Aggregation behavior of juvenile Neptunea cumingii and eff ects on seed production*
YU Zhenglin , HU Zhi , , SONG Hao , XU Tao , YANG Meijie , , ZHOU Cong , , ZHANG Tao , **
1 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
4 CAS Engineering Laboratory for Marine Ranching, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China 5 University of Chinese Academy of Sciences, Beijing 100049, China
6 Shandong Fisheries Technology Extension Center, Jinan 250013, China
Abstract Neptunea cumingii is an important nutrition-rich economic species in China. Juveniles of N. cumingii suff er from high mortality at low temperatures, which has proved a limiting factor in raising seedlings in artifi cial habitats. Previous research has shown that N. cumingii displays aggregation behavior in response to adverse environmental changes. Therefore, we determined the eff ects of temperature, food, size of juvenile snails, substratum type, and density of juvenile snails on the aggregation behavior of N. cumingii. Results show that, at a low (4°C) or a high (22°C) temperature, juvenile snails adjusted to the inhospitable environment by exhibiting increased aggregation behavior. However, their aggregation behaviors diff ered at these two temperatures. There was no signifi cant diff erence in the aggregation rate, but the typical aggregation size was larger at 4°C than at 22°C. At 10°C or 16°C, aggregation behavior of juvenile snails reduced. Aggregation increased in the satiation treatment at 10°C and 16°C. Small-sized juveniles tended to have higher aggregation rates (92.22%) and a larger typical aggregation size. More juveniles were distributed in the bottom of shaded substrata. A larger typical aggregation size or higher density signifi cantly reduced the mortality of juvenile snails at a low temperature (4°C). These results broaden our understanding of gastropod aggregation behavior and can be used to develop and improve commercial breeding strategies and resource recovery for N. cumingii.
Keyword: aggregation behavior; Neptunea cumingii; environmental temperature; survival rate; seed production
1 INTRODUCTION
Neptunea cumingii Crosse is a large sea snail species of the family Buccinidae (Cai, 2001). It is an important fi shery resource found along the north coast of China, predominantly distributed in the Yellow and Bohai Seas (Miranda et al., 2009; Zhang et al., 2014). N. cumingii is a carnivorous snail, rich in many types of amino acids, glycogen, enzymes, and other nutrients, and has high economic and nutritional value (Cai, 2001). Owing to the current overfi shing of wild resources, the N. cumingii population has declined in large numbers; however, artifi cial breeding technology for this species is still in its infancy. Another major challenge for aquaculture is the low survival rate of juvenile snails, especially at low water temperatures in winter, because the most suitable water temperature is 8-20°C. This limiting factor has been highlighted in N. arthritica (Miranda et al., 2008), a species closely related to N. cumingii.
Winter is a stressful period for animals; living in cold environments requires individual morphological and physiological adjustments, as well as cooperative behavior from the group. Aggregation is an important group behavior (Sukhchuluun et al., 2018) that represents an ecological strategy adopted by animals in response to inhospitable environments. The “group eff ect” produced by aggregation behavior, such as reduced water loss (Yoder et al., 2002), reduced energy loss (Gilbert et al., 2007; Kotze et al., 2008; Nowack and Geiser, 2016), and antipredation defense (Hatle and Salazar, 2001), is of great signifi cance to animals (Su et al., 2007). Many organisms such as mammals, insects, and aquatic organisms exhibit aggregation behavior in winter, which is a proven strategy for resisting adversity and improving survival (Clark and Faeth, 1997; Scheibling and Lauzon-Guay, 2007; Kobak et al., 2009; Zhang et al., 2018).
In general, the aggregation behavior of organisms is aff ected by many factors, such as environmental temperature, light, biological specifi cations, and organism density (Lapointe and Sainte-Marie, 1992; Rojas et al., 2013; Xu et al., 2017). These factors have diff erent eff ects on the aggregation behavior of diff erent organisms. For example, sea urchin density and body size are the two most important factors aff ecting their aggregation behavior (Hagen and Mann, 1994), whereas the aggregation behavior of the gastropod Buccinum undatum Linnaeus is aff ected by water fl ow (Lapointe and Sainte-Marie, 1992). Moreover, Lasiopodomys brandtii mice develop aggregation behavior at low temperatures (Sukhchuluun et al., 2018).
