Inductive eff ect of bioactive substances on strobilation of jellyfi sh Aurelia coerulea*
WANG Nan , WANG Minxiao , , WANG Yantao , LI Chaolun ,
1 Key Laboratory of Marine Ecology and Environmental Sciences, Center of Deep-sea Research, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
2 Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China
3 University of Chinese Academy of Sciences, Beijing 100049, China
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Abstract The moon jellyfi sh Aurelia spp. is a worldwide distributed scyphozoan species that seasonally blooms in coastal waters. Although the strobilation is directly responsible for the scale of jellyfi sh bloom, the underlying mechanisms remain largely unknown. We exposed Aurelia coerulea polyps to 18 bioactive substances to test their ability to induce strobilation at the natural typically non-strobilation temperature of 21°C. Results revealed that A. coerulea polyps responded in four types. Type I—no change for estradiol, folic acid, iodine, retinoic acid, serotonin hydrochloride, and vitamin A. We suggested that 5-aza-2-deoxycytidine and N-acetyl-L-glutamic acid could not induce strobilation, since the percent of strobilated polyps in these two substances treatments was 3.3% and 1%, respectively. Type II—polyp body elongation for 3,5-diiodo-Ltyrosine, indole-3-acetic acid, L-dopamine, and noradrenaline treatments. Type III—transverse constrictions for L-thyroxine, progesterone, and melatonin treatments. Finally, Type IV—complete strobilation for 5-methoxy-2-methylindole, acetylcholine chloride, and indomethacin treatments, where the pre-strobilation periods were 2, 4, and 2 days, and the mean numbers of ephyrae released per strobila were 4.7, 5.7, and 5.7, respectively. The results reveal that indole derivatives, which contained methoxy or methyl pharmacophore, were the common strobilation inducer in the genus Aurelia. Iodinated organic compounds, catecholamine, acetylcholine chloride, and retinoic acid are species-specifi c strobilation inducer. Therefore, A. coerulea strobilation is regulated by neuronal and endocrine processes. Our fi ndings provide clues in understanding the mechanism of strobilation and contribute to developing specifi c strobilation antagonists in controlling moon jellyfi sh blooms.
Keyword: Aurelia coerulea; jellyfi sh; strobilation; bioactive substances; metamorphosis
1 INTRODUCTION
The scyphozoan jellyfi sh genus Aurelia is cosmopolitan species in neritic waters between 70°N and 40°S (Lucas, 2001). Under the pressure of global changes and human activities, mass occurrences of Aurelia spp. are frequently reported in the coastal waters in recent decades, causing economic losses in many marine realms. In China coastal waters, massive blooms of Aurelia coerulea medusae have occurred in harbors and inshore areas of the Bohai Sea and Yellow seas, such as in Dalian of Liaoning Province, Qinhuangdao of Hebei Province, and Weihai, Yantai, and Qingdao of Shandong Province (Liu et al., 2008a; Lu, 2009; Dong et al., 2010).
Aurelia spp. have complex life cycles, including asexual benthic polyp and sexual pelagic medusa stages (Arai, 1997). Mature medusae produce planulae, which settle on hard substrate and metamorphose into polyps. These perennial polyps can bud new polyps. In an adverse environment, polyps produce podocysts—the excystment, which represents another means of recruitment into the polyp population. Under certain environmental conditions, polyps can perform strobilation, during which a great number of ephyrae may arise from one strobila (Holst and Jarms, 2007), making strobilation the key process aff ecting the abundance of medusa population.
Strobilation is aff ected by temperature (Pascual et al., 2015; Sokołowski et al., 2016), salinity (Purcell et al., 2009), pH (Winans and Purcell, 2010), light (Liu et al., 2008b), dissolved oxygen (DO) (Ishii et al., 2008), and food availability (Wang et al., 2015). Of these, temperature is believed to be the primary factor to trigger the strobilation (Prieto et al., 2010). Strobilation in Aurelia polyps could be induced by manipulating water temperature to strobilationoccurred ranges, and in culture, by maintaining temperature within specifi c ranges (Kroiher et al., 2000; Wang et al., 2018). Extrinsic environmental changes must serve as cues perceived by polyps, leading to intrinsic biochemical or molecular regulation, but how environmental cues are biochemically transduced to create physiological responses during strobilation is poorly understood.
Hormones may serve as an important messenger regulating the development, especially strobilation. Several conservative hormones (e.g., steroids, iodinated organic compounds, neuropeptides, and indoleamines) have been identifi ed in cnidarian tissues (Tarrant, 2005). Thyroxine concentration has increased sharply following changes in water temperature (Gorbman, 1974). Iodinated compounds, Indomethacin and retinoic acid have been reported to induce the strobilation of A. aurita. (Spangenberg, 1967, 1971; Silverstone et al., 1977; Kuniyoshi et al., 2012; Fuchs et al., 2014).
To better understand the basic mechanism of strobilation in A. coerulea, we exposed its polyps to 18 candidate bioactive substances (including steroids, iodinated organic compounds, indole derivatives, reported from cnidarians) to evaluate their induction performance on strobilation at the non-strobilation temperature. Some of the tested bioactive substances were reported been identifi ed in cnidarian, including the strobilation inducing substances (Spangenberg, 1967, 1971; Silverstone et al., 1977; Kuniyoshi et al., 2012; Fuchs et al., 2014). We also identifi ed the strobilation-specifi c genes and pathways by comparison of the A. coerulea strobilation transcriptome of diff erent developmental stages (our unpublished data) and picked their related bioactive substances. Identifi cation of the bioactive substances capable of inducing strobilation may provide a better understanding of the strobilation mechanism and contribute to developing strobilation antagonists in controlling A. coerulea blooms.
