Corrosion behavior of Q235B carbon steel in simulated seawater pumped storage system under operational conditions*
YANG Dan , HUANG Yanliang , , , LI Jianqiu , GAO Yanming , ZHAO Xia , XU Weichen
1 CAS Key Laboratory of Marine Environmental Corrosion and Bio-fouling, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 University of Chinese Academy of Sciences, Beijing 100049, China
3 CSG Power Generation Co., Ltd., Guangzhou 511400, China
4 Open Studio for Marine Corrosion and Protection, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
5 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract Seawater pumped storage systems have bright prospect for energy storage in the coming years. The operational conditions of seawater pumped storage system are complex and harsh, where metal materials suff er from severe general and local corrosion. The corrosion behavior of Q235B carbon steel in simulated seawater pumped storage system under operational conditions was studied by potentiodynamic polarization, cyclic potentiodynamic polarization, and scanning electron microscope (SEM). The results confi rm that the working pressure aff ected the corrosion resistance of Q235B carbon steel during the whole immersion period. The pressure promoted the electrochemical reaction of corrosion process and the corrosion rate increased with pressure at the initial immersion period. However, the stable rust layer formed after longtime immersion at diff erent pressures increased the corrosion resistance of carbon steel, and decreased the corrosion degree of carbon steel. Meanwhile, the working pressure aff ected the pitting corrosion behavior of Q235B carbon steel during the whole immersion period. The pitting corrosion potential was more negative and the tendency of pitting corrosion was higher at 4 MPa during the whole immersion period. However, pressure also accelerated the formation rate of protective rust layer on the steel surface. Q235B carbon steel has higher susceptibility to pitting corrosion at 4 MPa in the static seawater.
Keyword: seawater pumped storage system; seawater pipeline; pressure; corrosion resistance; pitting corrosion
1 INTRODUCTION
Pumped storage system (PSS) is the most mature and the most competitive technology for energy storage at a large-scale all over the world. PSS plays an extremely signifi cant role in the power grid in China (Wu et al., 2019). Coastal regions have the abundant renewable resources and the advanced economies that required huge electricity support (Ozkan and Mayo, 2019). Wind farms, solar photovoltaic, solar thermal power stations, tidal power stations, and wave power stations are developed increasingly for electricity generation that will adjust and optimize the energy mix effi ciently in coastal regions and islands (Schaber et al., 2004; Wang et al., 2018). However, the renewable resources are stochastic and the production of renewable power is unsteady, which put a severe challenge on the national electric grids (Park and Hur, 2018). Combining the stable pumped storage system and renewable power systems enjoys great potential and vast development prospects (Katsaprakakis et al., 2013; Katsaprakakis and Christakis, 2014). The traditional hydro pumped storage system relies heavily on fresh water resources, which makes it more expensive and diffi cult to build traditional hydro pumped storage system in coastal regions and islands. Seawater pumped storage system (SPSS) will gradually spring up for providing the large-scale energy storage that will be required in coming years (Manfrida and Secchi, 2014). Using the sea as the lower reservoir directly, SPSS reduces much upfront costs and construction time compared with traditional hydro pumped storage system. SPSS built near the large power sources such as wind power, nuclear power and solar power or the coastal load center with relatively large power demand enables to storage and dispatch the electricity generated by renewable resources so that guaranteeing the fl exibility and stability of electric grids (McLean and Kearney, 2014).
The experts and scholars in the world have done some theoretical studies and project practices on the site selection, construction, operation, and maintenance of SPSS. The operational conditions of SPSS are complex and harsh, where constructional materials will suff er from severe general corrosion (Caines et al., 2015) and localized corrosion (Ma et al., 2015; Amann et al., 2018). Temperature (López-Ortega et al., 2018), chloride ion (Ma et al., 2009), dissolved oxygen (Xue et al., 2019), operational pressure (Tian et al., 2014), seawater velocity (Islam and Farhat, 2017) and biological activities (Liu et al., 2014) are the major factors that aff ect the corrosion of metal materials in marine environment. Therefore, the greater challenges for the structural materials selection in the seawater pumped storage system shall be addressed. The seawater falling from higher reservoir or being pumped to a higher reservoir must be transferred through the pipelines in SPSS. Seawater pipeline is one of the key structures in SPSS that infl uences the effi ciency even the operational stability of SPSS directly. Thus, the major issue with the seawater pipeline construction is the selection of a corrosion-resistant material. The anti-fouling coatings, anti-corrosion coatings, and cathodic protection will be applied in the inner pipeline surface. However, the seawater pipeline will corrode because of the failure of coating. Metal materials selection should take account of the compression intensity, fatigue resistance, corrosion resistance, and economy.
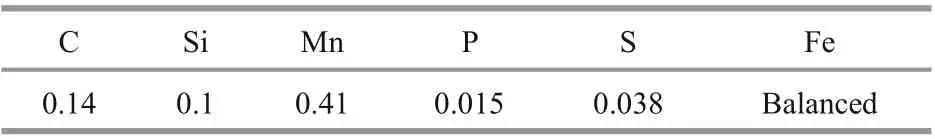
Table 1 The chemical composition of Q235B carbon steel (wt%)
In recent years, carbon steels have been widely used as construction materials in marine environment due to their remarkable strength, excellent ductility, superb formability and low cost. Corrosion is one of the persistent problems for marine infrastructures, which occurs due to chemical or electrochemical reactions between the marine environment and metals (Zhang et al., 2009). Except for the general corrosion, pitting corrosion of carbon steel is a major cause of failure (Yang et al., 2018). Pitting corrosion can be initiated when the protective fi lm on the metal surface is disrupted. The presence of certain aggressive anions, such as the chlorides can migrate to the active corrosion area and stabilize the pitting corrosion (Pardo et al., 2000). Pressure is also one of the major factors that infl uences the corrosion behavior in the marine environment. The operation pressure in SPSS is higher than normal pressure. The eff ect of working pressure on the corrosion behavior of carbon steel in SPSS needs to be concerned.
This study investigated the corrosion behavior of Q235B carbon steel in simulated seawater pumped storage station under operational conditions. The eff ect of pressure on the corrosion resistance of Q235B carbon steel in the seawater was measured by potentiodynamic polarization curves, and analyzed by the corrosion current density. The eff ect of pressure on the pitting corrosion behavior of Q235B carbon steel in the seawater was investigated by obtaining cyclic potentiodynamic polarization curves. This study aimed at examining the eff ect of pressure on the corrosion behavior of carbon steel. In addition, the results would contribute to the material selection of seawater pipeline for the seawater pumped storage system.
2 MATERIAL AND METHOD
2.1 Material and immersion electrolyte
Q235B carbon steel was used in the experiment with the chemical composition shown in Table 1. The specimens were in as received state without heat treatment before the tests.
Disc-shaped specimens for electrochemical tests with diameters of 18 mm were cut from the steel plate. The sample was embedded in a designed polytetrafl uoroethylene tube. The non-working surface of the sample was sealed with sealant, leaving a working area of 2.54 cm2. Prior to the experiments, the exposed surfaces of all samples were subsequently abraded with 400, 600, 800, and 1 000 grit silicon carbide emery papers. The samples were then rinsed with distilled water, degreased with absolute ethanol, and dried in a cool airfl ow. We induced electrical contact to each coupon by a metal conductor connected to the backside of each coupon.
The immersion electrolyte was seawater that was collected directly from Qingdao, China. After pumping the seawater, the high-pressure reaction kettle was full of seawater and closed tightly. And the static pressure was achieved by pushing water into the high-pressure reaction kettle using pneumohydraulic pressurization system. The seawater velocity was decided by running speed of agitator shaft in the kettle. For static seawater, the velocity was set at 0.