During the process of artifi cial cultivation, juvenile N. cumingii exhibit obvious aggregation behavior, especially at low water temperatures (personal observation). We hypothesize that this behavior is related to their adaptation to the harsh winter environment. Therefore, this study investigates the following research question: How do factors such as temperature, juvenile snail size, substratum type, and juvenile snail density aff ect the aggregation behavior of N. cumingii. The hypotheses tested within the study are as follows: (1) low temperature and satiation promote the aggregation of juvenile snails; (2) small juvenile snails aggregate at a higher frequency; (3) juvenile snails prefer a shaded substratum; and (4) high snail density promotes the aggregation of juvenile snails. The fi ndings of this study expand our understanding of the aggregation behavior of gastropods, which prevents low temperature death in juvenile snails. Therefore, this research is signifi cant for resource management and improving seedling breeding effi ciency.
2 MATERIAL AND METHOD
2.1 Juvenile culture and holding methods
Adult N. cumingii broodstock were collected from the Bohai Sea in Shandong Province, China. Adult male and female snails (50 males and 50 females in each cement pool) were kept in seawater at a temperature of 13-15°C to mate and lay eggs in the absence of light. In nature, the mating and spawning season extends from March to May. In the experiment, the hatching rate of egg capsules was more than 80%, and we obtained more than 6 000 juvenile snails. All experimental juvenile snails (shell height, SH, of 5-16 mm) were cultured under laboratory conditions (water temperature: 13-15°C; salinity: 29.8-31.7 with constant aeration) and fed on Ruditapes philippinarum mollusks (shell length, SL, of 10±0.5 mm) for 5 d prior to experimentation.
2.2 Collection of behavioral data
The experimental device was a plexiglass aquarium (370 mm×280 mm×310 mm). The behavior of juveniles was recorded with a timelapse camera (Brinno TLC-200) at 5-s intervals for 24 h. The experiment was repeated six times and diff erent juveniles were used in all replicates. At the end of each experiment, the aggregation of juvenile snails was counted and the aquaria were cleaned; new fi ltered seawater was used for each experiment. It was considered aggregation behavior only when two or more juveniles gathered, and their numbers were then counted. The formula for calculating the aggregation rate of juvenile snails is as follows:

where M is the aggregation rate, n is the number of aggregating juveniles, and N is the total number of juveniles.
However, the animals could form large or small aggregations but result in the same fi nal aggregation rate, therefore exhibiting clearly diff erent behavior. As the aggregation rate does not show this diff erence, the typical aggregation size was used to represent this diff erence. The formula for calculating the typical aggregation size is as follows:

where S is the typical aggregation size, n is the number of aggregating juveniles, and n1, n2, ··· are the number of individuals in each aggregation (including singletons) (Jarman, 1974). The greater the S value, the larger the typical aggregation size.
2.3 Eff ect of temperature and food on aggregation behavior
This experiment was designed to test whether temperature and food were contributing factors to aggregation behavior. Aggregation behavior was examined in four diff erent water temperature groups (4°C, 10°C, 16°C, and 22°C) and two food groups (satiation and hunger). In the satiation treatment, fi ve R. philippinarum (SL=10±0.5 mm) were introduced as prey in the center of the aquarium (gathered together) and their total wet weight ( W1) was measured before the experiment. Thirty N. cumingii juveniles (SH=5±1 mm) were then introduced into each experimental group. After the experiment, the remaining food was removed and its wet weight was measured ( W2) to calculate the daily food intake of juveniles. The formula for calculating the daily food intake ( Fd) of juveniles was as follows: Fd= W1- W2. The experiment was conducted at a salinity of 29.8-31.7 with constant aeration in the absence of light.
2.4 Eff ect of size on aggregation behavior
This experiment was designed to test whether the size of N. cumingii was a contributing factor to aggregation behavior. Aggregation behavior was examined in three diff erent size groups (SH=5±1 mm, 10±1 mm, and 15±1 mm). Five R. philippinarum (SL=10±0.5 mm) were introduced as prey in the center of the aquarium. Thirty N. cumingii juveniles were then introduced into each experimental aquarium (water temperature: 4°C, salinity: 29.8-31.7 with constant aeration in the absence of light).