2 MATERIAL AND METHOD
Sessile A. coerulea polyps were from the stock population reared at the Institute of Oceanology, Chinese Academy of Sciences. A. coerulea polyps were derived from planulae released by mature medusae from Jiaozhou Bay, Qingdao, in June 2014, and then cultured on corrugated plastic plates of 30 cm×50 cm placed in thermostatic aquaria of sandfi ltered seawater (under natural indoor light, salinity 30-31) maintained at 21°C, a temperature at which no strobilation naturally occurs (Feng et al., 2018), for about 6 months.
The corrugated plates with polyps were cut into pieces of about 3 cm×4 cm. Budded polyps, podocysts, and impurities on each piece were carefully removed with a dissecting needle, leaving 30 similarly sized healthy polyps. Each piece was placed in an individual glass beaker containing 200 mL of seawater that had been fi ltered through a 0.45-μm mixed cellulose ester membrane.
Eighteen bioactive substances reported to induce strobilation or related to this process were evaluated. Pre-experimentation was performed to determine appropriate culture water volumes and concentrations of bioactive substances to use in experiments.
In the preliminary experiment, the concentrations of the 18 bioactive substances were set to three gradients, i.e., 5, 10, and 100 μmol/L. The status of polyps was worst when they were treated in the highest concentration of most bioactive substances. Therefore, the concentration of most bioactive substances was chosen at 10 μmol/L. As the solubility of progesterone and estradiol in ethanol is low, to reduce the adverse reaction caused by ethanol solvent, the concentration of these two substances was set to 5 μmol/L. It was reported that when the concentration of iodine was 1:10-8, the polyp strobilation rate was 66%, and when the concentration was 1:10-7, the polyp strobilation rate rose to 100% (Spangenberg, 1967). To ensure the induction eff ect, 100 μmol/L was determined as the fi nal concentration of iodine in the experiment.
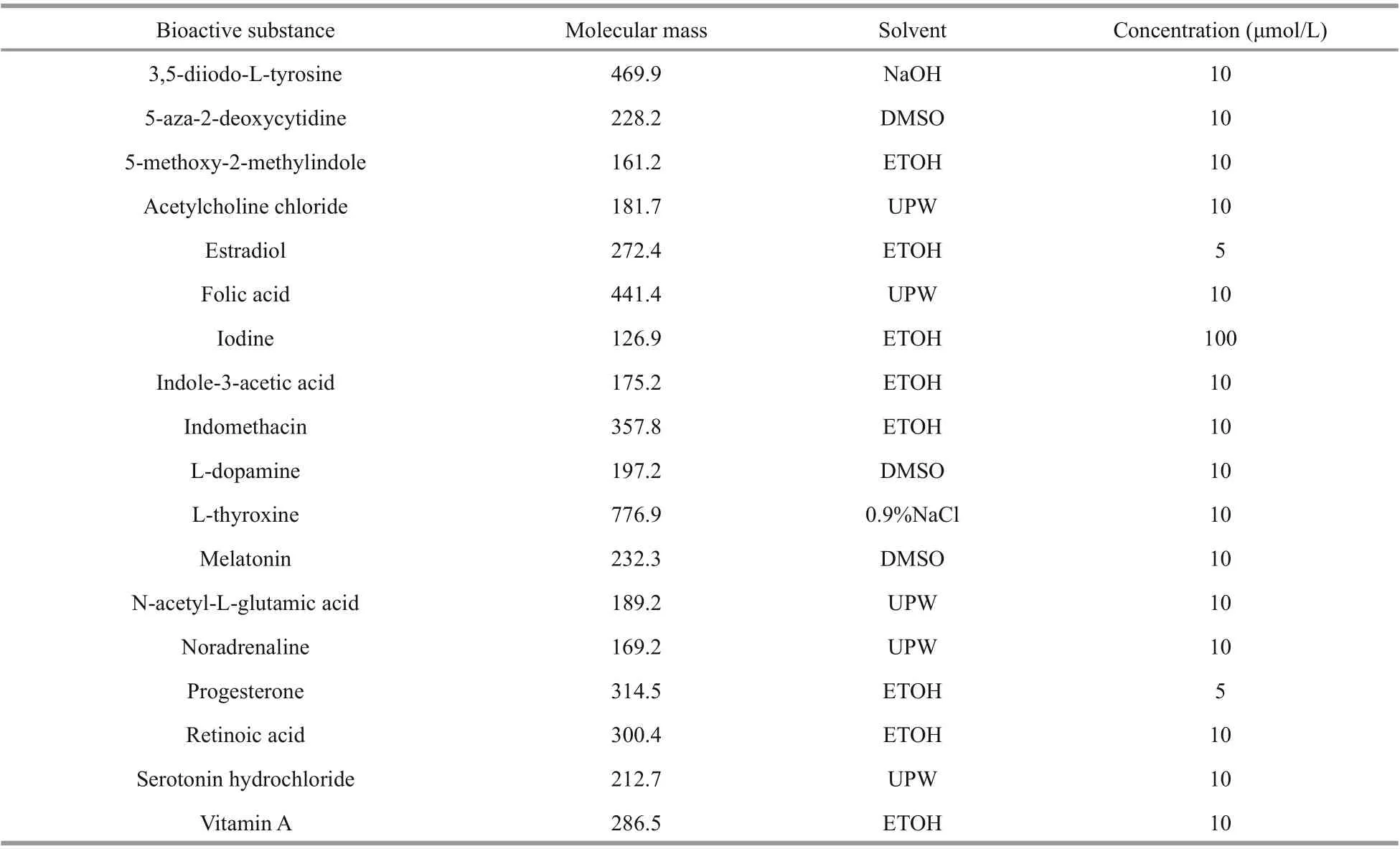
Table 1 Concentrations of 18 bioactive substances
Experimental concentrations were set to those at which bioactives were eff ective, and the amount of bioactive and solvent was non-toxic to polyps. Concentrations are detailed in Table 1. Three replicates were performed in each treatment and control. The controls contained an equal volume of solvent. Polyps in each beaker were fed suffi cient newly hatched Artemia nauplii for 1 hour every 3 days. Uneaten food together with water in the beakers was replaced with fresh, fi ltered seawater of the same temperature 1 hour later, after which bioactive substances were added. Polyps were observed daily under a dissecting microscope, morphological changes, the time when the fi rst transverse constriction formed, and ephyra production were recorded. Any induced strobilation was photographed. The experiment ran for 12 days.