2.2 Electrochemical measurements
The electrochemical experiments were conducted in a high-pressure reaction kettle fi lled with seawater. A traditional three-electrode system with a PS-08 multichannel potentiostat workstation was used to measure the electrochemical characteristics of the specimens. In the three-electrode system, the specimen, an Ag/AgCl/seawater electrode, and a Hastelloy rod were used as the working electrode (WE), the reference electrode (RE) and the counter electrode (CE), respectively. All potentials are presented in comparison to the Ag/AgCl/seawater electrode. All measurements were conducted at 25±1℃.
The solid Ag/AgCl electrode is widely used in seawater conditions, which has very small solubility, extremely high stability and reversibility in high temperature and pressure aqueous solution system, and even in the presence of hydrogen. The electrode surface was well protected using a special designed structure while in use. These characteristics make it very distinctive compared with other similar electrodes, such as Ag/AgCl/0.1-mol/L KCl, Ag/AgCl/1-mol/L KCl and Ag/AgCl/saturated KCl. The electrode potential of Ag/AgCl/seawater electrode is decided by Cl-concentration in seawater. The fabrication of the solid Ag/AgCl electrode under the pressure of 12 MPa makes it durable at least below this pressure.
2.2.1 Polarization curve
When the open circuit potential (OCP) of the specimen was stable, polarization curve test was conducted starting from 250 mV below the OCP to 250 mV above the OCP with a sweep rate of 10 mV/min. The electrochemical measurements were performed at normal pressure and 4 MPa to investigate the corrosion rate of Q235B carbon steel in simulated SPSS operation conditions.
2.2.2 Cyclic polarization curve
When the open circuit potential (OCP) of the specimen was stable, each specimen was polarized from 100 mV below the OCP to the potential corresponding to the anodic current density value of 1 mA/cm2and then reversing back to the potential that near the initial potential. The scan rate of cyclic polarization measurement was 10 mV/min.
Pitting corrosion is commonly found in carbon steel, especially at marine environment. For the steel with passive fi lm, the polarization curve shows a sudden and rapid increase of the anodic current density indicating breakdown of the passive fi lm and pit initiation. For the Q235B carbon steel, this point is not obvious. Practically, the potential of the corresponding point with the current density of 10 μA/cm2or 100 μA/cm2can be taken as the pitting potential, and record it as Eb10or Eb100respectively. This method was proved reasonable by our previous work (Yu et al., 2015).
The electrochemical measurements were performed at normal pressure and 4 MPa, which is the nominal pressure of seawater pipeline, to investigate the pitting corrosion behavior of Q235B carbon steel in simulated SPSS operation conditions.
2.2.3 Corroded surface observation
The potentiostatic polarization experiments were performed at the applied potential that was below and above the practical pitting potential Eb100at diff erent pressures for 1 h in seawater after 1-d immersion.
After 1 h of potentiostatic polarization, the specimen surface was cleaned with distilled water, and then the corrosion products were removed by immersion in acidic solution (100-mL HCl+0.3-g hexamethylene tetramine+100-mL distilled water) for 0-1 min. Finally, the specimens were cleaned with distilled water and dried with cold air. The characteristics of these cleaned specimen surfaces were examined by scanning electron microscope (SEM).
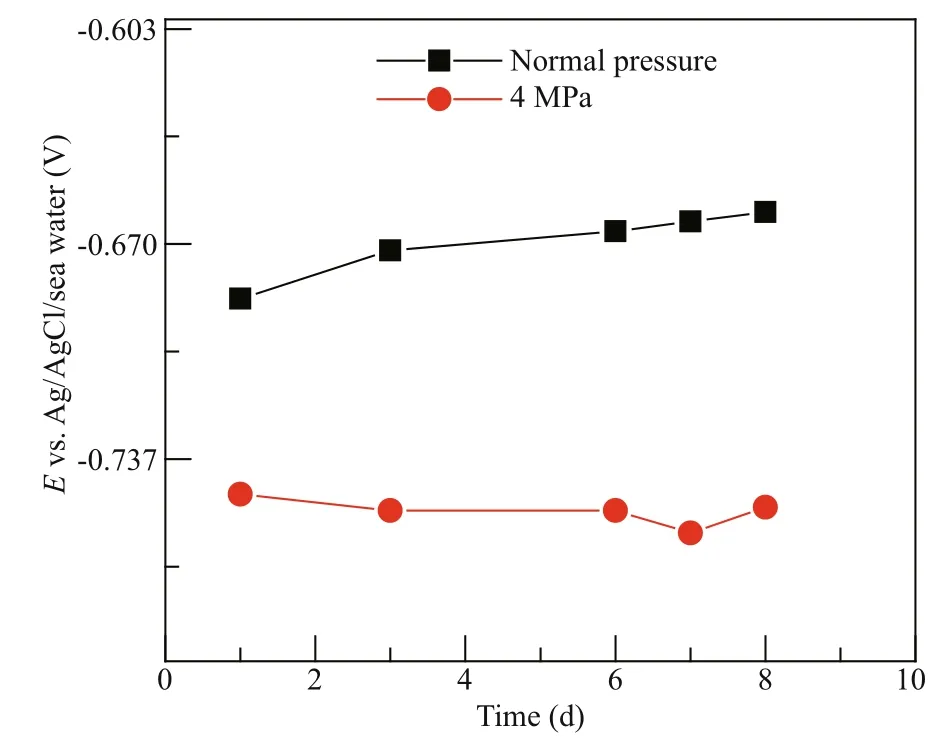
Fig.1 The stable OCP results of Q235B carbon steel at diff erent pressures
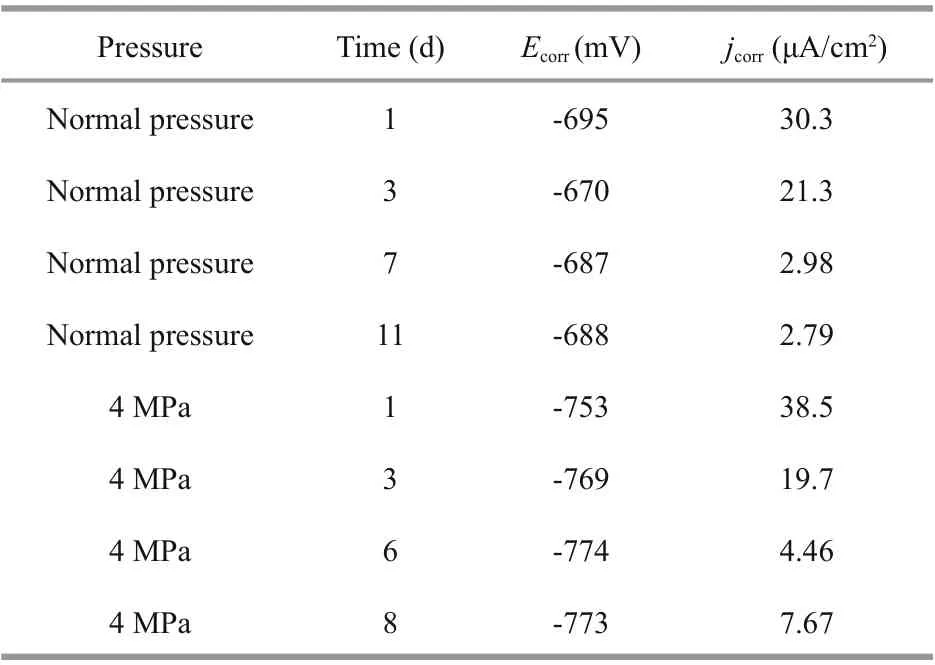
