3 External structure of AQP4
The AQP4 is mainly involved in the transport of intracellular and extracellular water molecules in the brain. It is expressed in epithelial cells, glial cells, endothelial cells, and also in retina, hippocampus, corpus callosum and other tissues. The AQP4 is an important membrane protein,which involved in water transport and water balance between glial cells, endothelial cells and epithelial cells [9,10].
1.2 Classification and function of AQP4
The AQP4 is divided into M1, M23and MZ subtypes. Under the electron microscope, the three subtypes are arranged in an orthogonal manner on the cell membrane, which is formed by the combination of M1, M23and MZin a certain proportion. This orthogonal arrangement can make AQP4 more effectively anchored on the intracellular protein[11].
A number of studies have shown that[7,12], the main function of AQPs is to mediate the transmembrane transport of free water and regulate the balance of osmotic pressure intracellular and extracellular, which is closely related to its molecular environment. The AQP4 has been proved not only as an aquaporin, but also as an adhesion molecule, participating in cell migration and nerve excitation, synaptic plasticity, learning and memory and other physiological functions[13].
2 Summary of brain edema
2.1 Molecular mechanism of brain edema
Brain edema refers to the increase of water inside and outside brain cells caused by various factors, which leads to the increase of brain volume. At present, there are many theories about the molecular mechanism of brain edema, including microcirculation disorder, Ca2+overload, blood-brain barrier destruction, and so on, which can affect the occurrence and development of brain edema [14]. With the in-depth study in recent years[15] , it has also been found that the occurrence and development of brain edema is closely related to AQP4, matrix metalloproteinase, tight junction protein, inflammatory cytokines and so on. The study of AQP4 on the molecular level of cerebral edema can open up new ideas for the treatment of cerebral edema.
2.2 Classification and pathological mechanism of brain edema
According to the different pathophysiological mechanism of brain edema, it is mainly divided into clinically common vascular brain edema and cytotoxic brain edema, while osmotic brain edema and interstitial brain edema are rare in clinic. When the blood-brain barrier is destroyed, the permeability of capillaries increases, and the extracellular exudation increases, resulting in vascular brain edema. Cytotoxic (intracellular) brain edema is mostly due to the damage of cell itself and cell membrane function. In most cases, various types of brain edema exist in a mixture.
2.3 Correlation between AQP4 and traumatic brain edema
The AQP4 plays a dual complex role in traumatic brain edema[16]. There are many types of edema in traumatic brain edema. With the extension of time, there are differences between AQP4 expression and brain edema types. Most scholars[17,18] believes that the expression of AQP4 is up-regulated in intracellular brain edema, the brain edema in AQP4 knockout experimental animals is significantly reduced and can block the secondary injury caused by brain edema, while the expression of AQP4 is down regulated in vascular brain edema, and the brain edema in AQP4 knockout experimental animals is significantly increased, which indicates that AQP4 plays an important role in eliminating fluid and alleviating edema in vascular edema. A few studies[19] believe that the expression of AQP4 is down-regulated in intracellular brain edema and up-regulated in vascular brain edema, which is helpful to accelerate water clearance, which is a self-protection mechanism of the body against brain edema. Tifenn Clement et al [20] found that the expression of AQP4 around vascular endothelial cells and on the foot process of astrocytes was up-regulated, while in the central area of the trauma core, the expression of AQP4 was significantly down-regulated. The experimental results showed that the change of AQP4 expression was related to the degree and time of brain edema. LI X[21] found that AQP4 knockout significantly reduced the damage of blood-brain barrier and brain edema. Lu Hong et al[22] showed that gene silencing of AQP4 can effectively inhibit intracellular edema and also inhibit the up-regulation of AQP4 expression caused by intracellular edema. The above studies showed that AQP4 is involved in the formation of brain edema, and the inhibition of AQP4 expression or knocking out the AQP4 gene, it can effectively prevent intracellular edema and block the secondary damage caused by AQP4.
At present, there are few reports on the molecular mechanism of pathological changes in the uninjured side of the brain. LU Hong[23] found that intracellular edema first appeared in the uninjured side, followed by vascular edema, then mainly intracellular edema of hybrid brain edema. Pathological changes lag behind the injured side in time. It is speculated that the body is under stress, through humoral neuromodulation to cause pathological changes on the uninjured side. However, the expression of AQP4 in the uninjured lateral brain needs further study.
The "trauma penumbra" refers to the peripheral tissues outside the trauma core area that have secondary trauma areas. The "trauma penumbra" can develop into two aspects of deterioration and improvement. If timely and correct intervention can be made to reverse the "trauma penumbra" to the maximum extent, it has become the focus of clinical research. So far, there are few reports about this kind of research, and limited data still show that cerebral hypoperfusion after injury is the pathophysiological basis of "traumatic penumbra", and cell edema is the main pathological change[24]. Ren Huanhuan[25] reported that the main pathological change of brain tissue in the "traumatic penumbra" at the early stage of trauma was vasogenic edema, and the down-regulation of AQP4 expression could alleviate the vascular edema, and then the up-regulation of AQP4 expression caused intracellular edema. The coexistence of the two kinds of edema aggravated the brain edema. On the basis of this study, Chen[26] applied gene silencing AQP4 to significantly reduce brain edema.