3 RESULT
3.1 Reactions of A. coreulea polyps to bioactive substances
Polyps reacted diff erently (e.g., body elongation, transverse constrictions formation, developing to strobila) to the 18 bioactive substances. The reactions of A. coreulea polyps were classifi ed into four types: (1) no change, (2) body elongation, (3) transverse constriction, and (4) complete strobilation. Polyps in controls containing only solvents showed no morphological changes. (1) No change
Estradiol, folic acid, iodine, retinoic acid, serotonin hydrochloride, and vitamin A did not induce any change in morphology, and polyps remained the same as those in the controls. (2) Body elongation
Polyps in the 3,5-diiodo-L-tyrosine, indole-3-acetic acid, L-dopamine, and noradrenaline treatments were obviously elongated compared with those in control groups (Fig.1). In the L-dopamine treatment, polyps body slightly elongated. (3) Transverse constrictions
Polyps in L-thyroxine, melatonin, and progesterone treatments formed transverse constrictions, but retained the strobila form without releasing ephyra. In two of the three L-thyroxine treatments, polyps developed the transverse constrictions. In the melatonin treatments, polyp body elongated, and there was one strobila formed in each of two replicates. In the progesterone treatment, polyps formed transverse constrictions with body pigmented.

Fig.1 Morphology of normal Aurelia coerulea polyps in controls with ethanol (a) and with dimethyl sulfoxide (b), and elongated polyps in treatments with 3,5-diiodo-L-tyrosine (c) and L-dopamine (d)
(4) Complete strobilation
Strobilation was accomplished in the 5-aza-2-deoxycytidine, acetylcholine chloride, 5-methoxy-2-methylindole, N-acetyl-L-glutamic acid, and indomethacin treatments. 5-methoxy-2-methylindole and indomethacin induced all the 30 polyps releasing ephyra in each replicate, acetylcholine chloride induced more than half polyps releasing ephyra in each replicate. The strobilation rate (the percent of experiment polyps that released ephyra) in the 5-aza-2-deoxycytidine and N-acetyl-L-glutamic acid treatments was 3.3% and 1%, respectively. Since the other polyps did not elongate or form transverse constrictions in these two treatments, these two bioactive substances were not divided as inducing complete strobilation.
3.2 Bio-active substances induced strobilation
Morphological changes in polyps in the acetylcholine chloride, 5-methoxy-2-methylindole, and indomethacin treatments were photographed (Fig.2). Polyps induced by three bioactive substance underwent a series of morphological changes: body elongation, transverse constrictions, strobila pigmented, strobilation, and fi nally released ephyra.
We defi ned the period from the start of experiment to the fi rst formation of transverse constriction as the pre-strobilation period. The durations of this stage in 5-aza-2-deoxycytidine, acetylcholine chloride, 5-methoxy-2-methylindole, N-acetyl-L-glutamic acid, and indomethacin treatments were depicted in Fig.3. The length of the pre-strobilation period was shortest in the 5-methoxy-2-methylindole and indomethacin treatments (<2 days), followed by the N-acetyl-L-glutamic acid treatment (4 days), acetylcholine chloride (7 days) and 5-aza-2-deoxycytidine (8 days).

Fig.2 The morphology of the Aurelia coerulea polyps in 5-methoxy-2-methylindole (A1-A4), indomethacin (B1-B4), and acetylcholine chloride (C1-C4) treatments

Fig.3 Duration of pre-strobilation period in the 5-aza-2-deoxycytidine, acetylcholine chloride, 5-methoxy-2-methylindole, N-acetyl-L-glutamic acid, and indomethacin treatments
In 5-aza-2-deoxycytidine, 5-methoxy-2-methylindole, acetylcholine chloride, indomethacin, and N-acetyl-L-glutamic acid treatments in which strobilation was induced, the number of ephyra released per strobila was 5.0±2.6, 4.7±1.5, 5.7±1.2, 5.7±0.8, and 0.3±0.3, respectively (Fig.4). In the N-acetyl-L-glutamic acid and 5-aza-2-deoxycytidine treatments, ephyra was not released in all replicates. The minimum and maximum mean numbers of ephyra released per strobila were 0.3 and 5.7 in the N-acetyl-L-glutamic acid and indomethacin treatments, respectively. The number of ephyra released per strobila did not diff er signifi cantly among these fi ve treatments ( P=0.176>0.05).