Table 2 Electrochemical parameters obtained from the potentiodynamic polarization curves of Q235B carbon steel at diff erent pressures
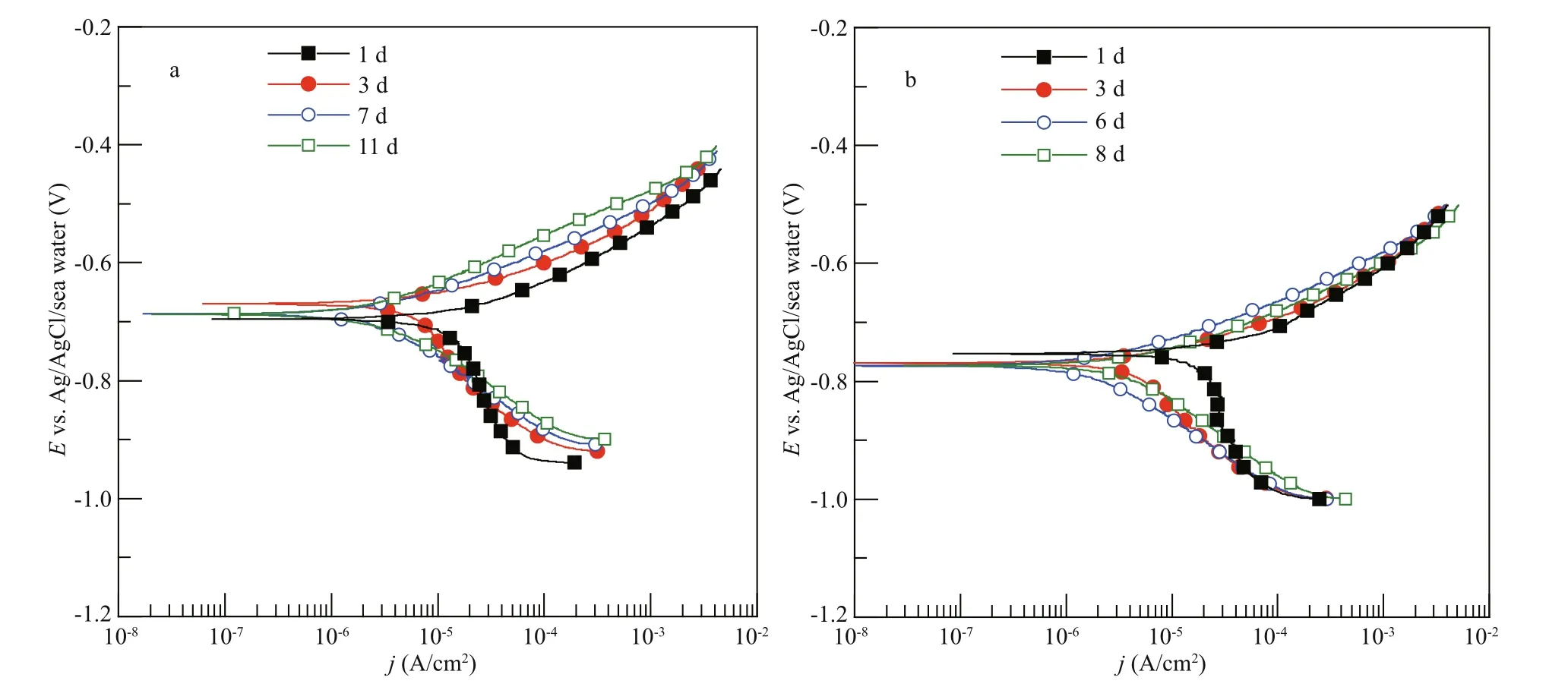
Fig.2 The potentiodynamic polarization curves of Q235B carbon steel at normal pressure (a) and 4 MPa (b)
3 RESULT
3.1 Open circuit potential measurements
Figure 1 shows the stable OCP results of Q235B carbon steel at the normal pressure and 4 MPa, respectively. The stable OCP values of Q235B carbon steel increased with immersion time at the normal pressure. The initial stable OCP of -687 mV increased to -660 mV at normal pressure. The stable OCP values of Q235B carbon steel were stable with time at 4 MPa. The signifi cant phenomenon was that the stable OCP shifted to negative direction with the increase of pressure. After 1-d immersion, the value of the stable OCP was -687 mV at the normal pressure while the value of the stable OCP was -753 mV at 4 MPa.
3.2 Polarization measurements
The corrosion kinetics of metal materials is characterized by potentiodynamic polarization (Kim et al., 2014). Potentiodynamic polarization curves of the Q235B carbon steel samples at diff erent pressures are illustrated in Fig.2. Table 2 shows the electrochemical parameters of corrosion potential ( Ecorr) and corrosion current density ( jcorr) obtained using the Tafel extrapolation method (Zhang et al., 2018). The Ecorrof Q235B carbon steel at normal pressure was more positive than the Ecorrat 4 MPa in initial period, that is after 1-d immersion. The jcorrof Q235B carbon steel decreased with the increasing of immersion time, and it decreased from 3.03×10-5A/cm2to 2.79×10-6A/cm2, indicating that the corrosion degree of Q235B in later experiment period decreased compared with initial period at normal pressure (Abd El-Raouf et al., 2018). Although the Ecorrof Q235B carbon steel at 4 MPa was fairly stable within test period, the jcorrof Q235B carbon steel decreased compared with initial period. The values of jcorrdecreased from 3.85×10-5A/cm2to 7.67×10-6A/cm2, indicating that the corrosion degree of Q235B in later experiment period decreased as well compared with initial period at 4 MPa.
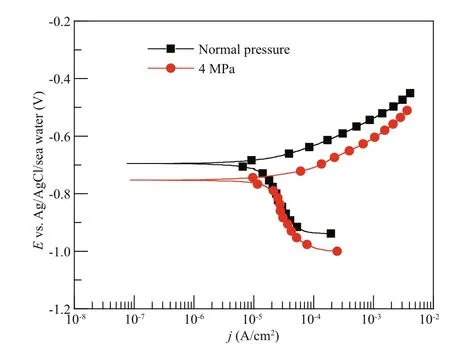
Fig.3 The potentiodynamic polarization curves of Q235B carbon steel at diff erent pressures after 1-d immersion
The potentiodynamic polarization curves of carbon steel at diff erent pressures after 1-d immersion are shown in Fig.3. The anodic polarization curves indicated active corrosion characteristic at normal pressure and 4 MPa. The cathodic polarization curves indicated oxygen reduction at normal pressure and 4 MPa. The corrosion potential of Q235B carbon steel decreased and the corrosion current density of Q235B carbon steel increased at 4 MPa compared with that at normal pressure. It is well known that the reduction of corrosion potential was caused by two aspects: the acceleration of anodic process and/or the inhibition of cathodic process. The pressure increased the concentration of dissolved oxygen while the corrosion products fi lm prevented the diff usion of dissolved oxygen to metal surface. It could be noticed the cathodic process was nearly unchanged at diff erent pressures as shown in Fig.3, which indicates that the cathodic oxygen reduction rate did not change with the increase of pressure. Consequently, the acceleration of anodic process might be proper explanation of the decreasing of corrosion potential (Yang et al., 2010).
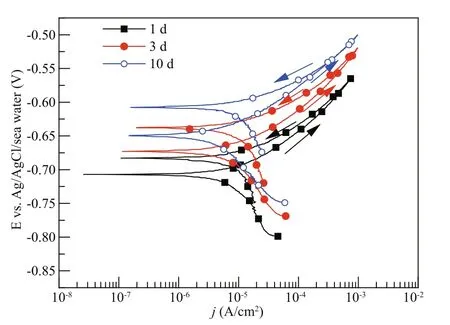
Fig.4 The cyclic potentiodynamic polarization curves of Q235B carbon steel at normal pressure
In addition, after more than 1-d immersion in the sea water, the slopes of cathodic polarization curves were somewhat diff erent from that after 1 d. This is because the formed scale of corrosion products hindered the oxygen transport through the scale to the metal surface.