2.5 Eff ect of substratum type on aggregation behavior
This experiment was designed to test whether substratum type was a contributing factor to aggregation behavior. Aggregation behavior was examined for six diff erent substratum types: 1. top of an oyster shell (the internal surface of a single valve); 2. bottom of an oyster shell (the external surface of a single valve); 3. top of a transparent corrugated plate; 4. bottom of a transparent corrugated plate; 5. top of a black corrugated plate; 6. bottom of a black corrugated plate. We placed an oyster shell, transparent corrugated plate, and black corrugated plate (the size of all three substrates was 90 mm× 40 mm, selected and measured the size of an oyster shell and then corrugated plates were cut to the appropriate size according to the size of the oyster shell) on the bottom of the same aquarium with no overlap. The substrates were in parallel contact with the bottom of the aquarium so that the juvenile snails could reach their bottom sides due to their corrugated shape. The depth of the corrugation was 2.0 cm. The experimental aquarium was illuminated from the top with an incandescent lamp with a light intensity of 800±50 lx (Illuminometer, LB-ZD809, China). Then, thirty N. cumingii juveniles (SH=5±1 mm) were introduced into the experimental aquarium (water temperature: 4°C, salinity: 29.8-31.7 with constant aeration). This experiment did not provide food. After the experiment, substratum preferences were calculated according to the distribution (numbers/percentages of all animals occupying particular materials whether aggregated or not) and aggregation rate (percentage of aggregated animals on each material, relative to the total number of individuals on the material).
2.6 Eff ect of density on aggregation behavior and mortality rate
This experiment was designed to test whether density was a contributing factor to aggregation behavior and mortality rates. The eff ect of aggregation behavior on mortality rate was examined in three diff erent experiments with diff erent numbers of N. cumingii juveniles (10, 30, and 50; SH=5±1 mm). Five R. philippinarum (SL=10±0.5 mm) were introduced as prey in the center of the aquarium. The experiment lasted for 15 d. The prey and the full volume of water were changed daily (water temperature: 4°C, salinity: 29.8-31.7 with constant aeration in the absence of light). The number of aggregating juveniles and typical aggregation size was calculated every 24 h. Then, the average values of these parameters over the entire experiment duration were calculated and the juvenile mortality was determined.

Fig.1 Aggregation rate of N. cumingii juveniles in diff erent temperature and food groups (a) and the average daily food intake in diff erent temperature groups (b)
2.7 Statistical analysis
The data were tested for normality (Shapiro-Wilk test) and homogeneity (Levene’s test). Data related to the aggregation rate and typical aggregation size in diff erent temperature and food groups were analyzed using two-way analysis of variance (ANOVA). Bonferroni-adjusted paired sample t-tests were used for post-hoc comparisons. When the interaction was signifi cant, one-way ANOVA was used to compare the temperature eff ect for each food group, and an independent sample t-test was used to compare the food eff ect for each temperature group. Data related to food intake, the size and density of juvenile snails were analyzed using one-way ANOVA. Tukey’s posthoc test was performed to identify signifi cant diff erences among groups. Data related to the eff ects of substratum type (distribution rate) were analyzed using repeated measures ANOVA (with the substratum as a within-subject factor). Bonferroni-adjusted paired sample t-tests were used for post-hoc comparisons among the substrata. Data related to the eff ects of substratum type (aggregation rate) were analyzed using a paired sample t-test, using only the substrata frequently visited by snails (see Section 3). All statistical computations were conducted using SPSS v. 16.0 software (SPSS Inc., Chicago, IL), where α-values of 0.05 were considered to be statistically signifi cant.
3 RESULT
3.1 Juvenile culture and holding
Adult N. cumingii broodstock began mating and laid eggs at 12°C. The base of the egg capsule was attached to the bottom or the wall of the cement pools. 20-80 yellow egg capsules were laid in a cluster and each egg capsule produced 1-2 juvenile snails. The larvae did not pass through the planktonic stage but directly metamorphosed into juvenile snails (SH=5-7 mm) in the egg capsule before hatching. Bivalves R. philippinarum were provided as food.
3.2 Eff ect of temperature and food on aggregation behavior
The aggregation rate diff ered signifi cantly among the diff erent temperature groups (4°C, 10°C, 16°C, and 22°C) ( F3,48=89.11, P <0.001) and between diff erent food groups (satiation and hunger) ( F1,48=19.73, P <0.001) (Fig.1a). Temperature and food had signifi cant interactive eff ects with the aggregation rate of snails ( F3,48=5.21, P=0.004). The aggregation rate of the 4°C group was higher than that of the 10°C ( P <0.001), 16°C ( P <0.001), and 22°C ( P=0.014) groups. The aggregation rate of the 22°C group was higher than that of the 10°C ( P <0.001), and 16°C ( P <0.001) groups.