Fig.4 The number of ephyra released per strobila in the 5-aza-2-deoxycytidine, 5-methoxy-2-methylindole, acetylcholine chloride, indomethacin, and N-acetyl-L-glutamic acid treatments
The duration of pre-strobilation period, strobilation rate, and number of ephyra released per strobila, indicate indomethacin and 5-methoxy-2-methylindole were the strongest strobilation inducer of the fi ve compounds. Polyps induced by indomethacin and 5-methoxy-2-methylindole synchronously moved into a pre-strobilation period in 2 days. The strobilation inducing capacity of acetylcholine chloride was medium. Neither N-acetyl-L-glutamic acid nor 5-aza-2-deoxycytidine had signifi cant ability to induce strobilation.
4 DISCUSSION
4.1 Strobilation induced by bioactive substances
In the strobilation, A. coerulea polyps underwent a series of metamorphose process, fi nally released ephyrae. During the metamorphosis process, polyp body elongated, and transverse segments constricted amorally. After the polyp body was fully segmented, tentacles gradually degenerated, and the body became pigmented. There are seven bioactive substances involved in specifi c metamorphosis period, and three involved in the complete strobilation process.
4.1.1 Morphological changes induced by bioactive substances
3,5-Diiodo-L-tyrosine, indole-3-acetic acid, L-dopamine and norepinephrine only induced the elongation of A. coerulea polyps at the nonstrobilation temperature of 21°C, while diiodotyrosine was reported to induce Aurelia strobilation at 20°C (Silverstone et al., 1977). Exposure to norepinephrine previously caused irregular, premature metamorphosis of hydrozoan planulae (Van Marle et al., 1983). In our experiment, the reported bioactives lose the effi cacy on strobilation might be due to the jellyfi sh strain and the diverse intrinsic mechanism of regulation.
L-thyroxine, melatonin and progesterone could induce polyps to develop transverse segments. In vertebrates, the function of thyroxine hormones aff ects numerous physiological processes including growth, development, reproduction and metabolism. Thyroxine was also reported to help form proteinaceous skeletons in gorgonian (Kingsley et al., 2001) and induce strobilation in Aurelia (Spangenberg, 1971, 1974). However, in this study, the entail strobilation was incomplete and ephyra was not released in the L-thyroxine treatment. The lightsensitive hormone melatonin induced transverse segments formation. Light is an important environmental signal for strobilation (Purcell, 2007). The A. coerulea polyps perceived light cycle changes, causing intrinsic coordination of the light-sensitive hormone melatonin and its precursor serotonin concentrations, regulating the transverse constriction formation in strobilation.
The above seven bioactive substances (3,5-diiodo-L-tyrosine, indole-3-acetic acid, L-dopamine, norepinephrine, L-thyroxine, melatonin, progesterone) are possibly related to specifi c metamorphosis stages in the strobilation process. They cannot induce complete strobilation independently from polyp to ephyra at non-strobilation temperatures. Therefore, polyps ceased metamorphosis at specifi c stages. Hence, we do not know whether they can accelerate or enhance the strobilation output during the natural strobilation processes.
4.1.2 Complete strobilation induced by bioactive substances
Indomethacin and 5-methoxy-2-methylindole are the common strobilation inducer of diff erent Aurelia species (Kuniyoshi et al., 2012; Fuchs et al., 2014). Other indole derivatives 5-methoxy-2-methylindole acetic acid, 5-methoxyindole-2-carboxylic acid, and 2-methylindole could induce A. aurita polyps’ strobilation as well, which indicated that the indole ring was the minimal pharmacophore in the strobilation hormone (Fuchs et al., 2014). However, indole-3-acetic acid did not induce complete strobilation in our experiment. We suggest that indole ring modifi ed with methoxy or methyl pharmacophore groups is the core element of the strobilation hormone. Kuniyoshi et al. (2012) reported that indomethacin initiated strobilation in a dose-dependent manner (the higher the concentration, the earlier strobilation started); once strobilation started, it autonomously proceeded to the end with or without indomethacin.
Acetylcholine is a neurotransmitter. The ability of acetylcholine chloride to induce strobilation has rarely been reported. We report this neurotransmitter as capable of inducing strobilation, suggesting that strobilation may be related to neuronal conduction. Acetylcholine chloride was reported to be an active inducer in veined rapa whelk Rapana venosa larval metamorphosis (Yang et al., 2015), the neuronal and neuroendocrine activities are thought to control the metamorphosis process of the bivalve larvae (García-Lavandeira et al., 2005). More research is needed to identify the functions of acetylcholine chloride and its mechanisms of action in Aurelia jellyfi sh strobilation.
The environment cues for Aureila strobilation have been reported to be temperature (Pascual et al., 2015; Wang and Li, 2015; Sokołowski et al., 2016). When A. coerulea polyps were exposed to decreasing temperature to 10, 13, and 15°C, the length of prestrobilation period was about 19, 18, and 12 days, respectively (Wang et al., 2015). While when the polyps were treated with 5-methoxy-2-methylindole, acetylcholine chloride, and indomethacin, release of ephyra accelerated, and the length of the prestrobilation period was signifi cantly shorter than that for temperature-triggered strobilation. The production of ephyra released per strobila in the treatments (around 5, except for 0.3 in the N-acetyl-L-glutamic acid treatment) was lower than those (around 8) be induced at natural strobilation temperatures (Wang et al., 2012, 2015). The reduced ephyra production when strobilation was induced with bioactives may be related to the shorter feeding duration in the prestrobilation period, increased respiration at 21°C, and relatively lower food availability. During the strobilation season, energy accumulated by polyps was mainly allocated to respiration, budding, release of ephyra, and individual growth. Providing more food produced larger A. coerulea polyps and greater ephyra production at a constant strobilation temperature (Wang et al., 2018). Respiration consumption increased with increasing temperature. At 21°C, A. coerulea polyps required more food to cover energy consumed by respiration. However, bioactives shortened the duration of the prestrobilation period, negatively aff ecting polyp feeding duration and energy accumulation. In addition, ephyra production was directly related to food supply (Thein et al., 2013; Schiariti et al., 2014; Feng et al., 2015; Sun et al., 2015). Our feeding frequency (every 3 days) aff ected food availability, resulting in fewer polyps releasing ephyra, and the lowered ephyra production.