3.3 Cyclic polarization measurements
Figure 4 presents the cyclic potentiodynamic polarization curves of Q235B carbon steel at normal pressure after 1-d, 3-d, and 10-d immersion, respectively. The potential was scanned positively from 100 mV below the OCP to a pre-defi ned potential and then scanned back with the anodic current density limitation of 1 mA/cm2. The anodic potentiodynamic curves at positive and negative scanning direction are compared. It is obvious that the reverse anodic curve has the lower value of current density, indicating that no pitting expected on the Q235B carbon steel surface after 1-d, 3-d, and 10-d immersion, respectively (Zaid et al., 2008).
Although no pitting is expected on the Q235B carbon steel in marine environment through the evaluation of cyclic polarization curves, the pitting can still be observed in practice. For passivation systems having a region where the current density is constant and low, the pitting potential is defi ned as the lowest potential when pits are shown. On the polarization curve, the pitting potential is at the point when the current exhibits a sharp increase. This textbook explanation of pitting corrosion can be found anywhere. In practice, non-passivation system such as carbon steel in seawater, the appearance of pitting corrosion is also common (Jeff rey and Melchers, 2007, 2009). The reason for the occurrence of pitting corrosion in non-passivation system is that, although the overall corrosion rate is high, there are points where the localized material is even more active, leaving pits being visualized. Empirically, the pitting corrosion potential ( Eb100) of carbon steel was defi ned as the potential where the current density reached 100 μA/cm2(Shi et al., 2016). Table 3 shows the measured Ecorrand Eb100of Q235B carbon steel at normal pressure. The pitting corrosion potentials shifted to the higher values with the increasing of immersion time at normal pressure. Moreover, the diff erence between the Eb100and Ecorrincreased from 64 mV after 1-d immersion to 74 mV after 10-d immersion, showing enhanced pitting corrosion resistance in seawater in the later immersion period (Yang et al., 2017).
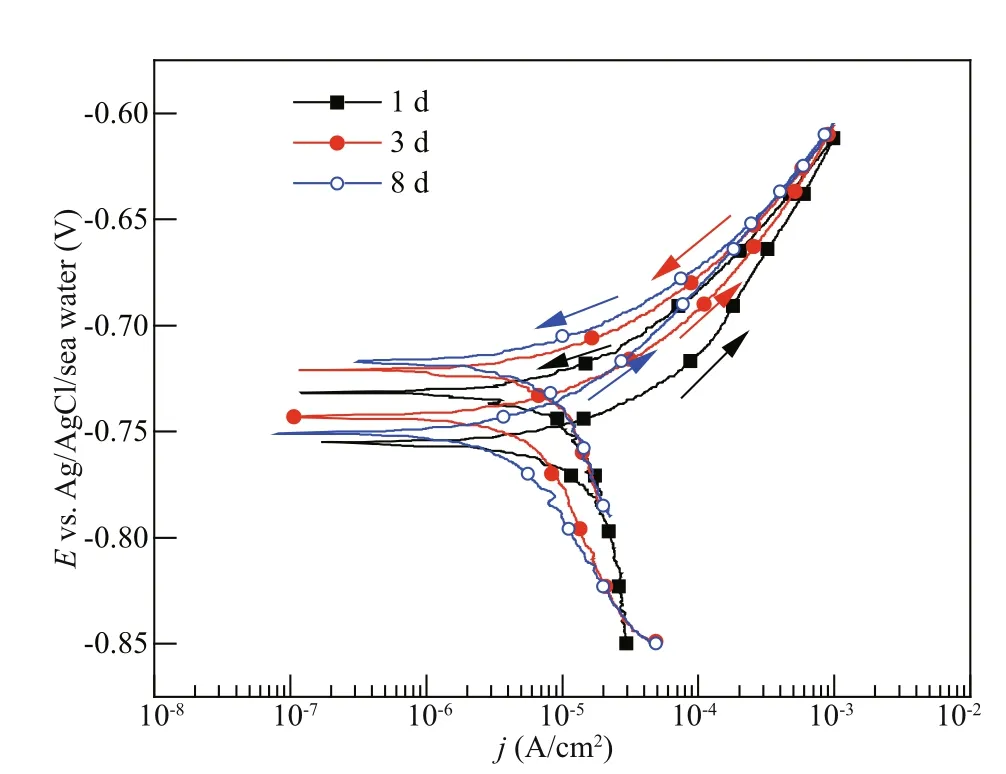
Fig.5 The cyclic potentiodynamic polarization curves of Q235B carbon steel at 4 MPa
Figure 5 presents the cyclic potentiodynamic polarization curves of Q235B carbon steel at 4 MPa after 1-d, 3-d, and 8-d immersion, respectively. The anodic potentiodynamic curves at positive and negative scanning direction are compared. It is obvious that the reverse anodic curve has the lower value of current density, indicating that no pitting expected on the Q235B carbon steel surface after 1-d, 3-d, and 8-d immersion, respectively. Table 4 shows the measured Ecorrand Eb100of Q235B carbon steel at 4 MPa. Similarly, the pitting corrosion potentials shift to the higher values with the increasing of immersion time at 4 MPa. The diff erence between Eb100and Ecorrincreased from 40 mV after 1-d immersion to 68 mV after 8-d immersion, showing increased pitting corrosion resistance in seawater in the later immersion period.