In both satiation ( F3,20=27.33, P <0.001, Fig.1a) and hunger ( F3,20=72.95, P <0.001, Fig.1a) groups, theaggregation rate was aff ected by temperature. In the satiation group, the aggregation rate of the 4°C group was higher than that of the 10°C ( P <0.001), 16°C ( P <0.001), and 22°C ( P=0.018) groups. Moreover, the aggregation rate of the 22°C group was higher than that of the 10°C ( P=0.003) and 16°C ( P=0.001) groups. In the hunger groups, the aggregation rates of the 4°C and 22°C groups were higher than those of the 10°C ( P <0.01) and 16°C ( P <0.001) groups. The aggregation rate of the 10°C group was higher than that of the 16°C ( P=0.018) group. Diff erences in aggregation rate between satiated and hungry groups varied according to temperature. At 10°C and 16°C, the aggregation rate of the satiation group was higher than that of the hunger group (10°C: t=2.34, P=0.042; 16°C: t=6.50, P <0.001). However, at 4°C and 22°C, there was no signifi cant diff erence between the satiated and hungry groups (4°C: t=1.51, P=0.162; 22°C: t=0.46, P=0.659).
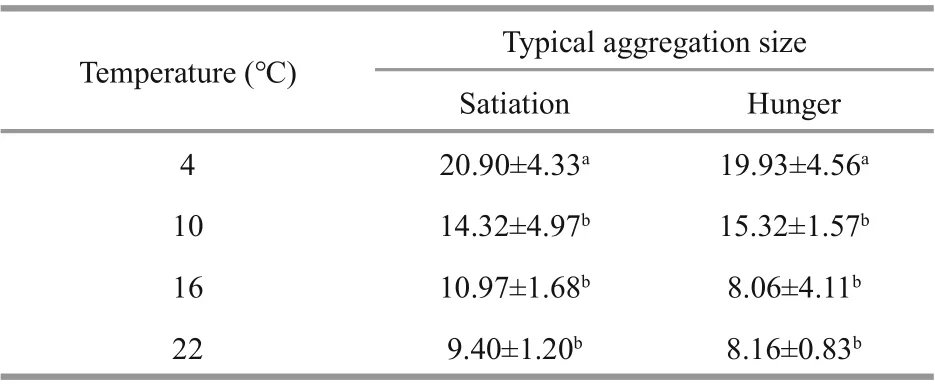
Table 1 Mean (±SE) typical aggregation size in diff erent temperature and food groups

Table 2 Mean (±SE) typical aggregation size in diff erent size groups
The typical aggregation size diff ered signifi cantly among the diff erent temperature groups (4°C, 10°C, 16°C, and 22°C) ( F3,48=14.45, P <0.001) but not between diff erent food groups (satiation and hunger) ( F1,48=0.01, P=0.977) (Table 1). The typical aggregation size of the 4°C group was higher than that of the 10°C, 16°C, and 22°C groups (satiation: 4-10°C, P=0.017, 4-16°C and 4-20°C, P< 0.001; hunger: 4-10°C, P=0.025, 4-16°C, P=0.016, 4-20°C, P< 0.001).

Fig.2 Aggregation rate of N. cumingii juveniles of diff erent sizes
The average daily food intake was also aff ected by temperature ( F3,20=24.29, P <0.001, Fig.1b). The average daily food intake of the 16°C group was higher than that of the 4°C ( P <0.001), 10°C ( P=0.003), and 22°C ( P <0.001) groups. The average daily food intake of the 10°C group was higher than that of the 4°C ( P=0.003) group. At 16°C, juveniles had the highest average daily food intake (3.56 g/d) but the lowest aggregation rate (60.56%), whereas at 4°C, juveniles had the lowest average daily food intake (0.34 g/d) but the highest aggregation rate (92.22%).
3.3 Eff ect of size on aggregation behavior
The aggregation rate was aff ected by the varying sizes of the juvenile snails; aggregation rates decreased with increasing juvenile size ( F2,10=12.59, P=0.001, Fig.2). The aggregation rate of the 5±1 mm group was higher than that of the 10±1 mm ( P=0.018) and 15±1 mm ( P <0.001) groups. However, there was no signifi cant diff erence between the 10±1 mm and 15±1 mm groups ( P=0.190). The typical aggregation size was also aff ected by juvenile size ( F2,15=7.39, P=0.006, Table 2). The typical aggregation size of the 5±1 mm group was higher than that of the 10±1 mm ( P=0.029) and 15±1 mm ( P=0.006) groups.