4.2 The potential mechanism of Aurelia spp. strobilation
4.2.1 The common Aurelia spp. strobilation inducer Indole derivatives were the common strobilation inducer of Aurelia spp. Indomethacin induced strobilation in A. coerulea from Jiaozhou Bay, A. aurita from the Seto Inland Sea (Kuniyoshi et al., 2012), and the Roscoff strain of A. aurita (Fuchs et al., 2014). 5-methoxy-2-methylindole induced strobilation in A. aurita (Roscoff strain), A. aurita (Red Sea strain), and our A. coerulea (Fuchs et al., 2014; Brekhman et al., 2015). According to the recent taxonomy, A. aurita in Kuniyoshi et al. (2012) might be A. coerulea due to its geographical origin, the Seto Inland Sea, Japan (Dong, 2019). Further, 5-methoxy-2-methylindole and indomethacin were reported to trigger metamorphosis across a broad diversity of species in Scyphozoa and Cubozoa, except for the coronate scyphozoan and hydrozoan (Helm and Dunn, 2017; Yamamori et al., 2017). So far, the potential mechanism of indole derivatives on strobilation is poorly understood. It was confi rmed that the indomethacin-induced strobilation was not related to the reducing levels of prostaglandin (Kuniyoshi et al., 2012). In addition, Fuches et al. (2014) suggested that the indole derivatives could induce the strobilation of A. aurita, because they contained similar structure (indole and tryptophan) with the protein of “strobilation hormone” precursor. This study suggests that the indole ring modifi ed with methoxy or methyl pharmacophore is the core element of the strobilation inducer in the A. coerulea, indicating the methylation process participates in the strobilation. The indole derivatives induction mechanism is convergent, their role in strobilation deserves further study.
4.2.2 The species-specifi c strobilation inducer
Iodinated organic compounds, including monoiodotyrosine, diiodothyronine, triiodothyronine, and thyroxine, are the thyroid hormones. Tyrosine is an essential raw component in thyroid hormone synthesis. It is synthesized in thyroid acinar cells by iodine and tyrosine through a process of iodine collection, iodine activation, and tyrosine iodination. Related thyroid hormones were detected in Aurelia polyps, but triiodothyronine was not been found in its polyps or in the culture medium.131I-treated Aurelia polyps reveal synthesis of monoiodotyrosine occurred within 8 h, and diiodotyrosine and thyroxine within 24 h; monoiodotyrosine and diiodotyrosine were detected up to the segmentation period, but not in ephyrae (Spangenberg, 1974). Thyroid hormones can regulate development, metabolic stimulation, and metamorphosis in vertebrates (Johnson, 1997). In cnidarians, iodinated organic compounds help form proteinaceous skeletons and aff ect strobilation and metamorphosis (Spangenberg, 1967, 1971; Szmant-Froelich, 1974; Silverstone et al., 1977; Kingsley et al., 2001). In this study, iodine had no induce eff ect on A. coerulea strobilation, 3,5-diiodo-L-tyrosine acted mainly on the process of polyp elongation during strobilation, and thyroxine acted on subsequent transverse constrictions. Iodine and various iodocompounds (monoiodotyrosine, diiodothyronine, triiodothyronine, and thyroxine) were active in initiating strobilation in the A. aurita located near the Puerto Rico (Silverstone et al., 1977). Iodine, thyroxin, triiodothyronine, diiodotyrosine, monoiodotyrosine, and thyroglobulin induced strobilation in temperature-preconditioned A. aurita sampled in the Texas Gulf (Spangenberg, 1967, 1971). These results revealed that iodinated organic compounds were species-specifi c strobilation inducers. In A. coerulea strobilation, the iodinecontained thyroid hormones mainly acted on the prophase of morphological changes, including the polyp body elongation and transverse constrictions formation.
Catecholamine is the neurogenic substance containing catechol and amine groups, including norepinephrine, adrenaline, and dopamine. These three catecholamines are all converted from the precursor tyrosine. In our pre-experiment, tyrosine itself had no A. coerulea strobilation inducing capacity, which is consistent with previous fi ndings (Fuchs et al., 2014). The ability of L-dopamine, and norepinephrine to elongate the polyp body of A. coerulea increased gradually. There are few reports on catecholamine in cnidarians. Hydrozoan Halocordyle disticha and the sea pansy Renilla koellikeri contained and could synthesize diverse catecholamines (Kolberg and Martin, 1988; Pani and Anctil, 1994), which induced the metamorphosis in H. disticha (Edwards et al., 1987). As the catecholamine-related studies in Aurelia spp. were rare, catecholamine was suggested to be the speciesspecifi c inducer in A. coerulea, working on the polyp body elongation.
Acetylcholine plays an essential role in bridging neuron-neuron and neuron-muscular synapses in marine invertebrates (Kuffl er et al., 1984). In bivalves, acetylcholine chloride has been reported to induce larval settlement and metamorphosis (Zhao et al., 2003; Yang et al., 2015). We report acetylcholine chloride as capable of inducing A. coerulea strobilation, suggesting that the activation of strobilation is related to the acetylcholine-involved nerve conduction process. Serotonin, another neurotransmitter derived from tryptophan, was reported to experimentally induce metamorphosis of hydrozoan larvae (McCauley, 1997). However, serotonin hydrochloride failed to induce any morphological changes in A. coerulea, indicating it is not a strobilation inducer in A. coerulea. The eff ect of serotonin on strobilation in other Aurelia species is still unknown.