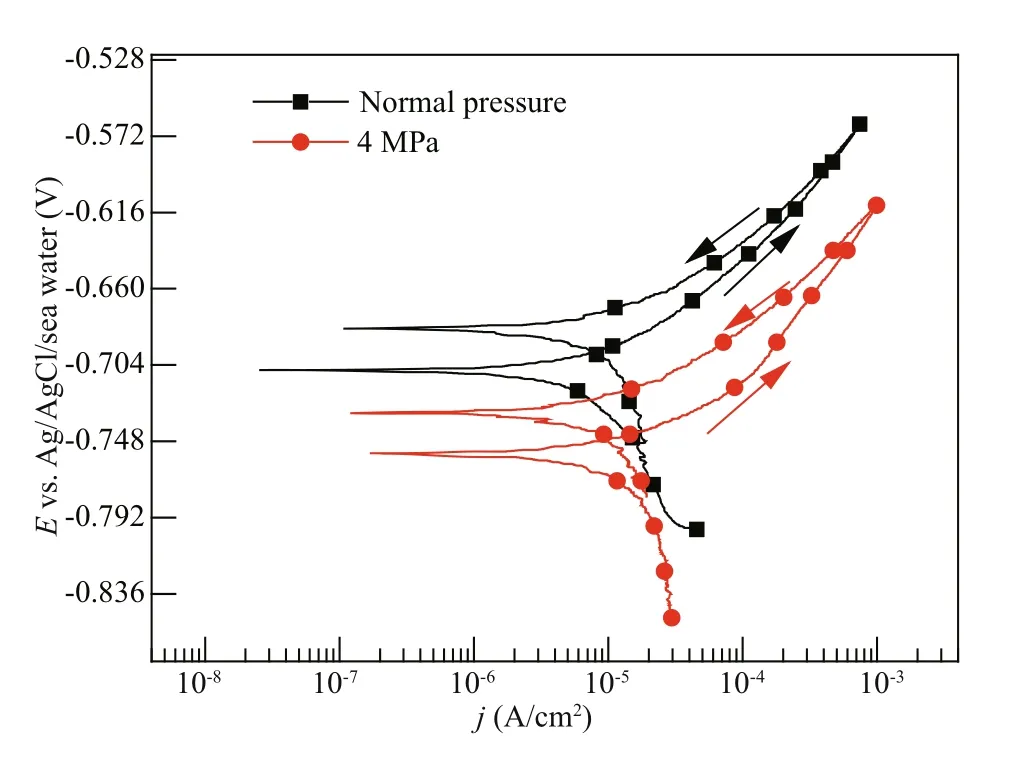
Fig.6 The cyclic potentiodynamic polarization curves of Q235B carbon steel at diff erent pressures after 1-d immersion
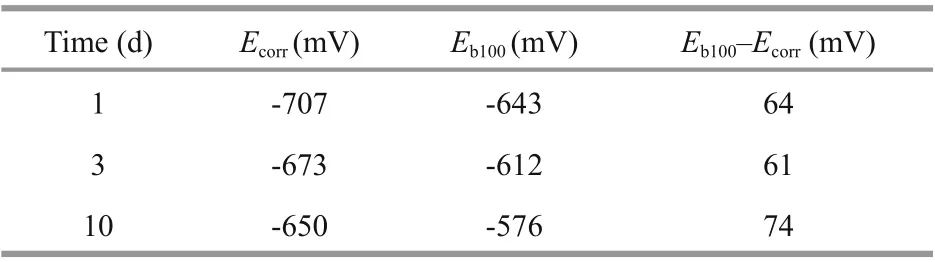
Table 3 Parameters obtained from the cyclic potentiodynamic polarization curves of Q235B carbon steel at normal pressure
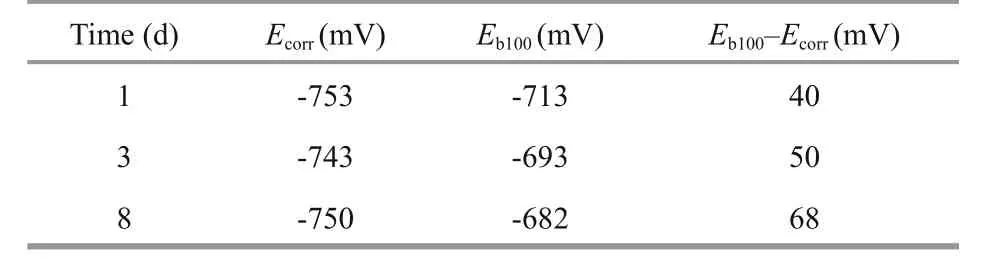
Table 4 Parameters obtained from the cyclic potentiodynamic polarization curves of Q235B carbon steel at 4 MPa
Figure 6 presents the cyclic potentiodynamic polarization curves of Q235B carbon steel at diff erent pressures after 1-d immersion. As can be seen in Tables 3 & 4, the Eb100as well as the diff erence between Eb100and Ecorrdecreased as the pressure increased, revealing that the carbon steel at higher pressure has the higher tendency of pitting corrosion in the initial immersion period.
3.4 Corroded surface observation by SEM
To verify the validity of using practical pitting potential Eb100in evaluating the pitting behavior, the potentiostatic polarization experiments at the fi xed potentials that below and above the practical pitting potential Eb100at diff erent pressures after 1-d immersion were also performed. Figure 7 shows the SEM photographs of Q235B after potentiostatic polarization at diff erent applied potentials for 1 h at normal pressure after 1-d immersion with corrosion products being removed. Clearly, the general corrosion was characterized for Q235B steel at the potential of -700 mV that is below the Eb100. The corrosion pits were detected at the anodic potential of -400 mV that is above the Eb100. Similar phenomena were observed at 4 MPa but larger pits were visualized as shown in Fig.8b.

Fig.7 SEM photographs of Q235B after potentiostatic polarization at diff erent applied potentials for 1 h at normal pressure after 1-d immersion
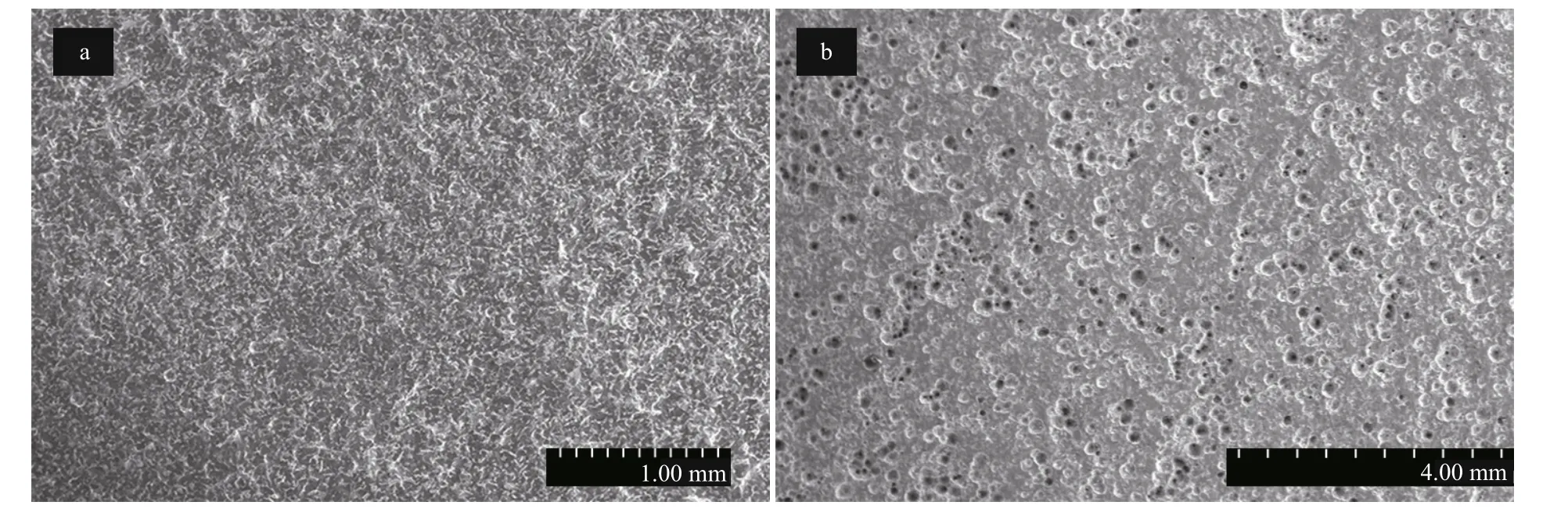
Fig.8 SEM photographs of Q235B after potentiostatic polarization at diff erent applied potentials for 1 h at 4 MPa after 1-d immersion
4 DISCUSSION
4.1 Eff ect of pressure on the corrosion resistance of Q235B carbon steel
Figure 9 shows the variations of Ecorrand jcorras a function of immersion time at diff erent pressures. Figure 9a shows that the values of Ecorrat 4 MPa are apparently lower than that at normal pressure within the immersion period, which implies that the electrochemical reaction are promoted by working pressure under simulated SPSS operational conditions. Figure 9b shows the values of jcorrwith immersion time at 4 MPa and normal pressure. The value of jcorris higher at 4 MPa than that at normal pressure after the initial 1 d. This indicates that the pressure accelerated the corrosion of carbon steel during the initial immersion period.