3.4 Eff ect of substratum type on aggregation behavior

Fig.3 Typical aggregation size and mortality rate of N. cumingii juveniles in diff erent experimental groups
The distribution was aff ected by diff erent substratum types ( F5,30=57.10, P <0.001, Table 3). The percentage at the bottom of the oyster shell (38.89%) and at the bottom of the black corrugated plate (32.78%) was higher than that at the top of the oyster shell ( P=0.003, P=0.001, respectively), the top of the transparent corrugated plate ( P=0.003, P< 0.001, respectively), the bottom of the transparent corrugated plate ( P <0.001, P=0.005, respectively), and the top of the black corrugated plate ( P=0.003, P=0.006, respectively). Only a few juvenile snails gathered on the top of the oyster shell (2.22%), the top (3.89%) and bottom (2.78%) of the transparent corrugated plate, and the top of the black corrugated plate (3.33%). The aggregation rate of juveniles predominantly occurred at the bottom of the oyster shell (86.09%) and the bottom of the black corrugated plate (82.11%), with no signifi cant diff erence between the two materials ( t=0.79, P=0.467).
3.5 Eff ect of density on aggregation behavior and mortality rate
Juvenile snail density signifi cantly aff ected the typical aggregation size ( F2,15=43.03, P< 0.001, Fig.3) and mortality of juvenile snails ( F2,15=20.67, P< 0.001, Fig.3). With an increase of juvenile density, the typical aggregation size increased and the mortality rates decreased. The typical aggregation size in the 50-snail group (19.82) was higher than that in the 10-snail group (4.22, P <0.001) and 30-snail group (11.83, P=0.001). Moreover, the typical aggregation size of the 30-snail group was higher than that of the 10-snail ( P=0.001) group. The mortality rate in the 10-snail group (28.33%) was higher than that in the 30-snail group (18.89%, P=0.006) and 50-snail group (12.00%, P <0.001). Moreover, the mortality rate of the 30-snail group was higher than that of the 50-snail group ( P=0.041).
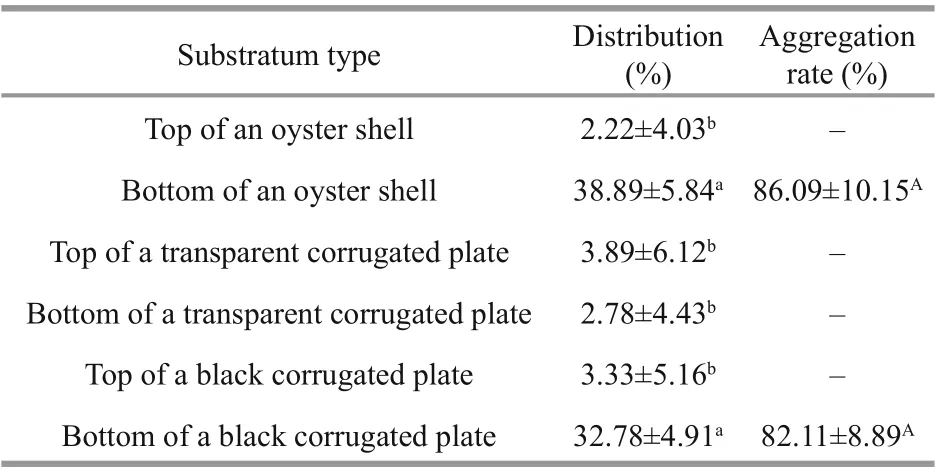
Table 3 Mean (±SE) distribution and aggregation rate of N. cumingii juveniles on diff erent substrata
4 DISCUSSION
4.1 Eff ect of temperature and food on aggregation behavior
Both temperature and food are important factors that aff ect aggregation behavior in various species. For example, mice (endothermic animals) show more obvious aggregation behavior in cold conditions than warm conditions as it enables them to lose less heat and produce heat for one another (Batchelder et al., 1983). Aggregation is a social thermoregulatory behavior, which is important for reducing heat loss and energy expenditure and maintaining body temperature to survive the cold (Gilbert et al., 2010; Sukhchuluun et al., 2018). However, heterothermic animals have diff erent heat demands and cold defense mechanisms. The insect Pyrrhocoris apterus aggregates signifi cantly during overwintering to lower their metabolic rates (Su et al., 2007). Additionally, the gastropod Nerita atramentosa has shown aggregation behavior in winter in intertidal habitats, where the temperature of aggregated snails is 2°C warmer than that of solitary individuals. The proposed reason for this phenomenon was that aggregation can increase thermal inertia (higher thermal inertia means that snails cool down more slowly) and thus reduce thermal exchanges rates between aggregated snails and environment (Chapperon and Seuront, 2012). Increased aggregation was found in the satiation treatment at 10°C and 16°C. However, this eff ect disappeared at suboptimum temperatures (4°C and 22°C). At 10°C and 16°C, some snails aggregated around the food sources, which increased the aggregation rate of the satiation treatment. It should be noted that aggregating around food is not a real aggregation with conspecifi cs, i.e. driven by preferences for individuals of own species, because each individual behaves independently of the others and the presence of conspecifi cs may be even disadvantageous for them. On the contrary, true aggregations are formed to defend from cold, predators and for reproduction purposes. Both can lead to a clumped distribution, but their mechanisms are diff erent. In the experiment on temperature and food, both these mechanisms can be involved.