Retinoic acid is a derivative of retinol. Neither retinoic acid nor vitamin A induced strobilation in our A. coerulea polyps. However, in A. aurita (Roscoff strain), retinoic acid, and retinol were capable of inducing strobilation (Wang, 2013; Fuchs et al., 2014). In strobilation, extrinsic environmental cues perceived by Aurelia polyps lead to intrinsic biochemical or molecular regulation. Among the various environmental factors, temperature is the primary trigger initiating strobilation. As the gene involved in retinoic acid signaling are sensitive to temperature changes, the mechanism behind the strobilation triggered by temperatures can be explained. Strobilation is regulated by a retinoic acid reaction cascade leading to the expression of a strobilation-specifi c gene, CL390, functioned as a temperature-sensitive “timer,” encoding the precursor of the Aurelia strobilation hormone (Fuchs et al., 2014). However, for A. aurita Roscoff strain, a biological-activated CL390 small peptide fragment sequence was not detected (Brekhman et al., 2015). It is possible that the CL390-like sequence is strainspecifi c and depends on the geographic origin of the animal (Schroth et al., 2002). In our Jiaozhou Bay A. coerulea strain, other specifi c peptides provided the conformation function.
4.2.3 The potential A. coerulea strobilation antagonists Identifi cation of the strobilation inducers and their mechanisms contributes to developing specifi c strobilation antagonists, which can be used to control jellyfi sh blooms. Present studies found that in the retinoic acid signaling pathway, the retinoic acid cascade inhibitors 4-diethylaminobenzaldehyde (DEAB) slowed down strobilation induced by retinol, by blocking the production of retinoic acid from retinol aldehyde (Fuchs et al., 2014). Another retinoic acid cascade inhibitors UVI3003, which specifi cally prevents RxR activation, inhibited temperatureinduced strobilation (Fuchs et al., 2014). In this study, indole derivatives modifi ed with methoxy or methyl, the common strobilation inducer, mediated strobilation by a conserved induction pathway within the genus Aurelia. To fi nd out the common strobilation antagonists, the mechanisms of indole derivatives in strobilation inducing, and the gene sites upon which they act in strobilation deserves further investigation. Acetylcholine chloride is the specifi c A. coerulea strobilation inducer. Atropine and penehyclidine hydrochloride are clinical acetylcholine chloride antagonists. Atropine functioned as an acetylcholine M receptor antagonist; it might have potential as an antagonist for acetylcholine chloride-induced strobilation. More research combining pharmacological analysis and the specifi c transcriptome dataset to study the strobilation antagonists is required for controlling jellyfi sh blooms.
5 CONCLUSION
We report A. coerulea polyps to respond in four ways to 18 bioactive substances at 21°C. Type I—no change—as reported for serotonin hydrochloride, vitamin A, retinoic acid, folic acid, iodine and estradiol. As 5-aza-2-deoxycytidine and N-acetyl-Lglutamic acid treatments induced strobilation on individual polyps, we suggest that these two bioactive substances lack the ability to induce strobilation. Type II—where the polyp body elongates, as reported for 3,5-diiodo-L-tyrosine, indole-3-acetic acid, L-dopamine, and noradrenaline treatments. Type III—where formation of transverse constrictions occurs, as reported for L-thyroxine, progesterone, and melatonin treatments. Finally, Type IV—for which the complete strobilation process is induced, as reported for acetylcholine chloride, 5-methoxy-2-methylindole, and indomethacin treatments. The ability of 5-methoxy-2-methylindole and indomethacin to induce strobilation is strongest, with pre-strobilation period reduced to 2 days, and the strobilation rate to be high. The ability of acetylcholine chloride to induce strobilation was weaker (4 days for pre-strobilation), and the strobilation rate was lower. The number of ephyra released did not diff er signifi cantly among the three treatments that induced strobilation. Our results suggest that indole derivatives, which contained methoxy or methyl pharmacophore, are the common strobilation inducer in various Aurelia species. Iodinated organic compounds, catecholamine, and acetylcholine chloride are species-specifi c or strainspecifi c strobilation inducer. It indicates that neuronal and endocrine processes participate in the A. coerulea strobilation. Additionally, these results provide clues for identifying the mechanisms behind strobilation in Aurelia spp. which may be used to develop strobilation antagonists to control moon jellyfi sh blooms.
6 DATA AVAILABILITY STATEMENT
The data that support the fi ndings of this study are available from the corresponding author upon reasonable request.
References
Arai M N. 1997. A Functional Biology of Scyphozoa. Chapman & Hall, London.
Brekhman V, Malik A, Haas B, Sher N, Lotan T. 2015. Transcriptome profi ling of the dynamic life cycle of the scypohozoan jellyfi sh Aurelia aurita. BMC Genomics, 16(1): 74.
Dong Z J, Liu D Y, Keesing J K. 2010. Jellyfi sh blooms in China: dominant species, causes and consequences. Marine Pollution Bulletin, 60(7): 954-963.
Dong Z J. 2019. Blooms of the moon jellyfi sh Aurelia: causes, consequences and controls. In: Sheppard C ed. World Seas: An Environmental Evaluation. 2nded. Academic Press, Amsterdam. p.163-171.
Edwards N C, Thomas M B, Long B A, Amyotte S J. 1987. Catecholamines induce metamorphosis in the hydrozoan Halocordyle disticha but not in Hydractinia echinata. Roux’s Archives of Developmental Biology, 196(6): 381-384.