The values of jcorrdecrease with the immersion time. However, the value of jcorris not always greater at 4 MPa than normal pressure at the later immersion stage. It depends on the properties of the corrosion rust layer (Xu et al., 2015). During the initial immersion stage, the corrosion products formed rapidly on the carbon steel surface and much part of the corrosion products formed was the stable rust layer (Tamura, 2008; Ding et al., 2017). The stable rust layer reduced the contact between carbon steel surface and seawater, especially the dissolved oxygen and chloride ion. The stable rust layer prevented the electrochemical progress, resulting in the decrease of
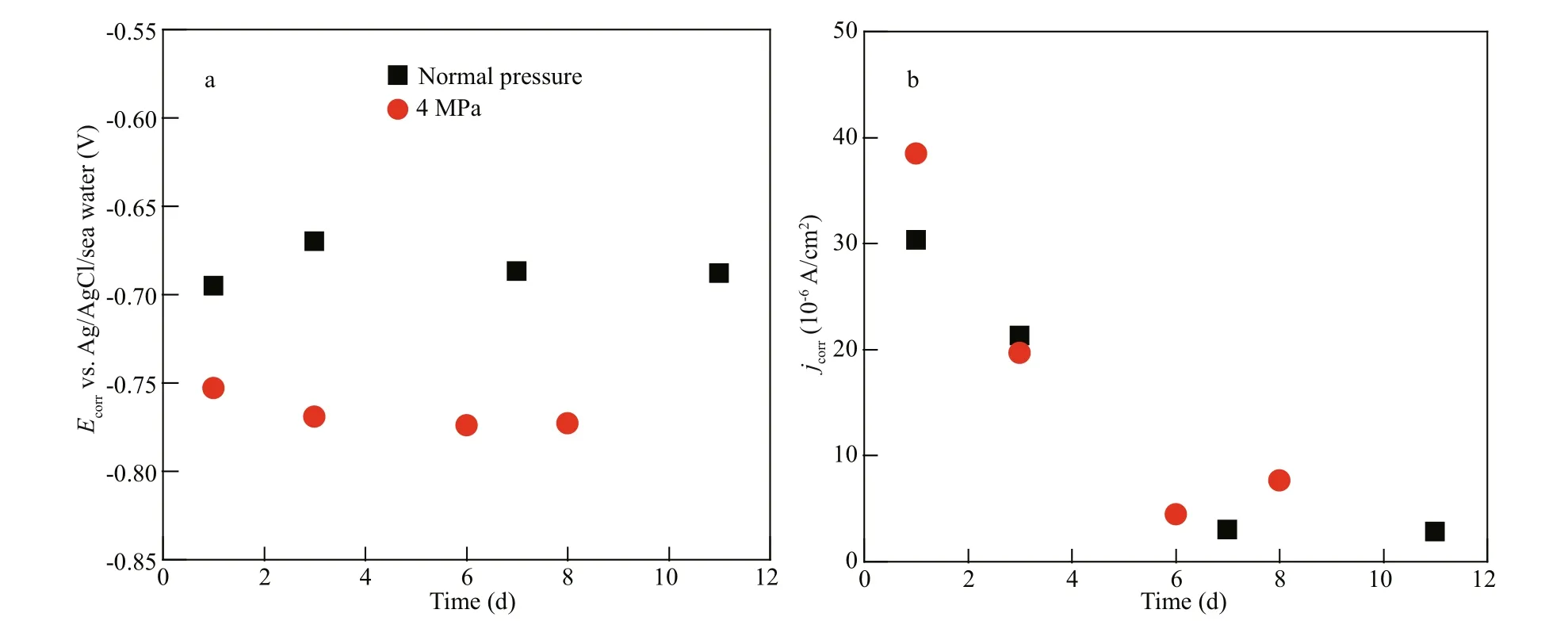
Fig.9 Electrochemical parameters obtained from the potentiodynamic polarization curves of Q235B carbon steel at diff erent pressure
jcorrvalues with the immersion time. The stable rust layer formed during the initial immersion stage is of the inner structure of rust layer. However, the outer rust layer is the loose and porous structure because of the chloride ion (Marcus et al., 2008). The outer rust layer is unstable and easy to fall off in the seawater in the later immersion period (Kamimura et al., 2006), making the obtained jcorrvalues somewhat scattering.
4.2 Eff ect of pressure on the pitting corrosion behavior of Q235B carbon steel
The diff erence between the Eb100and Ecorr( Eb100- Ecorr) can account for the tendency of pitting corrosion initiation (Dastgerdi et al., 2019). The higher the Eb100- Ecorr, the lower the tendency of pitting corrosion initiation. The values of Eb100- Ecorrwere calculated and listed in Tables 3 & 4. The variation of the Eb100- Ecorrvalues at diff erent immersion time and diff erent pressures are reported in Fig.10. Figure 10 shows that the Eb100- Ecorrdecreases by increasing pressure from normal pressure to 4 MPa. The tendency of pitting corrosion initiation increases at higher pressure, which implies that the electrochemical reactions of Q235B carbon steel are promoted by pressure.
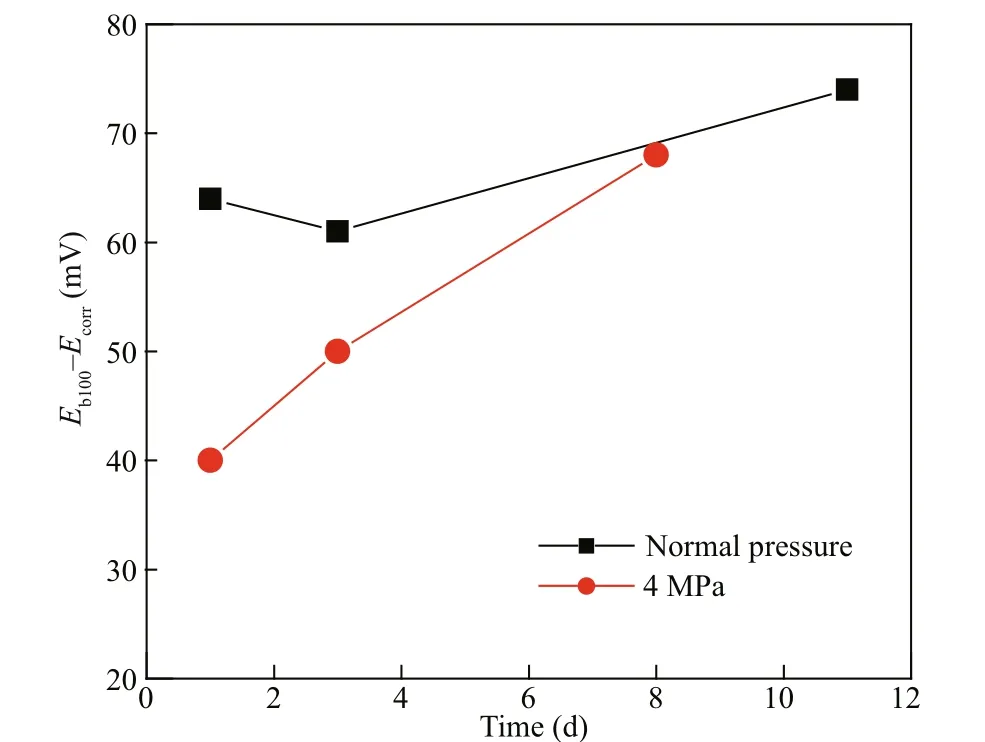
Fig.10 The variation of E b100- E corr the diff erence values at diff erent immersion time and diff erent pressures
The value of Eb100- Ecorrincreased at later immersion period compared with initial immersion period, which was a similar phenomenon at normal pressure and 4 MPa. This can be explained by the protective functions of stable rust layer enhancement with immersion time. Q235B carbon steel reveals mainly general corrosion in seawater. The protective rust layer consists of corrosion products formed on the steel surface. The main components of the rust layer of carbon steel in marine environment are Fe2O3, Fe3O4, and FeOOH. At the initial stage of corrosion, Fe(OH)2forms on the surface and is oxidized to magnetite/transition metal ferrites. And the outer Fe(OH)2is oxidized to Fe(OH)3rust by dissolved oxygen. Then, the dehydration and crystallization of Fe(OH)3rust proceed with further aging and transforms to α-FeOOH solid rust fi lm. With the progress of corrosion, the corrosion rate decreases due to the increase in the thickness of the α-FeOOH solid rust layer. The formation rate of new Fe(OH)3rust slows down while the transformation of Fe(OH)3rust into α-FeOOH rust continues. Finally, the α-FeOOH solid rust covers the whole surface, completing the formation of the protective rust fi lm (Monnier et al., 2011). The stable protective rust layer prevents the carbon steel from further corrosion in seawater by blocking the contact of carbon steel surface and marine environment (Chen and Zhang, 2019). The protective rust layer enhances with immersion time and improves the localized corrosion resistance within experiment period (Rocca et al., 2019), showing an increased Eb100- Ecorrwith immersion time. Chen et al. (2020) studied the passive fi lm formed on the reinforcement surface in alkali activated fl y ash (AAFA). It was found that the passive fi lm possessed bilayer structure with FeOOH-rich outer layer and FeO-rich inner layer in simulated AAFA pore solution. A passive fi lm can also be formed on the surface of steel in chloride solution containing nitrite (Li et al., 2019). With the addition of sodium nitrite, the main components of the passive fi lm were FeOOH, FeO, and Fe3O4. In the outer layer, FeOOH was the main component, whereas FeO and Fe3O4were found in relatively lower proportions. The inner layer was relatively dense because of FeO and Fe3O4. The components of rust layer formed in marine environment and the passive fi lm components of carbon steel are identical. However, the protection eff ect of the rust layer formed in marine environment is comparatively weak, since the protective ability is hindered by the presence of large concentration of chloride in the environment, which results in a fairly higher corrosion rate. The higher corrosion rate causes disappearance of typical passive region on the polarization curve, leaving active anodic dissolution region joins the transpassive region. The practical pitting potential is in fact the potential where active anodic dissolution region joins the transpassive region. When the potential is higher than the practical pitting potential, that is the potential falls in the transpassive region, the pits develop. This explains why the pitting corrosion can still occur in systems exhibiting no typical polarization curves with clear passive region.