The most suitable water temperature for N. cumingii is 8-20°C (Cai, 2001). In this study, the aggregation rate of juveniles was lower in water temperatures of 10°C (52.78%) and 16°C (41.44%), but higher at 22°C (78.89%) and 4°C (85.56%). This indicates that aggregation behavior plays an important role at a water temperature of 4°C or 22°C for juvenile snails. During actual production, we found that a temperature above 22°C increased the mortality of snails (Production data, unpublished). Of the studied temperatures, 10°C and 16°C were deemed more suitable for juvenile snails as they ate more food (Fig.1b) to gain more energy; thus, the aggregation rate was signifi cantly reduced. However, although there was no signifi cant diff erence in the aggregation rate between 4°C and 22°C, the typical aggregation size was signifi cantly diff erent. Snails formed a small number of large groups at 4°C and a large number of small groups at 22°C. Large groups at low temperature may benefi t juvenile snails by reducing heat loss, whereas small groups at high temperature may aid heat dissipation through the water. The question arises: why did juvenile snails exhibit high aggregation rate at high temperature? This may be because aggregation brings other benefi ts; for example, aggregating juveniles might be better protected against predators (Hatle and Salazar, 2001).
4.2 Eff ect of size on aggregation behavior
The size of an organism has signifi cant eff ects on its aggregation behavior. Smaller juvenile snails of N. cumingii had high aggregation rates (92.22%) and larger typical aggregation sizes at 4°C (Fig.2 & Table 2). Larger snails created groups of smaller size, which generated a similar mass and level of protection as larger groups of smaller individuals. This may be because the diff erent life stages of species have diff erent tolerance to low water temperature, and smaller individuals are more strongly aff ected than larger ones because of a higher energy demand per unit weight due to the large surface area-to-volume ratio. In contrast, larger individuals are more tolerant of low water temperature, and smaller individuals require more aggregation to survive (Mehner and Wieser, 1994; Kotze et al., 2008; Sukhchuluun et al., 2018).
4.3 Eff ect of substratum type on aggregation behavior
Marine invertebrates are susceptible to light in many aspects such as feeding, growth, and survival. Miao et al. (2016) found that higher light intensity can inhibit the growth of juvenile shellfi sh Sinonovacula constrict. Xu et al. (2017) indicated that light could reduce the aggregation rate of juvenile Mytilus coroscus mussels. In this study, the substratum type had a signifi cant eff ect on the distribution of juvenile snails, but no signifi cant eff ect on their aggregation rate. Juvenile snails were predominantly distributed on shaded substrata (bottom of opaque substrata), which may indicate that juvenile snails prefer a dark environment.
4.4 Eff ect of density on aggregation behavior and mortality rate
Density is an important factor aff ecting the aggregation behavior of organisms. For example, an increase of urchin density led to an increase in aggregation size (Hagen and Mann, 1994). The aggregation rate of juvenile Mytilus coroscus mussels also increased with an increase of density (Xu et al., 2017). However, it is diffi cult to determine if the N. cumingii snails aggregated due to a change in available space caused by the increase of density or because they actively clumped together according to the experiment conditions. Aggregation can signifi cantly increase the survival rates of organisms overwintering at low temperature due to a reduction in activity, respiration, and metabolic rate (Tojo et al., 2005). At the same time, aggregation improves heat preservation by reducing individual energy expenditure (Ancel et al., 1997; Sukhchuluun et al., 2018). In this study, it was found that the mortality of juvenile N. cumingii decreased signifi cantly with an increase in typical aggregation size. This was similar to the fi ndings of Su et al. (2007), who found that increased aggregation improved the survival rates of Pyrrhocoris apterus.