Feng S, Wang S W, Sun S, Zhang F, Zhang G T, Liu M T, Uye S I. 2018. Strobilation of three scyphozoans ( Aurelia coelurea, Nemopilema nomurai, and Rhopilema esculentum) in the fi eld at Jiaozhou Bay, China. Marine Ecology Progress Series, 591: 141-153.
Feng S, Zhang F, Sun S, Wang S W, Li C L. 2015. Eff ects of duration at low temperature on asexual reproduction in polyps of the scyphozoan Nemopilema nomurai (Scyphozoa: Rhizostomeae). Hydrobiologia, 754(1): 97-111.
Fuchs B, Wang W, Graspeuntner S, Li Y Z, Insua S, Herbst E M, Dirksen P, Böhm A M, Hemmrich G, Sommer F, Domazet-Lošo T, Klostermeier U C, Anton-Erxleben F, Rosenstiel P, Bosch T C G, Khalturin K. 2014. Regulation of polyp-to-jellyfi sh transition in Aurelia aurita. Current Biology, 24(3): 263-273.
García-Lavandeira M, Silva A, Abad M, Pazos A J, Sánchez J L, Pérez-Parallé M L. 2005. Eff ects of GABA and epinephrine on the settlement and metamorphosis of the larvae of four species of bivalve molluscs. Journal of Experimental Marine Biology and Ecology, 316(2): 149-156.
Gorbman A. 1974. Localized organic binding of radioiodine in Hydra. Acta Zoologica, 55(2): 97-100.
Helm R R, Dunn C W. 2017. Indoles induce metamorphosis in a broad diversity of jellyfi sh, but not in a crown jelly (Coronatae). PLoS One, 12(12): e0188601.
Holst S, Jarms G. 2007. Substrate choice and settlement preferences of planula larvae of fi ve Scyphozoa (Cnidaria) from German Bight, North Sea. Marine Biology, 151(3): 863-871.
Ishii H, Ohba T, Kobayashi T. 2008. Eff ects of low dissolved oxygen on planula settlement, polyp growth and asexual reproduction of Aurelia aurita. Plankton and Benthos Research, 3(S1): 107-113.
Johnson L G. 1997. Thyroxine’s evolutionary roots. Perspectives in Biology and Medicine, 40(4): 529-535.
Kingsley R J, Corcoran M L, Krider K L, Kriechbaum K L. 2001. Thyroxine and vitamin D in the gorgonian Leptogorgia virgulata. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 129(4): 897-907.
Kolberg K J, Martin V J. 1988. Morphological, cytochemical and neuropharmacological evidence for the presence of catecholamines in hydrozoan planulae. Development, 103(2): 249-258.
Kroiher M, Siefker B, Berking S. 2000. Induction of segmentation in polyps of Aurelia aurita (Scyphozoa, Cnidaria) into medusae and formation of mirror-image medusa anlagen. The International Journal of Developmental Biology, 44(5): 485-490.
Kuffl er S W, Nicholls J G, Martin A R. 1984. From Neuron to Brain. Sinauer Associates. Sunderland, MA. 651p.
Kuniyoshi H, Okumura I, Kuroda R, Tsujita N, Arakawa K, Shoji J, Saito T, Osada H. 2012. Indomethacin induction of metamorphosis from the asexual stage to sexual stage in the moon jellyfi sh, Aurelia aurita. Bioscience, Biotechnology, and Biochemistry, 76(7): 1 397-1 400.
Liu C Y, Wang W B, Zhou H, Yu X G. 2008a. Relationship between diff erentiation in strobilization and population fl uctuation in four marco-jellyfi sh species. Fisheries Science, 27(11): 592-595. (in Chinese with English abstract)
Liu W C, Lo W T, Purcell J E, Chang H H. 2008b. Eff ects of temperature and light intensity on asexual reproduction of the scyphozoan, Aurelia aurita (L.) in Taiwan. In: Pitt K A, Purcell J E eds. Jellyfi sh Blooms: Causes, Consequences, and Recent Advances. Springer, Dordrecht. p.247-258.
Lu J. 2009. Peninsula City Newspaper. 2009-7-8(A8). (in Chinese)
Lucas C H. 2001. Reproduction and life history strategies of the common jellyfi sh, Aurelia aurita, in relation to its ambient environment. In: Purcell J E, Graham W M, Dumont H J eds. Jellyfi sh Blooms: Ecological and Societal Importance. Springer, Dordrecht. p.229-246.
McCauley D W. 1997. Serotonin plays an early role in the metamorphosis of the hydrozoan Phialidium gregarium. Developmental Biology, 190(2): 229-240.
Pani A K, Anctil M. 1994. Evidence for biosynthesis and catabolism of monoamines in the sea pansy Renilla koellikeri (Cnidaria). Neurochemistry international, 25(5): 465-474.
Pascual M, Fuentes V, Canepa A, Atienza D, Gili J M, Purcell J E. 2015. Temperature eff ects on asexual reproduction of the scyphozoan Aurelia aurita s. l.: diff erences between exotic (Baltic and Red seas) and native (Mediterranean Sea) populations. Marine Ecology, 36(4): 994-1 002.
Prieto L, Astorga D, Navarro G, Ruiz J. 2010. Environmental control of phase transition and polyp survival of a massive-outbreaker jellyfi sh. PLoS One, 5(11): e13793.
Purcell J E, Hoover R A, Schwarck N T. 2009. Interannual variation of strobilation by the scyphozoan Aurelia labiata in relation to polyp density, temperature, salinity, and light conditions in situ. Marine Ecology Progress Series, 375: 139-149.