As can be observed in Fig.10, that the slope of the Eb100- Ecorrcurve at normal pressure is lower than that at 4 MPa. This agrees with the results declaring that the electrochemical reactions of Q235B carbon steel are promoted by pressure. Actually, the pressure may promote both the electrochemical process of general corrosion and pitting corrosion in the initial immersion period. Pressure also accelerates the formation of rust layer on the steel surface (Bouchar et al., 2017).
5 CONCLUSION
In this paper, the corrosion behavior of Q235B carbon steel in simulated seawater pumped storage station under operational conditions have been investigated by electrochemical measurements. The following conclusions can be reached from the present investigation.
Working pressure aff ects the corrosion resistance of Q235B carbon steel during the whole immersion period. At the initial immersion period, the pressure promotes the electrochemical reaction of corrosion process, resulting the corrosion rate increase. The stable rust layer formed in later immersion period at diff erent pressure decreased the corrosion rate of carbon steel.
Working pressure aff ects the pitting corrosion behavior of Q235B carbon steel during the whole immersion period with the tendency of pitting corrosion being higher at 4 MPa in the static seawater. The diff erence between Eb100and Ecorrincreases with immersion time because of rust layer accumulation both at normal pressure and 4 MPa.
6 DATA AVAILABILITY STATEMENT
The datasets during and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Abd El-Raouf M, Khamis E A, Abou Kana M T H, Negm N A. 2018. Electrochemical and quantum chemical evaluation of new bis(coumarins) derivatives as corrosion inhibitors for carbon steel corrosion in 0.5 M H2SO4. Journal of Molecular Liquids, 255: 341-353, https://doi.org/10.1016/ j.molliq.2018.01.148.
Amann T, Waidele M, Kailer A. 2018. Analysis of mechanical and chemical mechanisms on cavitation erosion-corrosion of steels in salt water using electrochemical methods. Tribology International, 124: 238-246, http://doi.org/10. 1016/j.triboint.2018.04.012.
Bouchar M, Dillmann P, Neff D. 2017. New insights in the long-term atmospheric corrosion mechanisms of low alloy steel reinforcements of cultural heritage buildings. Materials ( Basel), 10(6): 670, https://doi.org/10.3390/ma10060670.
Caines S, Khan F, Shirokoff J, Qiu W. 2015. Experimental design to study corrosion under insulation in harsh marine environments. Journal of Loss Prevention in the Process Industries, 33: 39-51, https://doi.org/10.1016/j.jlp.2014.10.014.
Chen R, Hu J, Ma Y W, Guo W H, Huang H L, Wei J X, Yin S H, Yu Q J. 2020. Characterization of the passive fi lm formed on the reinforcement surface in alkali activated fl y ash: Surface analysis and electrochemical evaluation. Corrosion Science, 165: 108393, https://doi.org/10.1016/j.corsci.2019.108393.
Chen S Q, Zhang D. 2019. Corrosion behavior of Q235 carbon steel in air-saturated seawater containing Thalassospira sp. Corrosion Science, 148: 71-82, https://doi.org/10.1016/ j.corsci.2018.11.031.
Dastgerdi A A, Brenna A, Ormellese M, Pedeferri M, Bolzoni F. 2019. Experimental design to study the infl uence of temperature, pH, and chloride concentration on the pitting and crevice corrosion of UNS S30403 stainless steel. Corrosion Science, 159: 108160, https://doi.org/10.1016/j.corsci.2019.108160.
Ding J W, Tang B, Li M Y, Feng X F, Fu F L, Bin L Y, Huang S S, Su W, Li D N, Zheng L C. 2017. Diff erence in the characteristics of the rust layers on carbon steel and their corrosion behavior in an acidic medium: Limiting factors for cleaner pickling. Journal of Cleaner Production, 142: 2 166-2 176, https://doi.org/10.1016/j.jclepro.2016.11.066.
Islam M A, Farhat Z. 2017. Erosion-corrosion mechanism and comparison of erosion-corrosion performance of API steels. Wear, 376-377: 533-541, https://doi.org/10.1016/j.wear.2016.12.058.
Jeff rey R, Melchers R E. 2007. The changing topography of corroding mild steel surfaces in seawater. Corrosion Science, 49(5): 2 270-2 288, https://doi.org/10.1016/j.corsci.2006.11.003.
Jeff rey R, Melchers R E. 2009. Corrosion of vertical mild steel strips in seawater. Corrosion Science, 51(10): 2 291-2 297, https://doi.org/10.1016/j.corsci.2009.06.020.
Kamimura T, Hara S, Miyuki H, Yamashita M, Uchida H. 2006. Composition and protective ability of rust layer formed on weathering steel exposed to various environments. Corrosion Science, 48(9): 2 799-2 812, https://doi.org/10.1016/j.corsci.2005.10.004.
Katsaprakakis D A, Christakis D G, Stefanakis I, Spanos P, Stefanakis N. 2013. Technical details regarding the design, the construction and the operation of seawater pumped storage systems. Energy, 55: 619-630, https://doi.org/10.1016/j.energy.2013.01.031.
Katsaprakakis D A, Christakis D G. 2014. Seawater pumped storage systems and off shore wind parks in islands with low onshore wind potential. A fundamental case study. Energy, 66: 470-486, https://doi.org/10.1016/j.energy. 2014.01.021.
Kim E J, Jeong Y H, Choe H C, Brantley W A. 2014. Electrochemical behavior of hydroxyapatite/TiN multilayer coatings on Ti alloys. Thin Solid Films, 572: 113-118, https://doi.org/10.1016/j.tsf.2014.08.035.
Li X Z, Liu J Z, Wang J M, Geng J D. 2019. Microstructure of passive fi lm on steel in synthetic concrete pore solution in presence chloride and nitrite. International Journal of Electrochemical Science, 14(9): 8 624-8 638, https://doi.org/10.20964/2019.09.43.
Liu F L, Zhang J, Sun C X, Yu Z H, Hou B R. 2014. The corrosion of two aluminium sacrifi cial anode alloys in SRB-containing sea mud. Corrosion Science, 83: 375-381, https://doi.org/10.1016/j.corsci.2014.03.003.