5 CONCLUSION
We investigated the aggregation behavior of juvenile N. cumingii gastropods and demonstrated that, at both low and high temperature, juvenile snails adjusted to the inhospitable environment through aggregation behavior. Food increased the aggregation rate of snails. Small juveniles exhibited more active aggregation behaviors. More juveniles were distributed on the bottom of substrata, i.e., in the shade. These fi ndings enable us to recommend culture practices that will increase the survival rates of juvenile snails in winter. First, it is recommended to provide shelter, such as oyster shells or black corrugated plates. Second, it is advisable to increase the breeding density appropriately in order to increase the typical aggregation size. In this study, we only studied the aggregation behavior of small juvenile snails (SH<15±1 mm), neglecting adult snails of larger size. In the future, we will continue to study the aggregation behavior of adult snails and explain the aggregation mechanism from the perspective of energy and physiological changes. In summary, this study broadens our understanding of gastropod aggregation behavior and can be used to develop or improve commercial breeding strategies for N. cumingii.
6 DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ancel A, Visser H, Handrich Y, Masman D, Le Maho Y. 1997. Energy saving in huddling penguins. Nature, 385(6614): 304-305, https://doi.org/10.1038/385304a0.
Batchelder P, Kinney R O, Demlow L, Lynch C B. 1983. Eff ects of temperature and social interactions on huddling behavior in Mus musculus. Physiology & Behavior, 31(1): 97-102, https://doi.org/10.1016/0031-9384(83)90102-6.
Cai Q H. 2001. Resource protection measures and processing methods of Naptunea cumingii. China Fisheries, (10): 73, https://doi.org/10.3969/j.issn.1002-6681.2001.10.051. (in Chinese)
Chapperon C, Seuront L. 2012. Keeping warm in the cold: on the thermal benefi ts of aggregation behaviour in an intertidal ectotherm. Journal of Thermal Biology, 37(8): 640-647, https://doi.org/10.1016/j.jtherbio.2012.08.001.
Clark B R, Faeth S H. 1997. The consequences of larval aggregation in the butterfl y Chlosyne lacinia. Ecological Entomology, 22(4): 408-415, https://doi.org/10.1046/j. 1365-2311.1997.00091.x.
Gilbert C, Blanc S, Giroud S, Trabalon M, Le Maho Y, Perret M, Ancel A. 2007. Role of huddling on the energetic of growth in a newborn altricial mammal. American Journal of Physiology- Regulatory, Integrative and Comparative Physiology, 293(2): R867-R876, https://doi.org/10.1152/ajpregu.00081.2007.
Gilbert C, McCaff erty D, Le Maho Y, Martrette J M, Giroud S, Blanc S, Ancel A. 2010. One for all and all for one: the energetic benefi ts of huddling in endotherms. Biological Reviews, 85(3): 545-569, https://doi.org/10.1111/j.1469- 185X.2009.00115.x.
Hagen N T, Mann K H. 1994. Experimental analysis of factors infl uencing the aggregating behaviour of the green sea urchin Strongylocentrotus droebachiensis (Müller). Journal of Experimental Marine Biology and Ecology, 176(1): 107-126, https://doi.org/10.1016/0022-0981(94)90200-3.
Hatle J D, Salazar B A. 2001. Aposematic coloration of gregarious insects can delay predation by an ambush predator. Environmental Entomology, 30(1): 51-54, https://doi.org/10.1603/0046-225X-30.1.51.
Jarman P J. 1974. The social organisation of antelope in relation to their ecology. Behaviour, 48(1-4): 215-267, https://doi.org/10.1163/156853974X00345.
Kobak J, Poznańska M, Kakareko T. 2009. Eff ect of attachment status and aggregation on the behaviour of the zebra mussel Dreissena polymorpha. Journal of Molluscan Studies, 75(2): 119-126, https://doi.org/10.1093/mollus/eyn046.
Kotze J, Bennett N C, Scantlebury M. 2008. The energetics of huddling in two species of mole-rat (Rodentia: bathyergidae). Physiology & Behavior, 93(1-2): 215-221, https://doi.org/10.1016/j.physbeh.2007.08.016.
Lapointe V, Sainte-Marie B. 1992. Currents, predators, and the aggregation of the gastropod Buccinum undatum around bait. Marine Ecology Progress Series, 85: 245-257, https://doi.org/10.3354/meps085245.
Mehner T, Wieser W. 1994. Energetics and metabolic correlates of starvation in juvenile perch ( Perca fl uviatilis). Journal of Fish Biology, 45(2): 325-333, https://doi.org/10.1111/ j.1095-8649.1994.tb01311.x.
Miao Z Q, Liu Z H, Xu J L, Zhou H B, Yan X J. 2016. Eff ects of light intensity on growth of juvenile Sinonovacula constricta (Lamarck, 1818). Journal of Ningbo University ( Natural Science & Engineering Edition), 29(2): 1-5. (in Chinese with English abstract)
Miranda R M, Fujinaga K, Ilano A S, Nakao S. 2009. Eff ects of imposex and parasite infection on the reproductive features of the Neptune whelk Neptunea arthritica. Marine Biology Research, 5(3): 268-277, https://doi.org/10.1080/17451000802419422.