3. Correlation between AQP4 and multimodal MRI
3.1 Introduction of multimodal MRI sequences
Multimodal magnetic resonance imaging is an imaging method that can accurately diagnose traumatic brain edema (especially traumatic penumbra). It mainly includes perfusion weighted imaging (PWI) and diffusion-weighted imaging (DWI). In recent years, aquaporin magnetic resonance molecular imaging has become a research hotspot.
DWI can be used to evaluate the diffusion of water molecules in human body by quantitative parameters, and the apparent diffusion coefficient (ADC) is usually used to reflect the diffusion of water molecules, and it can be used to distinguish intracellular brain edema from vascular brain edema. PWI mainly observed the microcirculation perfusion, which changed earlier than morphology.Aquaporin magnetic resonance imaging is a molecular imaging technology. Its main principle is based on the active transport of water molecules by aquaporin. The traditional view is that the distribution of water inside and outside the cell membrane is realized by free diffusion. With the continuous study of AQPs, the concept of active transport of water distribution inside and outside the cell membrane has been established. Aquaporin magnetic resonance imaging technology is to collect images obtained with consecutive different b values, and the expression of AQPs can be quantitatively obtained through post-processing [27]. A number of studies[5] have found that the higher the b value, the better the obtained MR images can express the microscopic images of water molecule transport. However, at present, this imaging technology is mainly limited to liver fibrosis, cerebral ischemia, isolated cells and so on[28,29,30], it has not been reported in brain trauma model.
3.2 Correlation between AQP4 and ADC
A large number of studies have shown that the expression of AQP4 plays an important role in physiological and pathological processes. The study[31] show that ADC value can identify the type of brain edema, AQP4 expression is negatively correlated with the traditional ADC value, so it can be concluded that the expression of AQP4 has a certain correlation with the type of brain edema. Yi HL et al [32] showed that AQP4 expression and ADC changes can reflect the changes of cerebral edema in rats during hyperacute and acute cerebral infarction, and there was a certain correlation between AQP4 expression and the change of ADC value. Li JH et al [28] Performed magnetic resonance functional imaging on cells in vitro, and the results showed that high b value (> 1700s / mm2) could display the transport information of aquaporin, and obtain the expression and distribution of AQP4 in cell membrane. Li QJ et al [29] performed water channel protein magnetic resonance molecular imaging on rat liver fibrosis model, and the results showed that water channel protein magnetic resonance molecular imaging could detect liver fibrosis earlier, and the expression of aquaporin was significantly correlated with ADC value. Chen QY et al [30] studied the correlation between aquaporin magnetic resonance molecular imaging and AQP4 expression by establishing a cerebral ischemia model, and the results showed that the ADC value of ultra-high b value was negatively correlated with AQP4. The possible reason for the above results is that the ADC value calculated by single exponential model cannot accurately reflect the diffusion of water molecules in the body, which is easily affected by microvascular perfusion, and the ultra-high b value can eliminate this effect, secondly, the ultra-high b value has better contrast, the tissue diffusion is enhanced, and the T2 transmission effect is weakened. Aquaporin magnetic resonance molecular imaging can show the expression and distribution of AQP4 in vivo.
4. Summary and Prospect
The condition of brain trauma changes rapidly and the pathological changes are complex. To accurately grasp the course of the disease and give timely targeted treatment is the key to effective treatment of traumatic brain injury. Brain edema is a serious complication of brain injury. In recent years, some studies have found that the occurrence, development and elimination of traumatic brain edema are closely related to AQP4. Multimodal magnetic resonance imaging technology can provide reliable imaging basis for accurate diagnosis of traumatic brain edema, especially aquaporin magnetic resonance molecular imaging, which can show the expression and distribution of AQP4, and provide new ideas for early clinical treatment of brain edema and drug development at the molecular level.
 Journal of Hainan Medical College2020年17期
Journal of Hainan Medical College2020年17期
- Journal of Hainan Medical College的其它文章
- Analysis of two cases of patent ductus arteriosus ligation in preterm identical twins and literature review
- Role of 18F-FDG SPECT / CT imaging in the diagnosis and initial staging of lymphoma
- The value of real-time three-dimensional echocardiography in evaluating left ventricular function for different degrees of heart failure
- Association between polymorphism of MC4R rs489693 gene and disorder of glucose and lipid metabolism in schizophrenia patients treated with olanzapine
- Target prediction and mechanism of Shiyangning in treatment of perianal eczema
- Meta-analysis of traditional Chinese medicine and western medicine in treating retinal vein occlusion complicated by macular edema