López-Ortega A, Bayón R, Arana J L, Arredondo A, Igartua A. 2018. Infl uence of temperature on the corrosion and tribocorrosion behaviour of High-Strength Low-Alloy steels used in off shore applications. Tribology International, 121: 341-352, https://doi.org/10.1016/j.triboint.2018.01.049.
Ma H C, Liu Z Y, Du C W, Wang H R, Li X G, Zhang D W, Cui Z Y. 2015. Stress corrosion cracking of E690 steel as a welded joint in a simulated marine atmosphere containing sulphur dioxide. Corrosion Science, 100: 627-641, https://doi.org/10.1016/j.corsci.2015.08.039.
Ma Y T, Li Y, Wang F H. 2009. Corrosion of low carbon steel in atmospheric environments of diff erent chloride content. Corrosion Science, 51(5): 997-1 006, https://doi.org/10. 1016/j.corsci.2009.02.009.
Manfrida G, Secchi R. 2014. Seawater pumping as an electricity storage solution for photovoltaic energy systems. Energy, 69: 470-484, https://doi.org/10.1016/j.energy.2014.03.040.
Marcus P, Maurice V, Strehblow H H. 2008. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corrosion Science, 50(9): 2 698-2 704, https://doi.org/10. 1016/j.corsci.2008.06.047.
McLean E, Kearney D. 2014. An evaluation of seawater pumped hydro storage for regulating the export of renewable energy to the national grid. Energy Procedia, 46: 152-160, https://doi.org/10.1016/j.egypro.2014.01.168.
Monnier J, Burger E, Berger P, Neff D, Guillot I, Dillmann P. 2011. Localisation of oxygen reduction sites in the case of iron long term atmospheric corrosion. Corrosion Science, 53(8): 2 468-2 473, https://doi.org/10.1016/j.corsci.2011. 04.002.
Ozkan C, Mayo T. 2019. The renewable wave energy resource in coastal regions of the Florida peninsula. Renewable Energy, 139: 530-537, https://doi.org/10.1016/j.renene. 2019.02.090.
Pardo A, Otero E, Merino M C, López M D, Utrilla M V, Moreno F. 2000. Infl uence of pH and chloride concentration on the pitting and crevice corrosion behavior of high-alloy stainless steels. Corrosion, 56(4): 411-418, https://doi.org/10.5006/1.3280545.
Park B, Hur J. 2018. Spatial prediction of renewable energy resources for reinforcing and expanding power grids. Energy, 164: 757-772, https://doi.org/10.1016/j.energy. 2018.09.032.
Rocca E, Faiz H, Dillmann P, Neff D, Mirambet F. 2019. Electrochemical behavior of thick rust layers on steel artefact: Mechanism of corrosion inhibition. Electrochimica Acta, 316: 219-227, https://doi.org/10. 1016/j.electacta.2019.05.107.
Schaber C, Mazza P, Hammerschlag R. 2004. Utility-scale storage of renewable energy. The Electricity Journal, 17(6): 21-29, https://doi.org/10.1016/j.tej.2004.05.005.
Shi J J, Sun W, Jiang J Y, Zhang Y M. 2016. Infl uence of chloride concentration and pre-passivation on the pitting corrosion resistance of low-alloy reinforcing steel in simulated concrete pore solution. Construction and Building Materials, 111: 805-813, https://doi.org/10. 1016/j.conbuildmat.2016.02.107.
Tamura H. 2008. The role of rusts in corrosion and corrosion protection of iron and steel. Corrosion Science, 50(7): 1 872-1 883, https://doi.org/10.1016/j.corsci.2008.03.008.
Tian W L, Liu L, Meng F D, Liu Y, Li Y, Wang F H. 2014. The failure behaviour of an epoxy glass fl ake coating/steel system under marine alternating hydrostatic pressure. Corrosion Science, 86: 81-92, https://doi.org/10.1016/j.corsci.2014.04.038.
Wang K X, Chen S, Liu L C, Zhu T, Gan Z X. 2018. Enhancement of renewable energy penetration through energy storage technologies in a CHP-based energy system for Chongming, China. Energy, 162: 988-1 002, https://doi.org/10.1016/j.energy.2018.08.037.
Wu Y N, Zhang T, Xu C B, Zhang X Y, Ke Y M, Chu H, Xu R H. 2019. Location selection of seawater pumped hydro storage station in China based on multi-attribute decision making. Renewable Energy, 139: 410-425, https://doi.org/10.1016/j.renene.2019.02.091.
Xu Q F, Gao K W, Wang Y B, Pang X L. 2015. Characterization of corrosion products formed on diff erent surfaces of steel exposed to simulated groundwater solution. Applied Surface Science, 345: 10-17, https://doi.org/10.1016/j.apsusc.2015.03.143.
Xue F, Wei X, Dong J H, Wang C G, Ke W. 2019. Eff ect of chloride ion on corrosion behavior of low carbon steel in 0.1 M NaHCO3solution with diff erent dissolved oxygen concentrations. Journal of Materials Science & Technology, 35(4): 596-603, https://doi.org/10.1016/j.jmst.2018.10.001.
Yang Y G, Zhang T, Shao Y W, Meng G Z, Wang F H. 2010. Eff ect of hydrostatic pressure on the corrosion behaviour of Ni-Cr-Mo-V high strength steel. Corrosion Science, 52(8): 2 697-2 706, https://doi.org/10.1016/j.corsci.2010.04.025.
Yang Z X, Kan B, Li J X, Su Y J, Qiao L J, Volinsky A A. 2017. Pitting initiation and propagation of X70 pipeline steel exposed to chloride-containing environments. Materials ( Basel), 10(9): 1 076, https://doi.org/10.3390/ma10091076.
Yang Z X, Kan B, Li J X, Su Y J, Qiao L J. 2018. Hydrostatic pressure eff ects on corrosion behavior of X70 pipeline steel in a simulated deep-sea environment. Journal of Electroanalytical Chemistry, 822: 123-133, https://doi.org/10.1016/j.jelechem.2018.05.010.
Yu X M, Huang Y L, Yadav A P, Qu W J, De Marco R. 2015. Study on the temperature dependence of pitting behaviour of AISI 4135 steel in marine splash zone. Electrochemistry, 83(7): 541-548, https://doi.org/10. 5796/electrochemistry.83.541.
Zaid B, Saidi D, Benzaid A, Hadji S. 2008. Eff ects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corrosion Science, 50(7): 1 841-1 847, https://doi.org/10.1016/j.corsci.2008.03.006.
Zhang T, Yang Y G, Shao Y W, Meng G Z, Wang F H. 2009. A stochastic analysis of the eff ect of hydrostatic pressure on the pit corrosion of Fe-20Cr alloy. Electrochimica Acta, 54(15): 3 915-3 922, https://doi.org/10.1016/j.electacta.2009.02.010.
Zhang Z, Li W W, Zhang W P, Huang X D, Ruan L, Wu L. 2018. Experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies of methionine and valine as corrosion inhibitors on carbon steel in phase change materials (PCMs) solution. Journal of Molecular Liquids, 272: 528-538, https://doi.org/10. 1016/j.molliq.2018.09.081.
 Journal of Oceanology and Limnology2020年5期
Journal of Oceanology and Limnology2020年5期
- Journal of Oceanology and Limnology的其它文章
- Distribution, sources and burial fl ux of sedimentary organic matter in the East China Sea*
- Photoelectrochemical cathodic protection of Cu 2 O/TiO 2 p-n heterojunction under visible light*
- Antioxidant bisabolane-type sesquiterpenoids from algalderived fungus Aspergillus sydowii EN-434*
- Calcium isotopic signatures of depleted mid-ocean ridge basalts from the northeastern Pacifi c*
- Application of confocal laser Raman spectroscopy on marine sediment microplastics*
- Inductive eff ect of bioactive substances on strobilation of jellyfi sh Aurelia coerulea*