Miranda R M, Lombardo R C, Goshima S. 2008. Copulation behaviour of Neptunea arthritica: baseline considerations on broodstocks as the fi rst step for seed production technology development. Aquaculture Research, 39(3): 283-290, https://doi.org/10.1111/j.1365-2109.2008.01901.x.
Nowack J, Geiser F. 2016. Friends with benefi ts: the role of huddling in mixed groups of torpid and normothermic animals. Journal of Experimental Biology, 219(4): 590-596, https://doi.org/10.1242/jeb.128926.
Rojas J M, Castillo S B, Escobar J B, Shinen J L, Bozinovic F. 2013. Huddling up in a dry environment: the physiological benefi ts of aggregation in an intertidal gastropod. Marine Biology, 160(5): 1 119-1 126, https://doi.org/10.1007/s00227-012-2164-6.
Scheibling R E, Lauzon-Guay J-S. 2007. Feeding aggregations of sea stars ( Asterias spp. and Henricia sanguinolenta) associated with sea urchin ( Strongylocentrotus droebachiensis) grazing fronts in Nova Scotia. Marine biology, 151(3): 1 175-1 183, https://doi.org/10.1007/s00227-006-0562-3.
Su Y L, Lu Z Z, Song J, Miao W. 2007. Eff ect of overwintering aggregation on energy metabolism in the fi rebug, Pyrrhocoris apterus (Heteroptera: pyrrhocoridae). Acta Entomologica Sinica, 50(12): 1 300-1 303, https://doi.org/10.3321/j.issn:0454-6296.2007.12.014. (in Chinese with English abstract)
Sukhchuluun G, Zhang X Y, Chi Q S, Wang D H. 2018. Huddling conserves energy, decreases core body temperature, but increases activity in Brandt’s voles ( Lasiopodomys brandtii). Frontiers in Physiology, 9: 563, https://doi.org/10.3389/fphys.2018.00563.
Tojo S, Nagase Y, Filippi L. 2005. Reduction of respiration rates by forming aggregations in diapausing adults of the shield bug, Parastrachia japonensis. Journal of Insect Physiology, 51(10): 1 075-1 082, https://doi.org/10.1016/j.jinsphys.2005.05.006.
Xu J K, Peng L H, Gao W, Shen H D, Liang X, Yang J L. 2017. Eff ects of light intensity, water temperature and density on aggregation of juvenile mussel Mytilus coruscus. Journal of Dalian Ocean University, 32(3): 275-279, https://doi.org/10.16535/j.cnki.dlhyxb.2017.03.004. (in Chinese with English abstract)
Yoder J A, Hobbs III H H, Hazelton M C. 2002. Aggregate protection against dehydration in adult females of the cave cricket, Hadenoecus cumberlandicus (Orthoptera, Rhaphidophoridae). Journal of Cave and Karst Studies, 64(2): 140-144.
Zhang X F, Yang D Z, Zhou Y B, Yue Z H, Zhang L X, Xu K. 2014. Impacts of temperature and salinity on oxygen consumption rate and ammonia excretion rate in juvenile whelk Neptunea cumingii. Journal of Dalian Ocean University, 29(3): 251-255, https://doi.org/10.3969/J.ISSN.2095-1388.2014.03.010. (in Chinese with English abstract)
Zhang X Y, Sukhchuluun G, Bo T B, Chi Q S, Yang J J, Chen B, Zhang L, Wang D H. 2018. Huddling remodels gut microbiota to reduce energy requirements in a small mammal species during cold exposure. Microbiome, 6(1): 103, https://doi.org/10.1186/s40168-018-0473-9.
 Journal of Oceanology and Limnology2020年5期
Journal of Oceanology and Limnology2020年5期
- Journal of Oceanology and Limnology的其它文章
- Distribution, sources and burial fl ux of sedimentary organic matter in the East China Sea*
- Photoelectrochemical cathodic protection of Cu 2 O/TiO 2 p-n heterojunction under visible light*
- Antioxidant bisabolane-type sesquiterpenoids from algalderived fungus Aspergillus sydowii EN-434*
- Calcium isotopic signatures of depleted mid-ocean ridge basalts from the northeastern Pacifi c*
- Application of confocal laser Raman spectroscopy on marine sediment microplastics*
- Corrosion behavior of Q235B carbon steel in simulated seawater pumped storage system under operational conditions*