Purcell J E. 2007. Environmental eff ects on asexual reproduction rates of the scyphozoan Aurelia labiata. Marine Ecology Progress Series, 348: 183-196.
Schiariti A, Morandini A C, Jarms G, Von Glehn Paes R, Franke S, Mianzan H. 2014. Asexual reproduction strategies and blooming potential in Scyphozoa. Marine Ecology Progress Series, 510: 241-253.
Schroth W, Jarms G, Streit B, Schierwater B. 2002. Speciation and phylogeography in the cosmopolitan marine moon jelly, Aurelia sp. BMC Evolutionary Biology, 2: 1.
Silverstone M, Tosteson T R, Cutress C E. 1977. The eff ect of lodide and various lodocompounds on initiation of strobilation in Aurelia. General and Comparative Endocrinology, 32(1): 108-113.
Sokołowski A, Brulińska D, Olenycz M, Wołowicz M. 2016. Does temperature and salinity limit asexual reproduction of Aurelia aurita polyps (Cnidaria: Scyphozoa) in the Gulf of Gdańsk (southern Baltic Sea)? An experimental study. Hydrobiologia, 773(1): 49-62.
Spangenberg D B. 1967. Iodine induction of metamorphosis in Aurelia. Journal of Experimental Zoology, 165(3): 441-449.
Spangenberg D B. 1971. Thyroxine induced metamorphosis in Aurelia. Journal of Experimental Zoology, 178(2): 183-194.
Spangenberg D B. 1974. Thyroxine in early strobilation in Aurelia aurita. American Zoologist, 14(2): 825-831.
Sun M, Dong J, Purcell J E, Li Y L, Duan Y, Wang A Y, Wang B. 2015. Testing the infl uence of previous-year temperature and food supply on development of Nemopilema nomurai blooms. Hydrobiologia, 754(1): 85-96.
Szmant-Froelich A. 1974. Structure, iodination and growth of the axial skeletons of Muricea californica and M. fruticosa (Coelenterata: Gorgonacea). Marine Biology, 27(4): 299-306.
Tarrant A M. 2005. Endocrine-like signaling in cnidarians: current understanding and implications for ecophysiology. Integrative and Comparative Biology, 45(1): 201-214.
Thein H, Ikeda H, Uye S I. 2013. Ecophysiological characteristics of podocysts in Chrysaora pacifi ca (Goette) and Cyanea nozakii Kishinouye (Cnidaria: Scyphozoa: Semaeostomeae): eff ects of environmental factors on their production, dormancy and excystment. Journal of Experimental Marine Biology and Ecology, 446: 151-158.
Van Marle J, Van Wehren-Kramer J, Lind A. 1983. Properties of a catecholaminergic system in some coelenterates. A histochemical and autoradiographic study. Comparative Biochemistry and Physiology Part C: Comparative Pharmacology, 76(1): 193-197.
Wang N, Li C L, Liang Y, Shi Y Q, Lu J L. 2015. Prey concentration and temperature eff ect on budding and strobilation of Aurelia sp. 1 polyps. Hydrobiologia, 754(1): 125-134.
Wang N, Li C L, Wang Y T, Feng S. 2018. Carbon distribution strategy of Aurelia coerulea polyps in the strobilation process in relation to temperature and food supply. Journal of Oceanology and Limnology, 36(6): 2 216-2 230.
Wang N, Li C L. 2015. The eff ect of temperature and food supply on the growth and ontogeny of Aurelia sp. 1 ephyrae. Hydrobiologia, 754(1): 157-167.
Wang W. 2013. Regulation of metamorphosis and the evolution of life cycles: insights from the common moon jelly Aurelia aurita. Universität zu Kiel, Kiel.
Wang Y T, Sun S, Li C L, Zhang F. 2012. Eff ects of temperature and food on asexual reproduction of the Scyphozoan, Aurelia sp.1. Oceanologia et Limnologia Sinica, 43(5): 900-904. (in Chinese with English abstract)
Winans A K, Purcell J E. 2010. Eff ects of pH on asexual reproduction and statolith formation of the scyphozoan, Aurelia labiata. Hydrobiologia, 645(1): 39-52.
Yamamori L, Okuizumi K, Sato C, Ikeda S, Toyohara H. 2017. Comparison of the inducing eff ect of indole compounds on medusa formation in diff erent classes of medusozoa. Zoological Science, 34(3): 173-178.
Yang Z, Yu H, Yu R, Li Q. 2015. Induced metamorphosis in larvae of the veined rapa whelk Rapana venosa using chemical cues. Marine Biology Research, 11(10): 1 085-1 092.
Zhao B, Zhang S, Qian P Y. 2003. Larval settlement of the silver-or goldlip pearl oyster Pinctada maxima (Jameson) in response to natural biofi lms and chemical cues. Aquaculture, 220(1-4): 883-901.
 Journal of Oceanology and Limnology2020年5期
Journal of Oceanology and Limnology2020年5期
- Journal of Oceanology and Limnology的其它文章
- Distribution, sources and burial fl ux of sedimentary organic matter in the East China Sea*
- Photoelectrochemical cathodic protection of Cu 2 O/TiO 2 p-n heterojunction under visible light*
- Antioxidant bisabolane-type sesquiterpenoids from algalderived fungus Aspergillus sydowii EN-434*
- Calcium isotopic signatures of depleted mid-ocean ridge basalts from the northeastern Pacifi c*
- Application of confocal laser Raman spectroscopy on marine sediment microplastics*
- Corrosion behavior of Q235B carbon steel in simulated seawater pumped storage system under operational conditions*
